Plasma miRNA expression levels in women with preeclampsia and chronic arterial hypertension in the first trimester of pregnancy
Semenov Yu.A., Antonov V.N., Azarenkova E.A., Chizhovskaya A.V., Veryaskina Yu.A.
Objective: This study aimed to investigate the expression of several miRNAs in the plasma of patients with chronic arterial hypertension (CAH) without preeclampsia and pregnant women with CAH-associated preeclampsia (CAP) during the first trimester of pregnancy.
Materials and methods: The study included 100 patients: Group 1 (n=58) comprised pregnant women with CAH without preeclampsia, and Group 2 (n=42) included women with CAP. The plasma expression levels of 11 miRNAs were assessed in these patients at 12–14 weeks of gestation using quantitative real-time polymerase chain reaction. Statistical analysis was conducted using the IBM SPSS Statistics software (version 26.0, USA).
Results: Women with CAP exhibited contrasting changes in the expression of five out of 11 plasma miRNAs compared to the group without CAP. The expression levels of miRNA-20a (p<0.001) and miRNA-181a (p<0.001) were significantly decreased in the CAP group. Conversely, the expression levels of miRNA-221 (p<0.001), miRNA-146a (p=0.02), and miRNA-210 (p=0.041) were higher in this group than in women who did not develop preeclampsia.
Conclusion: Statistically significant changes in the expression levels of several miRNAs associated with cardiovascular pathology and placental dysfunction were identified in the first trimester of pregnancy in patients with CAH and CAP.
Authors' contributions: Semenov Yu.A., Antonov V.N. – conception and design of the study, editing of the manuscript; Veryaskina Yu.A. – data collection and processing; Azarenkova E.A., Chizhovskaya A.V. – statistical analysis and drafting of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical approval: The study was reviewed and approved by the Research Ethics Committee of the Ural Research Institute for Maternity and Child Care.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Semenov Yu.A., Antonov V.N., Azarenkova E.A., Chizhovskaya A.V., Veryaskina Yu.A. Plasma miRNA expression levels in women with preeclampsia and chronic arterial hypertension in the first trimester of pregnancy.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (10): 90-96 (in Russian)
https://dx.doi.org/10.18565/aig.2025.215
Keywords
Hypertensive complications during pregnancy remain one of the leading causes of maternal morbidity and mortality [1]. Despite increased attention from the medical community and improvements in diagnostic and prognostic methods, chronic arterial hypertension (CAH) complicates 1–2% of pregnancies [2]. The rising average age at conception among women, coupled with a higher prevalence of comorbidities, contributes to a steady increase in this statistic [3]. CAH is an independent risk factor for preeclampsia and adverse pregnancy outcomes [4]. The combination of CAH and preeclampsia significantly elevates the risk of severe perinatal and maternal complications, including premature birth, cesarean section, and the birth of a low-birth-weight fetus or one in a severe condition requiring treatment in a neonatal intensive care unit [5]. Preeclampsia occurs in approximately 20% of women with CAH; however, 80% of these patients are unaware of their increased risk of developing preeclampsia, likely due to the variability in the severity of preexisting endothelial dysfunction and vasoregulatory disorders associated with CAH [2]. This situation has sparked interest in identifying specific biomarkers that can effectively screen for preeclampsia early in pregnancy and objectively stratify the risk of adverse outcomes in pregnant women with underlying CAH.
Currently, numerous studies have focused on the use of miRNAs as diagnostic markers for preeclampsia. It has been established that miRNAs epigenetically regulate target gene expression primarily through a post-transcriptional mechanism that involves the destabilization of messenger RNA and suppression of protein synthesis [6]. Extensive investigations into miRNA transcriptomics in pregnant women with normal and abnormal pregnancies have revealed dozens of differentially expressed miRNAs [7]. These miRNAs play a crucial role in the proliferation, differentiation, invasion, migration, and apoptotic death of trophoblast cells, as well as in regulating angiogenesis and the immune response during pregnancy [8].
However, most research studies have focused on the role of miRNAs in the development of preeclampsia in patients without concomitant somatic conditions, particularly CAH. Furthermore, existing studies often fail to clearly differentiate between isolated preeclampsia and preeclampsia occurring in the context of CAH (CAP). Given the shared pathophysiological mechanisms underlying both preeclampsia and CAH, including endothelial dysfunction, vascular dysregulation, inflammatory processes, and changes in the body's immunological reactivity [2], the conclusions drawn from studies on the role of miRNAs in isolated preeclampsia cannot be extrapolated to the population of patients with CAH.
