Известно, что под контролем системы человеческих лейкоцитарных антигенов (Human Leukocyte Antigens, HLA) I класса находится взаимодействие иммунной системы в целом и всех иммунокомпетентных клеток организма в частности [1]. На всех сроках гестации как на вневорсинчатых клетках трофобласта (EVT), так и на материнских лейкоцитах в децидуальной ткани на границе системы «мать–плацента–плод» существует уникальная комбинация экспрессии HLA. Известно, что на вневорсинчатом трофобласте экспрессируются полиморфные неклассические антигены HLA I класса (HLA-E, HLA-F, HLA-G), а также происходит продукция небольшого количества классического полиморфного HLA-C, который может служить источником полного распознавания материнскими иммунными клетками [2]. Эти гены высокополиморфны, и их белковые продукты экспрессии обладают рядом функций, таких как процессинг и представление антигенов, индукция смещения от провоспалительного к клеточноопосредованному ответу, ингибирование рецепторов натуральных киллеров (NK-клеток) с последующим снижением иммунного ответа на границе системы «мать–плацента–плод» с обеспечением иммунной толерантности к плоду [3]. Молекулы HLA класса Ib, наиболее изученной из которых является HLA-G, обладают другими функциями, по сравнению с классическими молекулами HLA класса I, являясь иммуномодулирующими, толерогенными и противовоспалительными [4].
Известно, что ген HLA-G экспрессируется в тканях, ограниченных в первую очередь иммуно-привилегированными участками, такими как плацента [5, 6]. Так, его экспрессия напрямую оказывает влияние на ремоделирование тканей и сосудов, а секретируемые молекулы sHLA-G (растворимой формы HLA-G) клеток вневорсинчатого трофобласта регулируют эндоваскулярную и децидуальную инвазию, что в последующем защищает эмбрион от атаки материнскими клетками иммунной системы [4, 7, 8]. Таким образом, при нарушении данного процесса возникает риск прерывания беременности на ранних сроках, часто клинически проявляющийся симптомокомплексом угрожающего выкидыша или неразвивающейся беременностью.
Причины ранних репродуктивных потерь крайне разнообразны. Установлено, что генетический фактор является одной из ведущих причин невынашивания беременности на ранних сроках. Генетические причины невынашивания беременности включают в себя не только хромосомные перестройки, но и наличие наследственной предрасположенности [9]. Невынашивание беременности является мультифакториальным заболеванием – результатом действия «функционально ослабленных» аллелей множества генов на фоне неблагоприятных внешних и внутренних факторов [10].
Полиморфизм гена, или полиморфные аллели гена, представляет собой многообразие нуклеотидных последовательностей гена [11]. В настоящее время в мировой и отечественной литературе хорошо изучен аллельный полиморфизм более 100 генов, относящихся к «генной сети невынашивания беременности», которые затрагивают различные системы при функционировании организма. Данные полиморфные варианты генов могут являться как триггерами развития патологического процесса, так и предрасполагающими факторами для его формирования.
В мировой и отечественной литературе описаны ассоциации гена HLA-G с акушерскими патологиями, такими как бесплодие, невынашивание беременности и неудачи вспомогательных репродуктивных технологий (ВРТ), прямая связь с повышенным риском развития преэклампсии и синдрома задержки роста плода [6, 12, 13]. Известно, что полиморфные варианта гена могут затрагивать как его кодирующие, так и некодирующие области. Широко изученным полиморфизмом в некодирующей области была инсерция/делеция 14 пар нуклеотидов (п.н.) в экзоне 8 в положении 3741 (rs66554220), которая определяет альтернативный сплайсинг HLA-G, оказывая влияние на стабильность матричной РНК (мРНК) гена HLA-G [14]. По сравнению с генотипами 3741*ins/del 14 п.н. и 3741*del/del 14 п.н. гомозиготный генотип (+14 п.н./+14 п.н.) был связан с более низкими уровнями мРНК и растворимой формы HLA-G [15]. Было заключено, что полиморфизм HLA-G 3741*ins/del 14 п.н. может играть важную роль в модуляции экспрессии HLA-G, и женщины с привычными потерями плода и повторными неудачами имплантации при ВРТ могут иметь более высокую частоту пациенток со вставкой размером 14 п.н. В ряде эпидемиологических исследований изучалась связь между полиморфизмом 3741*ins/del 14 п.н. HLA-G и повторными неудачами имплантации [16–18]. Аллель с 3741*Ins/Ins и Ins/Del ассоциирован с пониженным количеством мРНК HLA-G с более низкими концентрациями мембран-ассоциированных и растворимых форм продуктов экспрессии гена HLA-G. По данным мировой и отечественной литературы установлена ассоциация аллеля 3741*ins/-14 п.н. с развитием синдрома привычной потери плода [19].
