New perspectives on ovulation induction with letrozole in women with polycystic ovary syndrome
Objective: To compare the efficacy and adverse event rates of letrozole in a stair-step and standard ovulation induction (OI) protocols in women with polycystic ovary syndrome (PCOS) and anovulatory infertility. Materials and methods: The study included 194 patients aged 28.7 (25;33), body mass index 21.5 (21;26) kg/m2. Of them, 106/194 (54.6%) women did not respond to the initial stimulation with 2.5 mg letrozole. They were divided into group 1 (n=52) and group 2 (n=54) undergoing stair-step protocol and standard protocol, respectively. During OI, the patients underwent ultrasound monitoring of ovarian and endometrial status. Furthermore, the clinical evaluation included estimated time of ovulation and ovulation and pregnancy rates. Results: The ovulation rates were equally high in both groups and was 92.3% on the stair-step protocol and 85.2% on the standard protocol (p=0.25). The time to ovulation was statistically significantly shorter in the stair-step protocol [33.9 (3.23) versus 59.5 (11.96) days; p<0.001]. The mean M echo was larger in the same group [0.2 (2.2) mm versus 9.3 (2.2) mm; p=0.01]. There was no statistical difference in pregnancy and live birth rates between the stair-step and standard protocols [17/52 (32.7%) versus 12/54 (22.2%); p=0.23 and 15/52 (28.8%) versus 11/54 (20.4%); p=0.31]. None of the patients developed ovarian hyperstimulation syndrome (OHSS). The incidence of multiple pregnancies in group 1 was 1/17 (5.9%) (p=0.31). Conclusion: The stair-step protocol with letrozole allows repeated stimulation without inducing a menstrual-like response and can be considered an effective treatment of anovulatory infertility in PCOS. It reduces the time to ovulation approximately twofold without increasing the risk of OHSS and multiple pregnancies, thus improving patients' quality of life.Chernukha G.E., Kaprina E.K., Golovanova A.A.
Keywords
Polycystic ovarian syndrome (PCOS) is the most common cause of reproductive endocrine disorders, accounting for about 75–80% of all cases of anovulatory infertility [1, 2]. The prevalence of PCOS ranges from 6 to 21%, depending on the diagnostic criteria and the population [3, 4].
Many clinicians focus on the use of assisted reproductive technologies rather than drug-induced ovulation (OI) in the treatment of infertility. However, the high cost of in vitro fertilization programs, the increased risk of ovarian hyperstimulation, and multiple pregnancies do not allow IVF to be considered the main treatment modality. According to the Russian clinical guidelines, the first-line OI for PCOS is the use of an estrogen receptor blocker, clomiphene citrate (CC) [5]. In 2018, letrozole (LT) as the first‐line treatment for PCOS was included in the international clinical guidelines as an alternative to CC because its effectiveness is approximately 1.5 times greater than that of CC [3, 6, 7]. Although the safety of LT as an OI has been proven in many studies, it is still classified as an off-label drug and, according to Russian clinical guidelines, can only be recommended for use after signing informed consent [5, 8–10]. LT, like CC, has antiestrogenic effects, although its mechanism of action is different. Letrozole is a selective aromatase inhibitor which prevents the conversion of androgens to estrogen, thus releasing the hypothalamic-pituitary axis from the negative feedback of estrogen, resulting in an increase of follicle stimulating hormone (FSH) secretion from the anterior pituitary. It is also believed that LT-associated accumulation of intraovarian androgens further increases follicular sensitivity to FSH and stimulates insulin-like growth factor-1, which may have a positive effect on folliculogenesis [11, 12].
The high rate of clomiphene resistance has prompted the search for new approaches to OI by modification of stimulation protocols. In addition to the combination of CC and metformin, a so-called stair-step protocol was proposed in which, in women who were not responsive to stimulation with 50 mg CC, this dose was doubled without menstrual induction [13]. Few studies have shown that the use of the stair-step protocol reduces the time to ovulation compared with the standard protocol and, according to some data, enables an increased ovulation and pregnancy rates [14–18]. In recent years, several articles have been published with controversial results on the use of the stair-step LT OI protocol in PCOS. For example, one study reported an increase in ovulation and pregnancy rates [19], while another did not find significant differences [20]. Taking into account the above data, this study was planned and conducted to optimize therapy regimens for anovulatory infertility in PCOS.