This study aimed to investigate the expression levels of several miRNAs associated with cardiovascular diseases and hypertensive disorders of pregnancy in the peripheral blood plasma of patients with CAH without preeclampsia, as well as in pregnant women with CAH during the first trimester.
Materials and methods
This prospective study involved 100 peripheral blood plasma samples from women with CAH at 12–14 weeks’ gestation. Based on pregnancy complications, the cohort was divided into two groups: Group 1 included women without preeclampsia (n=58), and Group 2 comprised patients whose pregnancies were complicated by CAP (n=42).
The inclusion criteria for Group 1 were singleton spontaneous pregnancy, preexisting hypertension before pregnancy, or hypertension recorded during antenatal care before 14 weeks.
For Group 2, the inclusion criteria were as follows: singleton spontaneous pregnancy, preexisting hypertension before pregnancy or recorded during antenatal care before 14 weeks, and a diagnosis of preeclampsia during the current pregnancy.
The exclusion criteria for both groups included stage 3 hypertension or secondary arterial hypertension, type 1 and type 2 diabetes mellitus, decompensated hepatobiliary and gastrointestinal diseases, systemic connective tissue diseases, and HIV infection.
The miRNA expression study was conducted at the Institute of Molecular and Cell Biology of the Siberian Branch of the Russian Academy of Sciences (Novosibirsk, Russia). The selected miRNAs were based on a literature review regarding their involvement in the pathogenesis of preeclampsia and cardiovascular disease. The expression levels of 11 miRNAs (miRNA-210, miRNA-145, miRNA-20a, miRNA-21, miRNA-181a, miRNA-143, miRNA-221, miRNA-126, miRNA-146b, miRNA-146a, and miRNA-122) in the plasma samples were determined in a stepwise manner: RNA extraction was performed first, followed by reverse transcription reaction, and then quantification using real-time polymerase chain reaction (RT-PCR).
Blood samples were collected in the morning on an empty stomach from a peripheral vein into disposable sterile tubes containing the anticoagulant ethylenediaminetetraacetic acid (EDTA). Plasma was then separated from blood cells by centrifugation for 10 minutes at 3000 rpm. The obtained plasma was cryopreserved at -200°C.
Nucleic acids were isolated from plasma using a specialized RealBest Extraction 100 reagent kit (Vector-Best, Russia).
The extraction procedure included the following steps: plasma samples were centrifuged at 13,000 rpm for 5 min. Next, 100 μl of the sample to be analyzed was added to a 1.5 ml Eppendorf vial. Then, 400 μl of lysis solution, 10 μl of sorbent, 10 μl of SE139, and 20 μl of tRNA were added to the mixture. The contents were mixed by shaking in a VORTEX apparatus for 10 s, followed by vigorous mixing in a TS-20 Thermoshaker (Biosan, Latvia) at 65°C and 13,000 rpm for 10 min. Subsequently, 500 μl of isopropanol was added to the mixture, which was kept at room temperature for 2 min. Centrifugation was repeated under the same conditions (13,000 rpm for 10 min). The supernatant was then removed, and the resulting sediment was washed twice: first with 500 μl of 70% ethanol and then with 300 μl of acetone. The sediment was dried for 2 min, after which it was dissolved in a special elution solution with thorough mixing in a VORTEX apparatus. The resulting product was incubated in a thermoshaker at 65°C and 13,000 rpm for 5 min. The product was then transferred to a new tube and supplemented with 20 μl of RNase inhibitor. The total RNA concentration was determined spectrophotometrically using a NanoDrop2000C instrument (Thermo Fisher Scientific, USA). Standardization of the study was ensured by selecting a reference gene, miRNA-16, whose determination was based on the arithmetic mean of the fluorescence signal cycle thresholds (Ct values).
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics version 26.0 (USA). The assumption of normality was assessed using the Shapiro–Wilk test. Since all variables were non-normally distributed, they are presented as Me (Q25%; Q75%), where Me is the median, and Q25% and Q75% represent the lower and upper quartiles, respectively. The nonparametric Mann–Whitney U test was used to assess the differences between two independent groups. The significance level was set at p≤0.05.
Results
The relative expression values of 11 miRNAs (miRNA-210, miRNA-145, miRNA-20a, miRNA-21, miRNA-181a, miRNA-143, miRNA-221, miRNA-126, miRNA-146b, miRNA-146a, and miRNA-122) were determined in the peripheral blood plasma samples. A comparative analysis of these miRNA expression levels allowed us to identify potential biomarkers involved in the pathogenesis of CAP. The significance of the identified differences (p-values) is presented in the summary table.
