Analysis of the prevalence and viral load of different human papillomavirus types in the regions of the Russian Federation
Objective. To analyze the pattern of HPV carriage and the characteristics of viral load of HPV type 21 in different regions of the Russian Federation.Donnikov A.E., Markelov M.I., Pestrikova T.Yu., Yurasova E.A., Kotelnikova A.V.. Voroshilina E.S., Plotko E.E., Belokhvostikova T.S., Bondareva V.P., Chernikova M.A., Vashchenko S.N., Chernikova V.V., Stankevich L.I., Khasina M.Yu., Galkina I.S.
Subjects and methods. A total of 32,650 patients were surveyed. DNA samples collected by scraping the epithelium of the cervical canal (in women) or the urethra (in men) were analyzed using real-time PCR detection.
Results. At least one type of HPV was detected in 38.9% of the patients; two or more types were simultaneously identified in 32.8%. The largest proportion of patients with two or more types of HPV was observed in women aged less than 25 and 55-60 years and in men aged 50-55-years. The most common HPV type 16 was detected in 27.3% of HPV-positive men and 25.6% of women. HPV type 44 was found to be most common in the Far Eastern District and the North Caucasus Federal District; HPV types 52, 53, 58 were in the Volga Federal District. Higher viral load was more frequently detected in young women.
Conclusion. There were regional differences in the prevalence of HPV types. The carriage of more than one type of HPV was shown to be related to age and sex. Viral load differences according to the type of HPV were described.
Keywords
Cervical cancer (CC) has the second highest incidence in Russia and the world among women’s oncological diseases [1, 2]. According to the data from the Ministry of Health of Russia, in 2016, 10,000 new cases of CC among the working-age female population were registered, and the number of women who died from cervical cancer was 2,900 [1].
In 95% of the CC cases highly carcinogenic types of human papillomavirus (HPV) are detected; in 70% of the cases they cause the CC development and precancerous pathological conditions [2, 3]. The problem of diagnosis and treatment of diseases caused by HPV is relevant due to the sharp increase in infection of the population with this pathogen, its significant contagiosity, diverse localization of lesions and proven high oncogenicity of certain HPV types [4].
Vaccination against HPV is the primary prevention of CC. Today, there are several vaccines on the market, differing in the range of HPV types for which immunity is produced. It is believed that cross-reactions result in high probability of developing immunity to some other types of HPV. However, according to experts, a significant effect in the population is observed in 10–12 years after the start of systematic vaccination, in this regard there is still a need for secondary prevention of CC, especially in Russia, where there are no state-sponsored HPV vaccination programs.
Screening is the basis for secondary prevention of CC, which is capable of detecting early precancerous changes in the epithelium of the cervical canal. In addition to traditional screening tests — PAP-test and acetic acid test, HPV testing is recommended by World Health Organization as a primary test because it has a greater clinical sensitivity and allows for interscreening intervals to be longer compared to traditional cytological screening [5].
It is known that HPV incidence varies depending on the region [6], which should be taken into account in the formation of the screening panel, but the use of different diagnostic systems does not allow us to assess the place of rare types in the HPV carriage structure with sufficient accuracy.
The literature presents a lively discussion of the diagnostic value of determining the HPV viral load [4, 7, 8], as well as the clinical significance of co-infection with several types of HPV [9], however, population-based studies in this area are virtually non-existent.
The purpose of this study is to analyze the incidence of various HPV types and the characteristics of the HPV viral load in different regions of the Russian Federation.
Patients and Methods
Currently there is an HPV-Quant-21 diagnostic panel on the market developed by “DNA-technology”, which makes possible to conduct genotyping of HPV for 21 types and a differentiated evaluation of the viral load.
This multicenter study included depersonalized data from patients who came to laboratories within the period of 2010–2016 to do the HPV-Quant-21 test.
The materials for the study were DNA samples collected by scraping the epithelium of the cervical canal (in women) or the urethra (in men). A total of 32,650 epithelial scrapes from patients were analyzed, including 31,547 samples from women and 1,013 samples from men. From the total number of patients, 1,703 patients with at least one type of HPV were selected for further testing.