Доказано, что нулевая аллель HLA-G*01:05N встречается в 25% случаев у пациенток с угрожающим выкидышем среди населения Восточного Азербайджана, по сравнению с группой контроля (25 и 3,2% соответственно, p<0,001). Также результаты Naghavian E. et al. в своем исследовании продемонстрировали, что 59% женщин с привычным невынашиванием беременности в Мазандаране имели гетерозиготный аллель HLA-G*01:05N [20]. Отмечено значительное увеличение частоты аллеля HLA-G*01:05N среди женщин с привычным невынашиванием беременности в Иранской популяции (5,1% в группе с привычным невынашиванием беременности и 0% в группе контроля, р=0,015) [21].
В настоящее время активно изучается полиморфизм однонуклеотидной замены C на G (-725*C>G-rs1233334) в промоторной области гена, которая находится на расстоянии 19 п.н. от активного ISRE сайта и на расстоянии 10 п.н. от нефункционального гамма-интерферон-активируемого сайта [22, 23]. Известно, что аллельный полиморфизм -725*G/- ассоциирован с увеличением риска невынашивания беременности [24], тогда как анализ экспрессии гена на клеточной культуре трофобласта показал повышенный уровень транскрипции гена HLA-G, несущего -725*G/- аллель [25].
Так как в различных популяциях генетическая структура населения имеет свои уникальные особенности, изучение полиморфизма гена HLA-G в популяции Северо-Западного региона Российской Федерации не теряет своей актуальности и в настоящее время.
Цель исследования: провести молекулярно-биологическое исследование 9 полиморфных аллелей гена HLA-G в группе беременных в I триместре гестации и популяции Северо-Западного региона РФ и определить связь с отягощенным акушерским анамнезом.
Материалы и методы
Проведено исследование, включавшее 62 беременных и 118 человек из популяции Северо-Западного региона РФ на базе ФГБНУ «НИИ АГиР им. Д.О. Отта» (Санкт-Петербург) за период с 2019 по 2022 гг. Молекулярно-генетический анализ был выполнен в Отделе геномной медицины научно-исследовательского института. С целью анализа полиморфных аллелей гена HLA I класса (G) у беременных в I триместре гестации были сформированы 2 группы: 1-я группа – 33 беременных с отягощенным акушерским анамнезом (один выкидыш и более, неразвивающаяся беременность, неудачи ВРТ), 2-я (контрольная) группа – 29 беременных без отягощенного акушерского анамнеза.
В популяционную группу (сравнения) вошли 118 человек (65 мужчин и 53 женщины) в возрасте от 20 до 40 лет, без хронических заболеваний на момент забора крови на станции переливания крови НИИ АГиР им. Д.О. Отта, проживающих в Северо-Западном регионе РФ, оценка генетической структуры по гену HLA-G которых была изучена и опубликована ранее [26].
Критериями включения являлись: возраст от 20 до 44 лет, 6–13 недель гестации, как отсутствие репродуктивных нарушений (роды в анамнезе или первые роды без установленного диагноза бесплодие), так и наличие отягощенного акушерского анамнеза (1 самопроизвольный выкидыш и более, неразвивающаяся беременность, неудачи ВРТ). Критериями исключения являлись: антифосфолипидный синдром, прегестационный сахарный диабет, носительство генов, ассоциированных с тромбофилией высокого риска, беременность, наступившая с помощью ВРТ при использовании донорской яйцеклетки, отказ женщины от участия в исследовании, тяжелая экстрагенитальная патология (врожденные или приобретенные пороки сердца, гипертоническая болезнь II стадии и более, хронические заболевания в стадии обострения, ОРВИ, новая коронавирусная инфекция).