The present study aimed to compare the efficacy and adverse event rates of letrozole in the stair-step and standard ovulation induction protocols in women with PCOS and anovulatory infertility.
Materials and methods
A total of 194 women with PCOS and anovulatory infertility [age 28.7 (25; 33) years; body mass index (BMI) 21.5 (21; 26) kg/m²] were included in a prospective randomized study. PCOS was diagnosed according to the Rotterdam criteria (2003) [21].
Patients were selected according to inclusion criteria (age 18 to 35 years; BMI 18.5 to 40 kg/m²) and exclusion criteria (tubal-peritoneal and male infertility factors; endocrine disorders; severe somatic diseases; congenital malformations, OI, and use of combined oral contraceptives during the last 3 months).
Before the study, all patients were tested for hormone levels on days 2–3 of their menstrual cycle, including anti-müllerian hormone (AMH), luteinizing (LH) and follicle stimulating hormone (FSH), thyroid stimulating hormone (TSH), prolactin, total and free testosterone, androstenedione, and sex hormone binding globulin (SHBG).
Pelvic ultrasound (US) was performed using a 2000 Toshiba SSA-240 (Japan) transvaginal 7.5 MHz convex transducer; the volume of the ovaries was determined and the number of antral follicles was counted. Follicular response was assessed on day 14 of the cycle. If a dominant follicle was more than 10 mm, ovulation was monitored by ultrasound 7 days and endometrial thickness was measured; serum progesterone levels were also determined. Pregnancy was diagnosed by the serum human chorionic gonadotropin (hCG) β-subunit on day 14 after ovulation.
For OI, all women initially received LT (Femara 2.5 mg, Novartis) at a dose of 2.5 mg/day on days 3 to 7 of the spontaneous or micronized progesterone-induced menstrual cycle. In 88/194 (45.4%) cases, the follicle size exceeded 10 mm, and these patients were not included in the further study. Women who did not respond to the starting dose of LT [106/194 (54.6%)] were divided into group 1 (n=52) and group 2 (n=54) undergoing stair-step protocol and standard protocol, respectively. Simple envelope randomization was used to randomly assign study participants into groups. The blinding procedure was not used in this study.
In group 1, in the absence of a dominant follicle on day 14 of the cycle, LT was administered at a double dose (5 mg) for 5 days without induced menstrual bleeding. Seven days after completion of treatment, a new pelvic ultrasound scan was performed to assess endometrial thickness and ovarian function; progesterone levels were measured (Figure A). In group 2 (standard protocol), in the absence of a dominant follicle on day 14 of the cycle, signs of ovulation by progesterone level (less than 3 ng/ml) and pelvic ultrasound examination 7 days later (cycle day 21), the dose of LT was increased to 5 mg/day from day 3 to day 7 of the menstrual cycle induced by micronized progesterone (300 mg/day) (Figure, scheme B). The estimated time to ovulation was counted from day 1 of the menstrual cycle until progesterone levels were determined. The maximum duration of OI did not exceed 6 cycles, and the LT dose was 7.5 mg. Clinical outcomes were ovulation rate, mean time to ovulation, pregnancy and live birth rate, multiple pregnancy rate, and endometrial thickness.