The materials for the study (scraping of epithelial cells from the urethra or cervical canal) were transferred to a plastic tube with a 1.5 ml volume with a transport medium using a disposable sterile probe. Storage and transportation of the material was carried out according to the current regulations. DNA extraction was conducted using a kit for isolating nucleic acids, PROBA-GS-PLUS developed by “DNA-Technology”, registration certificate No. FSR 2010/08696. Genotyping for 21 HPV types (6, 11, 16, 18, 26, 31, 33, 35, 39, 44, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82) and determining the viral load of each type were conducted using real-time PCR detection with a reagent kit developed by “DNA-Technology” (HPV Quant-21, registration certificate No. FSR 2010/08811). The detection amplifier DT-prime was used in accordance with the manufacturer’s (“DNA-Technology”) instructions. When considering the results, an absolute analysis type was used to estimate the number of viral particles in the sample.
Statistical treatment was carried out using the IBM SPSS Statistics version 21.0 software package.
Results and Discussion
The average age of patients was 29.5 ± 8.6 years (women 29.4 ± 8.6 years; men 30 ± 8.1 years). As patients were included in the study based on treatment demand, the incidence of HPV carriage in this sample was not analyzed. We have analyzed only the HPV structure and viral load in patients with at least one HPV type, namely 12026 women and 677 men.
To account for the geographical features, 12703 patients were divided into 8 groups according to the Federal District (FD) where the patient lived: Far East FD — 3282 patients, North West FD — 108 patients, North Caucasus FD — 87 patients, Siberian FD — 120 patients, Ural FD — 1732 patients, Central FD — 7265 patients, South FD — 90 patients, Volga FD — 19 patients.
HPV carriage structure
Differences in HPV carriage structure depending on gender
HPV type 16 is the most common among women and men, it was detected in 27.3% of HPV-positive men and in 25.6% of the women, and that fact confirms the results of previous studies [10]. The least common was type 26, it was found in 0.7% of men and 0.9% of women. Some types such as 6, 11, 44, 16, 18, 35, 39, 45, 51, 53, 56, 59, 66, 73 and 82 are more common among men. When comparing HPV carrier structure among men and women, statistically significant differences were observed for all types except HPV 26, 33 and 58 (Fig. 1).

The analysis of the HPV types distribution in the regions showed that HPV type 16 was the most common in all FDs. The spread of the proportion of patients with HPV type 16 was from 22.1% in the Central FD to 31.6% in the Volga FD. HPV type 44 was most common in the Far East (19.4%) and North Caucasus (19.5%) FD, while the lowest prevalence of HPV type 44 was in the North West FD (6.5%). Volga FD differs from other regions in high incidence of HPV type 68 (22.1%), while in other FDs the proportion of patients with type 68 does not exceed 12%. Also in Volga FD there was a high prevalence of HPV types 52 (15.8%), 53 (15.8%), 58 (15.9%) in relation to the rest of the FDs. Interestingly, in all regions of the Russian Federation the incidence of HPV type 73 is approximately the same, ranging from 4.6 to 7.8%, however, in the North Caucasus FD the proportion of patients with HPV type 73 is 1.1%
Distribution of patients by number of high carcinogenic risk (HCR) HPV types
Distribution of patients by the number of identified HPV types does not differ significantly between men and women (Fig. 2) and between patients from different age groups and different territories.
In all studied groups there is a decrease in the proportion of patients with an increase in the number of simultaneously identified HCR HPV types. In 67.2% of patients there was only one type of HCR HPV detected, 18.7% of patients presented with two types of HCR HPV simultaneously, 8.1% had three types of HCR HPV, 3.4% had four types of HCR HPV, 1.4% had five types of HCR HPV, and 0.7% had six types of HCR HPV. Seven or more types of HCR HPV were identified in isolated cases; however, 11 types of HCR HPV were identified in two patients.
Age-specific features of HPV carriage
The patients were divided into nine age groups: younger than 25 years (3188, 30.3%); 25 to 30 (3224, 30.6%); 30 to 35 (1946, 18.5%); 35 to 40 (1975, 9.3%); 40 to 45 (506, 4.8%); 45 to 50 (300, 2.8%); 50 to 55 (201, 1.9%); 55 to 60 (100, 0.9%) and over 60 years (95, 0.9%). The age of 2168 patients was not specified at the time of examination. The analysis of the number of simultaneously detected HPV types showed that the highest proportion of patients with two or more types of HCR HPV was in the age groups under 25 years (38.6% among women and 50.5% among men) and between 55 and 60 years of age in women (41.3%) and between 50 and 55 years of age in men (71.4%). For both women and men, there is a tendency to decline in the occurrence of two or more types of HCR HPV before 40-45 years, and to increase — from 45 to 60 years (Fig. 3). It is assumed that this is due to the fact that the debut of heterosexual relations in girls and boys, as well as the beginning of regular sexual activity, occurs approximately at the age of 21 years. Also, according to some authors, the second peak of sexual activity in men occurs between 15 and 21 years, and among women it is at the age of 45 years [11]. Another possible cause of the observed differences may be various reasons for seeking a doctor at different age periods. In adulthood, symptomatic patients are more likely to visit the doctor, while among the younger demographic, there are more “random’ patients who sought medical attention due to reproductive problems, to select contraceptives, etc.