Материалом для генетического исследования служила венозная кровь. Ее образцы получали путем пункции локтевой вены, забор проходил в одноразовые пластиковые пробирки с 2,5% раствор ЭДТА (в соотношении 1:10) консервантом. Выделение ДНК проводили стандартным методом фенол-хлороформ с модификациями.
Олигонуклеотидные праймеры подбирали путем использования программы Oligo v.6.31 (Molecular Biology Insights Inc., США), тогда как последовательность фрагментов гена HLA-G была получена из интернет-баз HLA Informatic Group, Anthony Nolan Research Institute (США).
Для определения полиморфных аллелей HLA-G*01:01, HLA-G*01:02, HLA-G*01:03, HLA-G*01:04, HLA-G*01:05N, HLA-G*01:06 и HLA-G*01:07 была разработана собственная система праймеров (за основу брали олигонуклеотидные последовательности французских ученых) [27].
Аллели HLA-G*0101G0107 представляют собой сочетания замен в одной нуклеотидной последовательности в кодирующей области гена: HLA-G*01:01 (31A, 54A, 110C, 130CC, 258C), HLA-G*01:02 (31A, 54G, 110C, 130CC, 258C), HLA-G*01:03 (31T, 54A, 110C, 130CC, 258C), HLA-G*01:04 (31A, 54A, 110A, 130CC, 258C), HLA-G*01:05N (31A, 54A, 110C, 130CA, 258C), HLA-G*01:06 (31A, 54A, 110C, 130CC, 258T), HLA-G*01:07 (31A, 54A, 110A, 130CC, 258T).
Для проведения мультиаллельспецифичной полимеразной цепной реакции (ПЦР) праймеры подбирали таким образом, чтобы концевой 3’ нуклеотид праймера соответствовал анализируемой однонуклеотидной замене, и ПЦР шла только при полной комплементации праймера с анализируемой ДНК, что позволяет судить о присутствии изучаемого аллеля. Структура олигопраймеров и идентифицируемые аллели представлены на рисунке.

Для идентификации полиморфизма 3741*ins/del 14 п.н. использованы последовательности праймеров, приведенные в работе ученых из Бразилии в 2008 г. [28].
Анализ полиморфизма -725*С/G проводили с использованием олигопраймеров, позволяющих создать искусственный сайт рестрикции для фермента Pce I (F: 5’-TGA ACC AGC CCT CCT AAT AA3’, R: 5-’ACC GAT TAG ATT CCT GA TCA TTC AGG GGT TAC CAA3’). Для анализа замены -725*С>G проводили гидролиз продукта ПЦР эндонуклеазой Pce I согласно рекомендациям фирмы-изготовителя («Cибэнзим», Новосибирск).
Для амплификации использовали программируемые термоциклеры фирм «ДНК-технология» (Москва). После окончания ПЦР специфичность амплификации и количество ДНК проверяли методом электрофореза в 7,5% полиакриламидном геле (ПААГ).
ПААГ окрашивали водным раствором бромистого этидия (0,5 мкг/мл), просматривали в ультрафиолетовом свете на трансиллюминаторе Macrovue (Pharmacia LKB, Великобритания) и фотографировали с использованием системы видео-гель-документации (Vilber Lourmat).
Статистический анализ
Статистическая обработка результатов проводилась с использованием программ STATISTICA 10 (StatSoft) и IBM SPSS Statistics (версия 26).
Перед описанием количественных данных была проведена проверка распределения. В данном случае проверку гипотезы о нормальном распределении осуществляли, используя критерий Шапиро–Уилка для выборок с числом наблюдений 50 и менее. Все необходимые условия, которые должны соблюдаться в нормально распределенных совокупностях, для использования параметрических методов (t-критерий Стьюдента) были соблюдены с обоснованием их применимости, к которым относятся нормальное распределение признаков и равенство дисперсий в сравниваемых группах – максимальная близость значений средней арифметической, показатели измерены в количественной шкале, соблюдено правило «трех сигм». Так, все выборки соответствовали критериям нормального распределения. При проведении попарного сравнения групп применялась поправка на множественные сравнения – поправка Бонферрони – перерасчет уровня значимости р для множественных парных сравнений. При использовании поправки Бонферрони традиционный уровень ошибки 1 типа делится на количество сравнений для получения нового критического уровня значимости. Число пар сравнения рассчитывается по формуле:
m=n(n-1)/2,
где n – количество групп – 3.