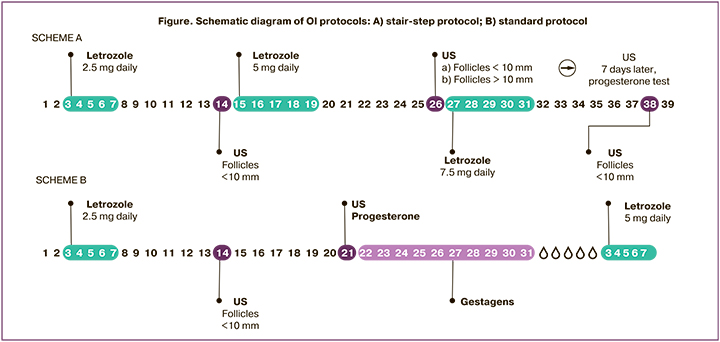
The study was conducted at the Department of Gynecological Endocrinology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation. The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P; all patients signed an informed consent to participate in the study.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics v.26 software (IBM Corporation). The distribution of continuous variables was tested for normality using the Kolmogorov–Smirnov test; skewness and kurtosis were calculated. Quantitative variables showing a normal distribution were expressed as means (M) and standard deviation (SD); otherwise, the median (Me) with the interquartile range (Q1; Q3) was reported. Categorical variables were presented as counts and percentages. Parametric Student's t-test, nonparametric Mann–Whitney U-criterion, and Fisher's test were used to compare the variables as appropriate. Relative risk with corresponding 95% confidence interval (95% CI) for binary outcomes, difference between means with corresponding 95% CI and the difference between medians with corresponding 95% CI estimated using nonparametric bootstrap (B=9999) were used as effect estimates when comparing groups.
Hypothesis of superiority was tested for all outcomes. In this study, no blinding procedure was performed. Differences were considered statistically significant at p<0.05. The sample size of the study was not calculated because it was a pilot study and no data were found in the literature to estimate the expected effect.
Results
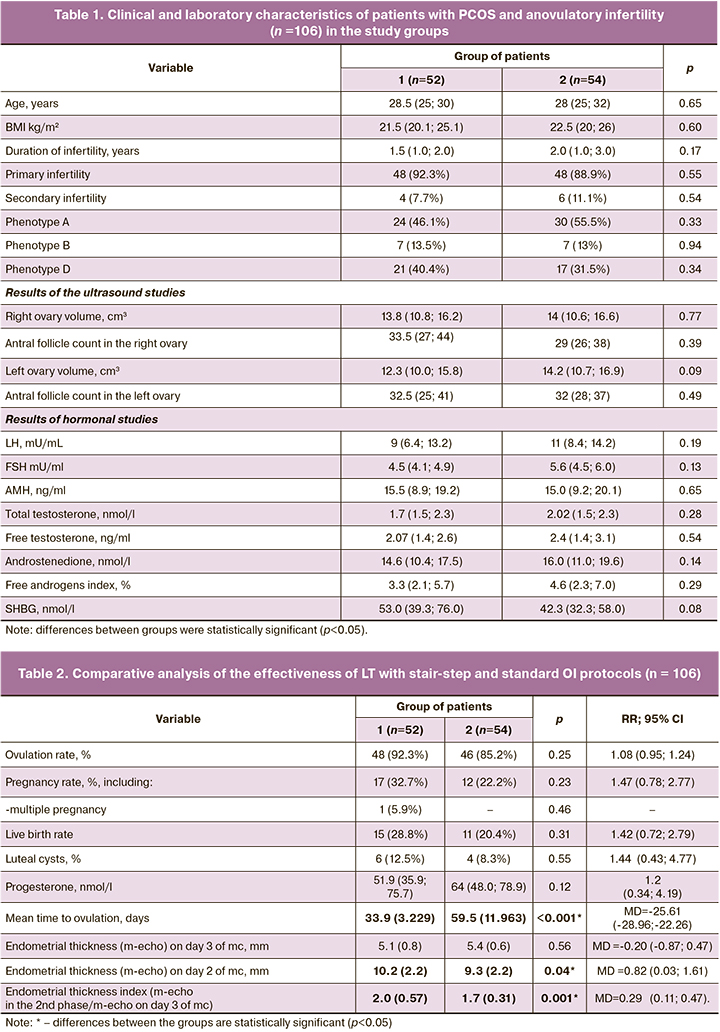
Baseline clinical and laboratory characteristics of the patients in the study are presented in Table 1. The patients were comparable for all main parameters (p>0.05).