This explains the presence of two characteristic peaks in the proportion of patients with two or more identified HPV types. The proportion of men with more than one type of HPV is higher than that of women by 0.9 — 38.6%, depending on age, possibly due to higher sexual activity among men. Interestingly, the highest proportion of men with two or more HCR HPV genotypes is in the age group of 50 to 55 years and is 71.4%.
Given the significant influence of age on HPV carriage, a receiver operating characteristic (ROC) analysis was carried out to determine the critical age at which differences in HPV carrier structure are the most pronounced. According to the results of the analysis, the threshold value is 27 years, respectively, all patients were divided into subgroups by age: before 27 and after 27 years. It should be noted that the threshold age, corresponding to the greatest differences in the HPV carriage structure, obtained in our study with the help of mathematical analysis is well consistent with recommendations determining the minimum age at which HPV screening is appropriate for the prevention of CC. According to various recommendations, this age corresponds to 25-30 [12].
Interestingly, the greatest differences were observed in low cancer risk HPV carriers between men and women in both age subgroups, whereas for HCR HPV the most significant were age characteristics (Fig. 4).
Features of viral load
When evaluating the viral load, only the carriers of the corresponding virus type were analyzed. In general (excluding age), the viral load of a certain type of HPV in men and women did not differ significantly. The exception was type 68, characterized by higher viral load in women — 3.4 (2.4 — 4.8) [LogGE/sample] than in men — 2.7 (2.1 — 3.8) [LogGE/sample]. However, the detailed analysis drew our attention to differences in the magnitude of viral load in different subgroups of patients (Table 1).
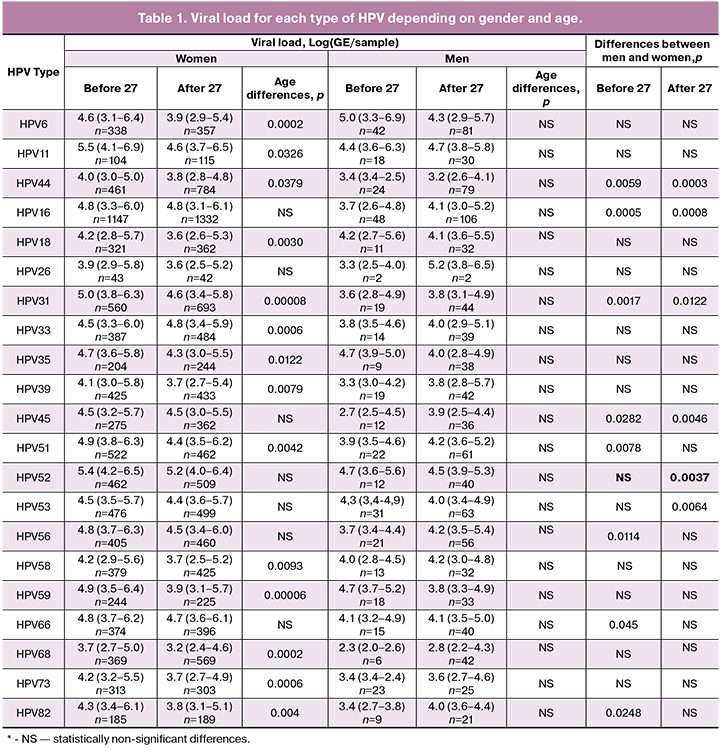
It is noteworthy that, in most cases, women experienced higher viral load under 27 years of age, while the risk of developing CC in this age group is significantly lower. The results obtained by us are consistent with the opinion of a number of foreign authors, who urge to be cautious about clinical interpretation of viral load [4, 7, 8]
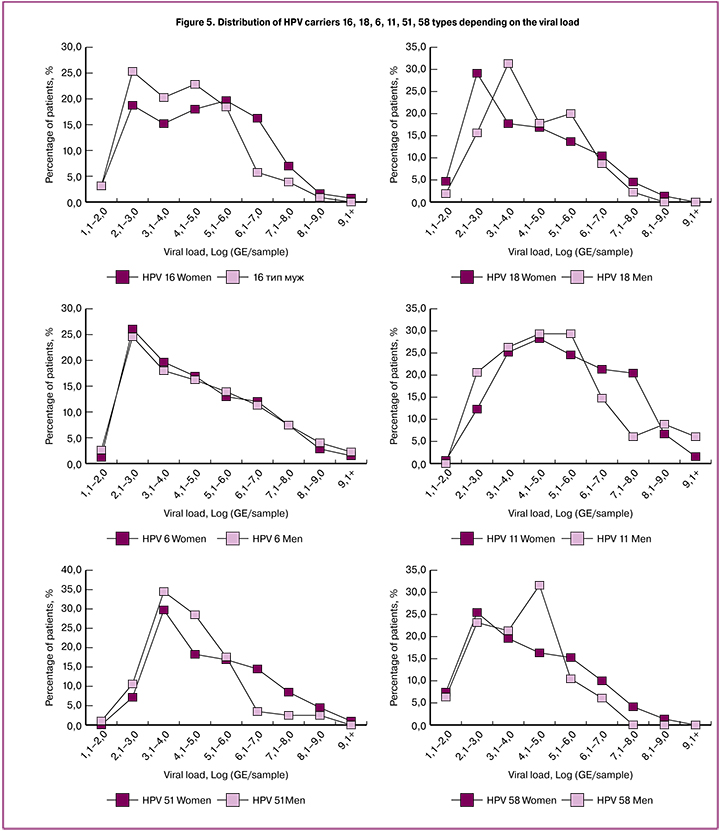