Для данного исследования было определено критическое значение t-критерия Стьюдента для требуемого уровня значимости. Так, при сравнении 3 групп различия между двумя средними величинами считали статистически значимыми при новом критическом уровне 0,05/3=0,017.
Достоверность различий частот определяли с помощью метода χ2 с учетом поправки Йейтса для парных сравнений с контрольной группой по стандартной формуле. В случае наличия достоверных отличий между контролем и исследуемой группой использовали также коэффициент отношения шансов (ОШ). Значение ОШ применительно к нашим данным показывает, во сколько раз вероятность наличия данного генотипа у пациентов с репродуктивными потерями превышает вероятность его наличия в контрольной группе или же во сколько раз выше вероятность иметь то или иное заболевание, обладая определенным генотипом.
Расчет частот аллелей производился исходя из закона Харди–Вайнберга. Статистически значимыми считали различия при р<0,05.
Результаты и обсуждение
Пациентки первых двух групп были сопоставимы по возрасту и индексу массы тела (ИМТ). Так, возраст женщин статистически не различался и варьировал в пределах от 20 до 44 лет (табл. 1). Средний возраст пациенток в 1-й и 2-й группах составил 33,4 (5,6) и 32,8 (5,3) года, ИМТ составил 24,2 (5,4) и 23,1 (5,1) кг/м2 соответственно.

При анализе гинекологического анамнеза всех обследованных у каждой 3-й беременной из группы 1 был установлен хронический эндометрит в анамнезе, что имело статистически значимые отличия от контрольной группы (10/33 (30,3%) и 1/29 (3,4%), p=0,006 соответственно) (табл. 1).
Пациентки из группы с отягощенным акушерским анамнезом в 9 раз чаще имели в анамнезе внутриматочные вмешательства – выскабливания и гистероскопии – по сравнению с контрольной группой, где данную процедуру выполнила только каждая 10-я женщина (28/33 (84,8%), 3/29 (10,3%) соответственно, p<0,001).
Другими патологиями были синдром поликистозных яичников, наружный генитальный эндометриоз, полип эндометрия, хронический сальпингоофорит и инфекции, передающиеся половым путем. Статистических различий по частоте гинекологической патологии в группах обследования не получено (p>0,05).
Среди осложнений в I триместре беременности в обеих группах на 1-м месте более чем в 50% случаев выявлена угроза ее прерывания. Рвота беременных наблюдалась в 2,2 раза чаще среди беременных из группы 1 по сравнению с контрольной группой (10/33 (30,3%), 4/29 (13,8%) соответственно, p=0,121). Другие осложнения I триместра (анемия беременных легкой степени, инфекция мочевыводящих путей, бактериальный вагиноз и ОРВИ) встречались в единичных случаях.
При анализе течения угрожающего выкидыша среди пациенток были выявлены особенности. Жалобы на тянущие боли внизу живота наблюдались у всех пациенток с угрожающим выкидышем из контрольной группы, тогда как в группе 1 только 76,4% беременных отмечали данный симптом у себя (16/16 (100%), 13/17 (76,4%) соответственно, p=0,115; табл. 1). Так, у пациенток из 1-й группы отслойка плодного яйца встречалась статистически чаще по сравнению с группой контроля (10/17 (58,8%), 1/16 (6,25%) соответственно, p=0,006). Таким образом, степень тяжести клинических проявлений угрозы прерывания беременности у пациенток с отягощенным репродуктивным анамнезом была значительно выше, чем у пациенток 2-й группы.