The analysis showed that the distribution of phenotypes in both groups was similar: every second case was diagnosed with classic phenotype A, about every 3rd case with nonandrogenic phenotype D; phenotype B was observed much less frequently; there were patients with ovulatory phenotype C. Excess body weight was observed in 14/52 (26.9%) patients in group 1 and 18/54 (33.3%) in group 2. Two patients in each group had grade I obesity (3.8 and 3.7%, respectively; p=0.47; p=0.97). Clinical hyperandrogenism was diagnosed in 25/52 (48%) patients in group 1 and in 27/54 (50%) cases in group 2 (p=0.84). Menstrual cycle disorders were observed in all patients. In group 1, 38/52 (73.1%) patients had primary oligomenorrhea, 12/52 (23.1%) had secondary oligomenorrhea, and 2/52 (3.8%) had secondary amenorrhea. In Group 2, these indicators were 45/54 (83.3%), 5/54 (9.3%), and 4/54 (7.4%), respectively (p=0.10; p=0.12; p=0.42).
The mean duration of infertility in groups 1 and 2 was 1.5 (1.0; 2.0) and 2.0 (1.0; 3.0), respectively (p=0.17). Patients with secondary infertility had spontaneous pregnancies: two patients underwent termination of pregnancy; 4 patients had a history of spontaneous pregnancies up to 12 weeks, and in 4 cases, the pregnancies ended in spontaneous births.
As shown in Table 2, the ovulation rate in response to 5 mg of LT was high and did not differ significantly between the groups (p=0.25; OR=1.08 (95% CI 0.95; 1.24). However, the time to ovulation in the stair-step protocol was almost twice shorter than in the standard protocol [33.9 (3,229) versus 59.5 (11,963); MD=-25.61 (95% CI -28.96; -22.26), p<0.001]. In the same group, the mean endometrial thickness in the second phase of the cycle was statistically significantly higher than in group 2 [MD=0.82 (95% CI 0.03; 1.61), p=0.04)], as was the endometrial thickness index [(2.0 (0.57) versus 1.7 (0.31); MD=0.29 (95% CI 0.11; 0.47), p<0.001]. No cases of thin endometrium (less than 7 mm) were detected in both groups. Progesterone levels less than 10 ng/mL were in 5/48 (10.4%) cases in group 1, and 4/46 (8.7%) cases in group 2 [p=0.77; OR=1.2 (95% CI 0.34; 4.19)]. Corpus luteum cysts were observed in approximately 1 in 10 patients; no statistical difference was found between groups [p=0.55; OR=1.44 (95% CI 0.43; 4.77)]. Cysts ranged in size from 3 to 4.5 cm; they regressed spontaneously within 1 to 2 subsequent cycles. No ovarian hyperstimulation syndrome was observed in either group during OI with LT. All the patients tolerated the drug well. The absence of ovulation during 3 cycles of stimulation when the maximum dose of the drug (7.5 mg) was reached was regarded as LT resistance and was observed in 3 patients: 1/52 (1.9%) in group 1 and 2/54 (3.7%) in group 2 [p=0.53; OR=0.52 (95% CI 0.05; 5.55)].
As shown in Table 2, the pregnancy rate was almost 1.5 times higher in group 1 than in group 2, but the difference did not reach statistical significance [p=0.23; OR=1.47 (95% CI 0.78; 2.77)]. Biochemical pregnancy was diagnosed in 2/17 (11.8%) patients in group 1. In group 2 there was 1/12 (8.3%) case of missed miscarriage before 12 weeks. Group 1 had 1/17 (5.9%) cases of multiple pregnancies (p=0.46). Live birth rates were comparable in both groups: 15/52 (28.8%) versus 11/54 (20.4%) in groups 1 and 2, respectively [p=0.31; OR=1.42 (95% CI 0.72; 2.79)].