We have also analyzed histograms of distributing carriers of each HPV type by the magnitude of viral load. This distribution was different from normal for all HPV types, at the same time HPV 16 and HPV 18 had a double-humped distribution with peaks of 102 — 103 and 105 — 106 GE/sample, whereas for HPV 6 and HPV 53, there was only one strong peak around 102–103 GE/sample for HPV 6 and 103–104 GE/sample for HPV 53. (Figure 5). This may be due to the peculiarities of biology of different HPV types. From a practical point of view, it is important to bear in mind that since HPV 6 is most often found in a small amount, this imposes increased sensitivity requirements for test systems for qualitative analysis of this type.
Conclusion
For the first time, the structure of HPV carriage and the features of viral load of 21 HPV types in different regions of the Russian Federation has been studied on a large sample using a single diagnostic panel. Interregional differences in the incidence of a number of HPV types have been identified, which should be taken into account when choosing a diagnostic platform for HPV screening.
We have identified a correlation between the share of carriers of more than one HPV type with the patient’s age, which may be associated with changes in sexual activity. The proportion of carriers of more than one HPV type is higher among men than among women.
The study did not assess the cytological picture, which does not allow us to come to any definite conclusion about the clinical and/or prognostic value of the viral load. However, in most cases, a higher viral load was observed in young women, for whom the risk of CC is significantly lower. At the same time, despite statistically significant differences in viral load between groups, individual values fluctuate widely, which leads to partial overlap of interquartile intervals. The above should be taken into account when assessing the viral load in a particular patient. Given the differences in viral load distribution of different HPV types, it is possible to conclude that there is a need for a differentiated approach for different types of virus, which certainly requires further research.
References
- Каприн А.Д., Старинский В.В., Петрова Г.В., ред. Злокачественные новообразования в России в 2016 году (заболеваемость и смертность). М.: МНИОИ им. П.А. Герцена - филиал ФГБУ «НМИЦ радиологии» Минздрава России; 2018. [Kaprin A.D., Starinsky V.V., Petrova G.V., ed. Malignant neoplasms in Russia in 2016 (morbidity and mortality). M .: Moscow them. P.A. Herzen branch of the FSUE «NMITS of radiology» of the Ministry of Health of Russia; 2018. (in Russian)].
- ВОЗ. Вирус папилломы человека (ВПЧ) и рак шейки матки. Информационный бюллетень № 380. Март 2015. [WHO. Human papillomavirus (HPV) and cervical cancer. Newsletter number 380. March 2015. (in Russian)].