При анализе частот аллелей гена HLA-G в группе 1 каждая 10-я беременная являлась носителем аллеля HLA-G*01:02 в отличие от группы сравнения (0/29 (0%)). Отмечено, что в группе с невынашиванием беременности частота аллеля HLA-G*01:05N была определена в 3,5 раза чаще, чем в популяционной выборке (табл. 2).
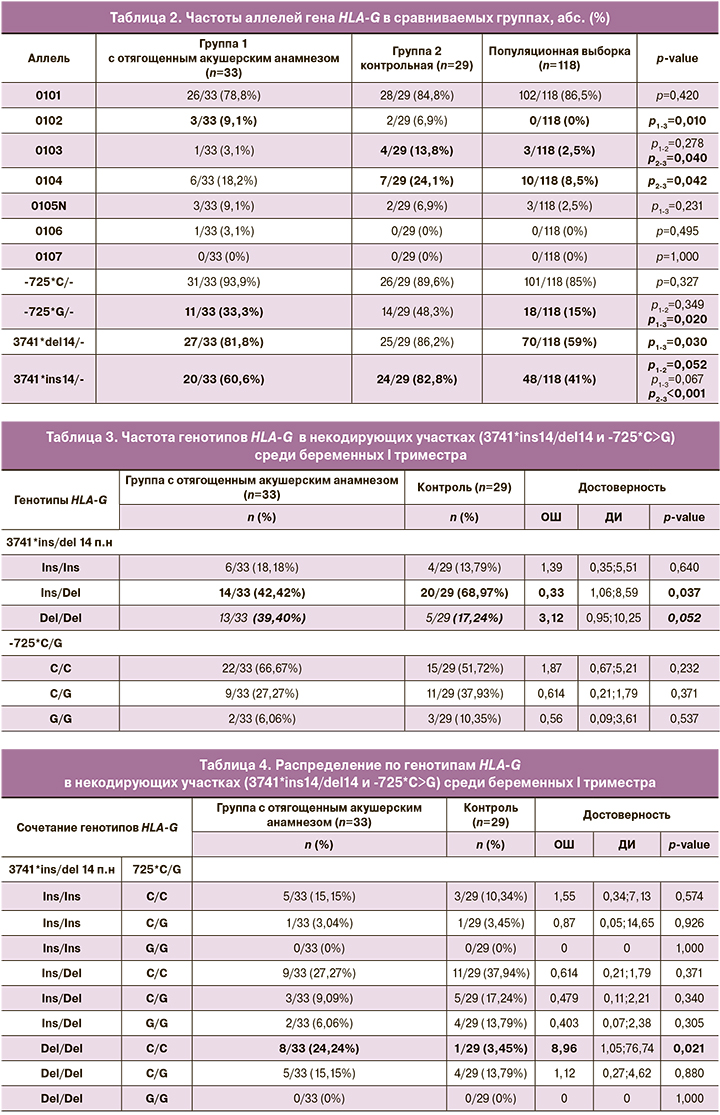
При анализе основных аллелей гена как в кодирующих (HLA-G*01:01–01:07), так и в некодирующих участках (-725*C>G и 3741*ins/del 14 п.н.), отмечено, что статистически значимые различия наблюдались между группой 1 и популяционной выборкой по аллелю HLA-G*01:02 (3/33 (9,1%) и 0/118 (0%) соответственно, p=0,010), по аллелю -725*G/- (11/33 (33,3%) и 18/118 (15%) соответственно, p=0,020) и по аллелю 3741*del14/- (27/33 (81,8%) и 70/118 (59%) соответственно, p=0,030).
При сравнении частот аллелей и генотипов полиморфизма 3741*ins/del 14 п.н. гена HLA-G среди двух групп беременных выявлено статистически значимое отличие между носителями гетерозиготных вариантов гена в группе 1 и контрольной (14/33 (42,4%) и 20/29 (68,9%) соответственно, p=0,037) (табл. 3). Отмечено, что частота генотипа Del/Del была установлена в 2,3 раза чаще в группе 1 по сравнению с группой контроля (13/33 (39,4%) и 5/29 (17,2%) соответственно, p=0,052). Частота аллеля Ins была установлена в 1,4 раза чаще в группе без репродуктивных нарушений (20/33 (60,6%) и 24/29 (82,7%), p=0,056) (табл. 2).