Analysis of obstetric complications showed that gestational diabetes mellitus was diagnosed in 3/15 (20%) patients in group 1 and 2/11 (18.1%) in group 2 [p=0.90; OR=1.10 (95% CI 0.22; 5.51)], gestational arterial hypertension was diagnosed in 1/15 (6.7%) patients in group 1, preterm birth between 34–36 weeks in 2/15 (13.3%) in group 1 and in 1/11 (9.1%) in group 2 [p=0.73; OR=1.47 (95% CI 0.15; 14.21)]. In group 1, 3/15 (20%) women and 4/11 (36.7%) in group 2 underwent cesarean section [p=0.35; OR=0.55 (95% CI 0.15; 1.97)]. Of these, one patient underwent surgery for multiple pregnancies, 3 for breech presentation, 2 for fetal hypoxia in labor, and 1 for ophthalmologic indications. The median birth weight was 3400 (3135; 3625) g in group 1 and 3340 (3330; 3545) g in group 2 (median difference = 60 (95% CI -350; 237), p=0.83). The Angar score at 1st minute in both groups was 8.6 (1.2) vs. 8.2 (1.3) [MD=0.4 (95% CI -0.64; 1.44), p=0.43] and at 5th minute 9.3 (0.6) vs 9.3 (0.6) [MD=0.2 (95% CI -0.21; 0.61), p=0.32].
Discussion
Among patients with type 2 chronic anovulation (according to the WHO classification), approximately 90% are patients with PCOS [2]. Since 2008, a three-line approach has been used to treat anovulatory infertility in PCOS [22]. Indirect ovulation inducers (LT, CC) are recommended as first-line therapy, gonadotropins or laparoscopic ovarian trilling are recommended as second-line therapy, and assisted reproductive technologies are the third line. Some of these therapies often require a long time to produce therapeutic effect, are expensive, carry a high risk of ovarian hyperstimulation syndrome and multiple pregnancy, and some are associated with the risk of surgical complications. As a result of the search for effective therapy regimens for anovulatory infertility more than ten years ago, American research scientists developed and applied in clinical practice a stair-step OI protocol with CC, which increased ovulation rates and reduced the time to ovulation [13]. The authors concluded that menstrual induction before increasing the CC dose in patients who did not respond to the initial dose of the ovulation trigger. In 2019, Thomas S. et al. compared the efficacy of CC and LT, which were used without inducing menstrual bleeding in patients resistant to CC. They found advantages of LT over CC regarding shorter time to ovulation, while ovulation and pregnancy rates were almost identical [19]. At the same time, in a later study, similar in design, the use of LT was statistically significantly more likely to cause ovulation and resulted in a higher rate of live births compared to CC [20]. In the domestic literature, there are only a few publications on the effectiveness of the standard OI protocol with LT [23, 24]. All this served as rationale for this study aimed at optimizing the treatment of anovulatory infertility in PCOS.
The findings of this study showed no ovulation at the initial 2.5 mg dose of LT dose in every 2nd case. The ovulation rate was twice as high at the 5-mg dose and was 92.3% on the stair-step protocol and 85.2% on the standard protocol. The ovulation rate was even higher than in one of the largest multicenter studies of LT efficacy, in which it was 61.7% [6]. This may be due to differences in BMI, since there were no obese women in our study. Ovulation rates in the stair-step protocol were comparable to those in studies that used a combination of LT with metformin and used hCG as an ovulation trigger [19, 20]. Despite the absence of differences in ovulation rates, the time to ovulation was almost twice shorter [33.9 (3.23) and 59.5 (11.96) days, p=0.04] when using the stair-step protocol. Taking into account that 23-54% of women with infertility suffer from anxiety and depressive disorders, it can be assumed that reduction of treatment duration and waiting time for OI results contribute to reduction of the incidence of these disorders and improvement of quality of life in patients with PCOS [25].
According to the study findings, LT has no adverse effect on the endometrium, which is evidenced by the absence of cases of thin endometrium. It should also be noted that the average M-echo in the second phase of the cycle was greater on the stair-step protocol than on the standard protocol. The endometrial thickness in group 1 was greater than in group 2 [2.0 (0.7) vs. 1.6 (0.5), (p=0.01)]. Previous studies showed that the expression levels of integrin αvβ3, L-selectin, leukemia inhibitory factor, and pinopod formation in endometrial epithelial and stromal cells were significantly higher in patients receiving LT compared to CC, which had a positive effect on pregnancy rate and its course [26, 27].