- Суламанидзе Л.А., Назарова Н.М., Прилепская В.Н., Бурменская О.В., Демура Т.А., Гордеев С.С., Чупрынин В.Д., Трофимов Д.Ю. Результаты ВПЧ-генотипирования эпителия шейки матки и анальной области у пациенток с цервикальными интраэпителиальными неоплазиями. Гинекология. 2016; 18(1): 45-8. [Sulamanidze L.A., Nazarova N.M., Prilepskaya V.N., Burmenskaya O.V., Demura T.A., Gordeev S.S., Chuprynin V.D., Trofimov D.Yu.The results of HPV genotyping of the epithelium of the cervix and anal region in patients with cervical intraepithelial neoplasias. Gynecology. 2016; 18 (1): 45-8. (in Russian)].
- Del Río-Ospina L., Soto-DE León S.C., Camargo M., Sánchez R., Moreno-Pérez D.A., Pérez-Prados A. et al. Multiple high-risk HPV genotypes are grouped by type and are associated with viral load and risk factors. Epidemiol. Infect. 2017; 145(7): 1479-90.
- ВОЗ. Комплексная профилактика рака шейки матки и борьба с ним — здоровое будущее для девочек и женщин. ВОЗ; 2013: 6-8. [WHO. Comprehensive prevention and control of cervical cancer is a healthy future for girls and women. WHO; 2013: 6-8. (in Russian)].
- Bruni L., Barrionuevo-Rosas L., Albero G., Serrano B., Mena M., Gómez D.et al. ICO Information Centre on HPV and Cancer (HPV Information Centre). Human papillomavirus and related diseases in the world. Summary Report July 2017.
- Cao M., Shah W., Qi J., Zhou Y., Wang Y., Chen H. Prognostic significance of human papillomavirus viral load in correlation with different therapeutic modalities in cervical cancer patients. Pathol. Res. Pract. 2016; 212(9): 804-10.
- Luo H., Belinson J.L., Du H., Liu Z., Zhang L., Wang C. et al. Evaluation of viral load as a triage strategy with primary high-risk human papillomavirus cervical cancer screening. J. Low. Genit. Tract Dis. 2017; 21(1): 12-6.
- Liu J., Liu W., Liu Y., Zhou X., Zhang Z., Sun Z. Prevalence of microorganisms co-infections in human papillomaviruses infected women in Northern China. Arch. Gynecol. Obstet. 2016; 293(3): 595-602.
- Назарова Н.М., Бурменская О.В., Суламанидзе Л.А., Прилепская В.Н., Павлович С.В., Трофимов Д.Ю. Распространенность типов вируса папилломы человека аногенитально области у пациенток с ВПЧ-ассоциированными заболеваниями шейки матки. Акушерство и гинекология. 2015; 12: 89-96. [Nazarova N.M., Burmenskaya O.V., Sulamanidze L.A., Prilepskaya V.N., Pavlovich S.V., Trofimov D.Yu. The prevalence of types of human papillomavirus anogenital area in patients with HPV-associated diseases of the cervix. Obstetrics and gynecology. 2015; 12: 89-96. (in Russian)].
- Ильин Е. П. Дифференциальная психофизиология мужчины и женщины. СПб.: Питер; 2007. [Ilyin E.P. Differential psychophysiology of men and women. SPb .: Piter; 2007. (in Russian)].
- Сычева Е.Г., Назарова Н.М., Прилепская В.Н., Бурменская О.В. “Малые” формы поражения шейки матки, ассоциированные с вирусом папилломы человека: диагностика, мониторинг, прогноз. Акушерство и гинекология. 2017; 9: 34-9. [Sycheva EG, Nazarova N.M., Prilepskaya V.N., Burmenskaya O.V. «Small» forms of cervical lesions associated with the human papilloma virus: diagnosis, monitoring, prognosis. Akusherstvo i Ginekologiya/Obstetrics and gynecology. 2017; 9: 34-9. (in Russian)].
Received 21.06.2018
Accepted 22.06.2018
About the Authors
Donnikov, Andrey Y., PhD, head of the laboratory of the molecular-genetics methods, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. E-mail: a_donnikov@oparina4.ruMarkelov, Mikahil I., student, N.I. Pirogov Russian National Research Medical University, Medical-Biological Department, division of the medical biochemistry.