Согласно рассчитанному коэффициенту ОШ, носители сочетанного генотипа Del/Del и C/C были определены в 9 раз чаще среди пациенток с репродуктивными нарушениями в анамнезе в отличие от группы контроля (ОШ=8,96; 95% ДИ l,05–76,74), что имело статистически значимые отличия в двух группах (8/33 (24,2%) и 1/29 (3,45%) соответственно, р=0,021; табл. 4). Так, среди всех 63 возможных сочетаний генотипов -725*C>G, 3741*ins/del 14 п.н. и аллелей гена HLA-G (HLA-G*01:01–01:07) отмечено, что наиболее часто встречающимся сочетанием в группе с отягощенным акушерским анамнезом является 3741del14/del14, -725*C/C, HLA-G*01:01 и 3741ins14/del14, -725*C/C, HLA-G*01:01.
По данным мировой литературы известно, что ген HLA-G обладает иммуномодулирующими свойствами [29], и изучение роли его полиморфизма позволит в будущем определить индивидуальный подход при назначении синтетической молекулы HLA-G в терапевтических целях [30]. По данным ряда исследователей, в зависимости от вариативных последовательностей в нуклеотидной цепи кодирующей области локуса гена HLA-G наблюдается 44 различных аллеля, частота которых, по-видимому, варьирует от популяции к популяции [31–33]. С помощью количественного анализа недавно было сообщено, что аллельные варианты гена HLA-G, такие как 01:05N, 01:06, 01:04:01, 01:01:08 и т.д., связаны с более низким уровнем продукции sHLA-G, известных как «низкосекреторные» аллели, и, таким образом, могут способствовать развитию привычного невынашивания беременности на ранних сроках гестации [34].
Кроме того, по данным Ober et al., аллель 725*G/- ассоциирован с привычным невынашиванием беременности, что противоречит данным о том, что этот однонуклеотидный полиморфизм связан с высоким уровнем экспрессии растворимой формы белковых продуктов экспрессии HLA-G [24]. В нашей работе статистически значимых различий в полиморфизме гена HLA-G -725*C/G между беременными с репродуктивными потерями в анамнезе и группой контроля выявлено не было.
В 2019 г. был опубликован систематический метаанализ статей, посвященных связи генотипа HLA-G и привычного невынашивания беременности [35]. Авторы пришли к выводу, что среди женщин, проживающих на территории Европейского союза, с генотипом 3741*ins/del 14 п.н. значительно чаще установлен диагноз привычного невынашивания беременности, хотя на основании ряда исследований имеются противоречивые данные, опровергающие заключение метаанализа [4, 14, 36]. В нашей работе отмечено, что генотип 3741*ins/del 14 п.н. встречался в 2,3 раза чаще в группе с невынашиванием беременности по сравнению с группой контроля.
Согласно данным группы польских ученых было установлено, что риск осложнений во время беременности зависит от аллелей HLA-G*0101, HLA-G*0108 и HLA-G*0106 и не зависит от 3741*ins/del 14 п.н. полиморфизм в 3’UTR гена HLA-G [37].
Заключение
Полиморфные варианты гена HLA-G, ассоциированные с пониженным количеством мРНК HLA-G, приводят к более низким концентрациям как растворимых, так и нерастворимых форм HLA-G, а также имеют ассоциации с развитием синдрома привычной потери плода и способны повышать риск данного осложнения I триместра беременности, поэтому необходимы дополнительные исследования для оценки их влияния на данную патологию и разработки новых стратегий профилактики на большей выборке пациентов.
Это позволит идентифицировать группы пациентов высокого риска, которым необходимы более тщательное наблюдение, персонифицированный подход к планированию и ведению беременности и более оптимизированному лечению.
Таким образом, результаты нашего исследования позволяют судить об ассоциации аллелей гена HLA-G с отягощенным акушерским анамнезом и могут быть важны для оценки риска развития ранних потерь беременности, а также для разработки новых стратегий профилактики и лечения данного осложнения беременности, однако необходимы дальнейшие исследования в этом направлении.