In patients undergoing OI, the pregnancy rate was comparable in both groups (32.7% vs. 22.2%, p=0.23); the lack of statistical significance may be due to the small sample size. The literature discusses the possibility of an adverse effect of gestagen-induced menstrual bleeding before OI in patients with PCOS, which may occur by affecting the hypothalamic-pituitary-ovarian axis, affecting endometrial structure and receptivity, which may reduce the likelihood of conception, and possibly of live births [28, 29]. It is also worth noting that in a study by Thomas et al., the pregnancy rate in the stair-step protocol using LT was twice as low as in our study. This significant difference is associated with the fact that the study included women of older reproductive age, resistant to CC, with a higher BMI [BMI 30.9 (4.7) kg/m2] [19]. The incidence of obstetric complications was comparable to that reported in the literature [30].
Conclusion
In patients with anovulatory infertility and PCOS, doubling the initial LT dose of 2.5 mg is associated with a double ovulation rate. The use of the stair-step protocol can be recommended as an effective and safe approach to OI, allowing a shorter time to ovulation and a greater endometrial thickness, without increasing the risk of ovarian hyperstimulation syndrome and multiple pregnancies.
References
- Balen A.H., Morley L.C., Misso M., Franks S., Legro R.S., Wijeyaratne C.N. et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum. Reprod. Update. 2016; 22(6): 687-708. https://dx.doi.org/10.1093/humupd/dmw025.
- ESHRE Capri Workshop Group. Health and fertility in World Health Organization group 2 anovulatory women. Hum. Reprod. Update. 2012; 18(5): 586-99. https://dx.doi.org/10.1093/humupd/dms019.
- Teede H.J., Misso M.L., Costello M.F., Dokras A., Laven J., Moran L. et al.; International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum. Reprod. 2018; 33(9): 1602-18. https://dx.doi.org/10.1093/humrep/dey256.
- Bozdag G., Mumusoglu S., Zengin D., Karabulut E., Yildiz B.O. The prevalence and phenotypic features of polycystic ovarysyndrome: a systematic review and meta-analysis. Hum. Reprod. 2016; 31(12): 2841-55. https://dx.doi.org/10.1093/humrep/dew218.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Синдром поликистозных яичников. М.; 2021. [Ministry of Health of the Russian Federation. Clinical guidelines. Polycystic Ovarian Syndrome. 2021. (in Russian)].
- Legro R.S., Brzyski R.G., Diamond M.P., Coutifaris C., Schlaff W.D., Casson P. et al.; NICHD Reproductive Medicine Network. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N. Engl. J. Med. 2014; 371(2): 119-29. https://dx.doi.org/10.1056/NEJMoa1313517.
- Franik S., Kremer J.A., Nelen W.L., Farquhar C. Aromatase inhibitors for subfertile women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2014; 24(2): CD010287. https://dx.doi.org/10.1002/14651858.CD010287.pub2.
- Tulandi T., Martin J., Al-Fadhli R., Kabli N., Forman R., Hitkari J. et al. Congenital malformations among 911 newborns conceived after infertility treatment with letrozole or clomiphene citrate. Fertil. Steril. 2006; 85(6): 1761-5. https://dx.doi.org/10.1016/j.fertnstert.2006.03.014.
- Sharma S., Ghosh S., Singh S., Chakravarty A., Ganesh A., Rajani S., Chakravarty B.N. Congenital malformations among babies born following letrozole or lomiphene for infertility treatment. PLoS One. 2014; 9(10): e108219. https://dx.doi.org/10.1371/journal.pone.0108219.
- Tatsumi T., Jwa S.C., Kuwahara A., Irahara M., Kubota T., Saito H. No increased risk of major congenital anomalies or adverse pregnancy or neonatal outcomes following letrozole use in assisted reproductive technology. Hum. Reprod. 2017; 32(1): 125-32. https://dx.doi.org/10.1093/humrep/dew280.