119021, Russia, Moscow, Bolshaya Pirogovskaya str. 9a. E-mail: m.markelov1994@mail.ru
Pestrikova,Tatyana Yu., MD, professor, head of the Department of Obstetrics and Gynecology, Far Eastern State Medical University the Ministry of health of the Russian Federation. 680000, Russia, Khabarovsk, Muraviev-Amur str. 35. Tel.: +79147719383. E-mail: typ50@rambler.ru
Yurasova, Elena A., MD, professor, Department of Obstetrics and Gynecology, Far Eastern State Medical University, Ministry of Health of Russia.
680000, Russia, Khabarovsk, Muraviev-Amur str. 35. Tel.: +79625838255. E-mail: urasovaea@yandex.ru
Kotelnikova, Anastacia V., assistant of the Department of Obstetrics and Gynecology, Far Eastern State Medical University, Ministry of Health of Russia.
680000, Russia, Khabarovsk, Muraviev-Amur str. 35. Tel.: +79244055777. E-mail: tempo-m @mail.ru
Voroshilina, Ekaterina S., MD, Professor, Ural State Medical University, Medical center «Garmonia».
620026, Russia, Ekaterinburg, Tveritina str. 16. Tel.: +73432510876. E-mail: voroshilina@gmail.com
Plotko, Evgeny E., MD, chief doctor of Medical Center «Harmony». 620026, Russia, Ekaterinburg, Tveritina str. 16. Tel.: +73432510876. E-mail: plotko@garmonia-mc.ru
Belokhvostikova, Tatiana S., MD, head of the Department of Clinical Laboratory Diagnostics, Irkutsk State Medical Academy.
664079, Russia, Irkutsk, Yubilejny dis., 100. E-mail: Belohvostikova2011@yandex.ru
Bondareva, Valentina P., MD, head of Laboratory Diagnistics Department, Stavropol Regional Clinical Consultative and Diagnostic Center.
355017, Russia, Stavropol city, Lenina str. 304. Tel.: +78652358542. E-mail: akldc@skkdc.ru
Chernikova, Maria A., head of the Department of Clinical Laboratory Diagnostics, DC «Laboratory diagnostics – Asclepius».
690033, Russia, Vladivostok, Gamarnik str. 3b. Tel.: +74232023003. E-mail: skibam@bk.ru
Vashchenko, Svetlana N., MD, gynecologist-endocrinologist, DC «Laboratory diagnostics – Asclepius».
690033, Russia, Vladivostok, Gamarnik str. 3b. Tel.: +7 4232023003. E-mail: svetlanaasklepiy@mail.ru
Chernikova,ViktoriaV., biologist, DC «Laboratory diagnostics – Asclepius». 690033, Russia, Vladivostok, Gamarnik str. 3b. Tel.: +74232023003. E-mail: viktoriachern@mail.ru
Stankevich, Liubov I., MD, Gontard & Cie Group (Switzerland, Russia, UAE) Group Medical Director. 125009, Russia, Moscow, Tverskaya str. 9; Group Labexa, Biologie medicale, Deputy Director Medical Affairs of group. 33600, Pessac, France, 208 avenue Pasteur. Телефон: +79031157005. E-mail: Lubov.stan@gmail.com
Khasina, Maria Yu., PhD, medical development director, LLC «Unilab». 690105, Russia, Vladivostok, Borodinskaya str. 46/50; associate professor,
School of Biomedicine, Far Eastern Federal University. 690922, Russia, Primorsky Krai, Fr. Russian, Ajax 10, FEFU campus. E-mail: 2985823@mail.ru
Galkina, Irina S., PhD in chemistry, Senior Researcher , Central Research Institute of Health Organization and Informatization; Chief Marketing Officer
of the Company DNA-Technology Co. 117587, Russia, Moscow, Varshavskoe shosse (high-way), 125Zh, Bld. 6, fl. 5. Tel.: +74959804555. E-mail: Galkina@dna-technology.ru.
For citation: Donnikov A.E., Markelov M.I., Pestrikova T.Yu., Yurasova E.A., Kotelnikova A.V.., Voroshilina E.S., Plotko E.E., Belokhvostikova T.S., Bondareva V.P., Chernikova M.A., Vashchenko S.N., Chernikova V.V., Stankevich L.I., Khasina M.Yu., Galkina I.S. Analysis of the prevalence and viral load of different human papillomavirus types in the regions of the Russian Federation. Akusherstvo i Ginekologiya/Obstetrics and Gynecology.2019; (4): 39-47. (in Russian)
https://dx.doi.org/10.18565/aig.2019.4.39-47