- Garcia-Velasco J.A., Moreno L., Pacheco A., Guillén A., Duque L., Requena A., Pellicer A. The aromatase inhibitor letrozole increases the concentration of intraovarian androgens and improves in vitro fertilization outcome in low responder patients: A pilot study. Fertil. Steril. 2005; 84(1): 82-7.https://dx.doi.org/10.1016/j.fertnstert.2005.01.117.
- Palomba S. Aromatase inhibitors for ovulation induction. J. Clin. Endocrinol. Metab. 2015; 100(5): 1742-7. https://dx.doi.org/10.1210/jc.2014-4235.
- Hurst B.S., Hickman J.M., Matthews M.L., Usadi R.S., Marshburn P.B. Novel clomiphene "stair-step" protocol reduces time to ovulation in women with polycystic ovarian syndrome. Am. J. Obstet. Gynecol. 2009; 200(5): 510.e1-4. https://dx.doi.org/10.1016/j.ajog.2008.10.031.
- Deveci C.D., Demir B., Sengul O., Dilbaz B., Goktolga U. Clomiphene citrate 'stair-step' protocol vs. traditional protocol in patients with polycystic ovary syndrome: a randomized controlled trial. Arch. Gynecol. Obstet. 2015; 291(1): 179-84. https://dx.doi.org/10.1007/s00404-014-3398-y.
- AbdelHamid A.M.S., Rateb A.M., Madkour W.A.I. Is clomiphene citrate stair-step protocol a good alternative to gonadotrophins in clomiphene-resistant PCO patients? Prospective study. J. Obstet. Gynaecol. Res. 2016; 42(5): 547-53. https://dx.doi.org/10.1111/jog.12940.
- Horowitz E., Levran D., Weissman A. Extension of the clomiphene citrate stair-step protocol to gonadotropin treatment in women with clomiphene resistant polycystic ovarian syndrome. Gynecol. Endocrinol. 2017; 33(10): 807-10. https://dx.doi.org/10.1080/09513590.2017.1320381.
- Agrawal K., Gainder S., Dhaliwal L.K., Suri V. Ovulation induction using clomiphene citrate using stair - step regimen versus traditional regimen in polycystic ovary syndrome women - A randomized control trial. J. Hum. Reprod. Sci. 2017; 10(4): 261-4. https://dx.doi.org/10.4103/jhrs.JHRS_15_17.
- Jones T., Ho J.R., Gualtieri M., Bruno-Gaston J., Chung K., Paulson R.J., Bendikson K.A. Clomiphene stair-step protocol for women with polycystic ovary syndrome. Obstet. Gynecol. 2018; 131(1): 91-5. https://dx.doi.org/10.1097/AOG.0000000000002418.
- Thomas S., Woo I., Ho J., Jones T., Paulson R., Chung K., Bendikson K. Ovulation rates in a stair-step protocol with Letrozole vs clomiphene citrate in patients with polycystic ovarian syndrome. Contracept. Reprod. Med. 2019; 4: 20.https://dx.doi.org/10.1186/s40834-019-0102-4.
- Sakar M.N., Oğlak S.C. Comparison of the efficacy of letrozole stair-step protocol with clomiphene citrate stair-step protocol in the management of clomiphene citrate-resistant polycystic ovary syndrome patients. J. Obstet. Gynaecol. Res. 2021; 47(11): 3875-82. https://dx.doi.org/10.1111/jog.14936.
- The Rotterdam ESHRE/ASRM-sponsored PCOS Consensus Workshop Group. Revised 2003 consessus on diagnostic criteria and longterm health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004; 19(1): 41-7.https://dx.doi.org/10.1093/humrep/deh098.
- Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Hum. Reprod. 2008; 23(3): 462-77. https://dx.doi.org/10.1093/humrep/dem426.
- Морчиладзе А.З., Савина В.А., Ткаченко Н.Н., Ярмолинская М.И. Применение ингибитора ароматазы летрозола для индукции овуляции у женщин с синдромом поликистозных яичников. Журнал акушерства и женских болезней. 2011; 60(2): 52-7. [Morchiladze A.Z., Savina V.A., Tkachenko N.N. Use of the aromatase inhibitor letrozole to induce ovulation in women with polycystic ovary syndrome. Journal of Obstetrics and Women Diseases. 2011; 2. (in Russian)].
- Кузнецова И.В., Фернандес Д.О., Гаврилова Е.А., Ведзижева Э.Р. Восстановление фертильности у пациенток с нормогонадотропной овуляторной дисфункцией и ожирением. Эффективная фармокотерапия. 2021; 17(43): 14-9. https://dx.doi.org/10.33978/2307-3586-2021-17-43-14-19. [Kuznetsova I.V., Fernandez D.O., Gavrilova E.A. Restoration of fertility in patients with normogonadotropic ovulatory dysfunction and obesity. Effective Pharmacotherapy. 2021; 17(43): 14-9. (in Russian)].
- Yin X., Ji Y., Chan C.L.W., Chan C.H.Y. The mental health of women with polycystic ovary syndrome: a systematic review and meta-analysis. Arch. Womens Ment. Health. 2021; 24(1): 11-27. https://dx.doi.org/10.1007/s00737-020-01043-x.
- Miller P.B., Parnell B.A., Bushnell G., Tallman N., Forstein D.A., Higdon H.L. 3rd, et al. Endometrial receptivity defects during IVF cycles with and without letrozole. Hum. Reprod. 2012; 27(3): 881-8. https://dx.doi.org/10.1093/humrep/der452.
- Ganesh A., Chauhan N., Das S., Chakravarty B., Chaudhury K. Endometrial receptivity markers in infertile women stimulated with letrozole compared with clomiphene citrate and natural cycles. Syst. Biol. Reprod. Med. 2014; 60(2): 105-11. https://dx.doi.org/10.3109/19396368.2013.862316.
- Diamond M.P., Kruger M., Santoro N., Zhang H., Casson P., Schlaff W. et al. Endometrial shedding effect on conception and live birth in women with polycystic ovary syndrome. Obstet. Gynecol. 2012; 119(5): 902-8.https://dx.doi.org/10.1097/AOG.0b013e31824da35c.
- Farhi J., Orvieto R., Homburg R. Administration of clomiphene citrate in patients with polycystic ovary syndrome, without inducing withdrawal bleeding, achieves comparable treatment characteristics and outcome. Fertil. Steril. 2010; 93(6): 2077-9. https://dx.doi.org/10.1016/j.fertnstert.2009.08.019.
- Chatzakis C., Tsakmaki E., Psomiadou A., Charitakis N., Eleftheriades M., Dinas K. et al. Different pregnancy outcomes according to the polycystic ovary syndrome diagnostic criteria: a systematic review and meta-analysis of 79 studies. Fertil. Steril. 2022; 117(4): 854-81. https://dx.doi.org/10.1016/j.fertnstert.2021.12.027.
Received 08.07.2022
Accepted 28.11.2022
About the Authors
Galina E. Chernukha, MD, Professor, Head of the Department of Gynecologic Endocrinology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(985)999-60-00, c-galina1@yandex.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.Elena K. Kaprina, Postgraduate Student at the Department of Gynecologic Endocrinology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(916)129-41-18, kaprina_elena@mail.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Аlina A. Golovanova, PhD, Obstetrician-Gynecologist, Medical Diagnostic Sonographer, Medok LLC, +7(916)675-00-97, aleeshka@mail.ru,
117403, Russia, Moscow, Nikolskaya str., 4, office 102.
Authors' contributions: Chernukha G.E., Kaprina E.K. – conception and design of the study; Kaprina E.K., Golovanova A.A. – data collection and analysis, statistical analysis; Kaprina E.K. – manuscript drafting; Chernukha G.E. – manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was conducted within the framework of the state task "Optimization of management strategy for patients with different phenotypes of PCOS based on the assessment of molecular genetic, morphological and endocrine-metabolic parameters".
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Chernukha G.E., Kaprina E.K., Golovanova A.A. New perspectives on ovulation induction with letrozole in women with polycystic ovary syndrome.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 12: 107-114 (in Russian)
https://dx.doi.org/10.18565/aig.2022.162