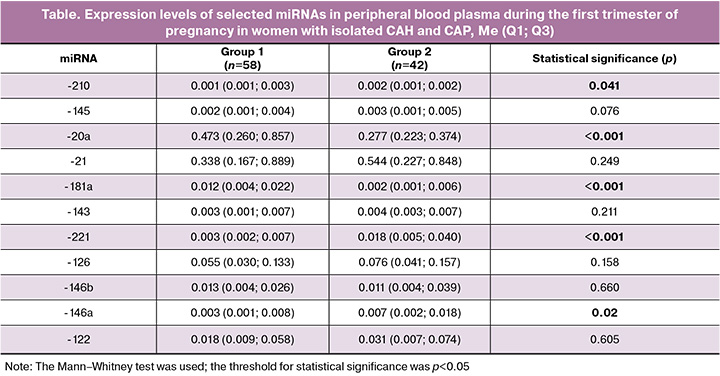
The data presented in the table demonstrate statistically significant contrasting changes in the expression levels of several miRNAs in plasma during the first trimester of pregnancy in patients with CAH and CAP.
A significant decrease in the expression levels of miRNA-20a and miRNA-181a was observed in the group of patients with CAP, whereas the expression levels of miRNA-221, miRNA-146a, and miRNA-210 were higher in this group than in women who did not develop preeclampsia.
Discussion
Current concepts define preeclampsia as a pathological condition that occurs in the second half of pregnancy, with maternal and/or placental factors playing a fundamental role in its pathogenesis. [10]. Experiments have established that a wide range of processes in the cells of the developing placenta, such as trophoblast differentiation, migration, proliferation, apoptotic cell death, vasculogenesis, angiogenesis, and regulation of cellular metabolism, are controlled by regulatory molecules known as miRNAs [11].
Modern scientific studies have demonstrated significant heterogeneity in the changes in placental and plasma miRNAs during the development of preeclampsia. This variability is compounded by differences in the studied samples, including gestational age, specific somatic status of patients, different pathogenetic forms of preeclampsia (early and late), and other factors. Given the importance of early preeclampsia risk stratification and the selection of personalized management strategies for patients with CAH, assessing plasma miRNA expression levels early in pregnancy shows promise for the identification of noninvasive prognostic markers.
However, a literature review by Lopes et al. [7] indicated that only 11 studies have been published on plasma miRNA expression levels in the first trimester of pregnancy among patients later diagnosed with preeclampsia. These studies did not distinguish between isolated preeclampsia and CAP. Notably, miRNA-221 and miRNA-146a expression levels were statistically significantly higher in the preeclampsia group than in the comparison group [12], which is consistent with our findings.
MicroRNA-221 is a crucial regulator of endothelial cell function [13], and its dysfunction has significant pathogenic implications in cardiovascular diseases [14] and preeclampsia [10]. The expression level of this miRNA has been extensively studied in patients with arterial hypertension, revealing a positive correlation between miRNA-221 levels and the presence of arterial hypertension [15]. However, its study in pregnant patients is limited, and most research has focused on placental tissue rather than circulating blood plasma [16]. In our study, we found a statistically significant increase in miRNA-221 expression in the blood plasma of patients with CAP compared to those with isolated CAH.
Another critical pathogenic factor in the development of preeclampsia is hypoxic placental damage. [10]. Placental hypoxia is a significant cause of oxidative stress, promotes the increased production of proinflammatory cytokines, and initiates programmed cell death processes in placental tissue [17]. Oxidative stress can influence the expression of specific miRNAs, particularly miRNA-210. Increased miRNA-210 expression in response to hypoxia suppresses extravillous trophoblast invasion during the first trimester by modifying mitochondrial function and exacerbating oxidativee stress through increased reactive oxygen species concentration [18]. Increased miRNA-210 expression in preeclampsia was observed in the study by Youssef et al. (2019) and in our own work; however, their study was conducted in the second half of pregnancy and did not consider the presence or absence of CAH among the participants [19]. The renin-angiotensin-aldosterone system (RAAS) is a key regulator of blood pressure and one of the primary mechanisms for detecting the onset of pregnancy in women. Dysfunction of the RAAS is a major cause of insufficient cytotrophoblast invasion and defects in spiral artery remodeling, which are the fundamental pathophysiological mechanisms underlying preeclampsia [20]. It has been established that miRNAs, particularly miRNA-146a and miRNA-181a, play significant roles in RAAS signaling [21].
Most studies on miRNA-146a expression levels have analyzed placental material, revealing multidirectional changes in its expression [10, 22]. Our study demonstrated a significant increase in miRNA-146a expression in women with CAP, likely associated with the pronounced dysregulation of the RAAS in early pregnancy in this group.
MiRNA-181a also regulates RAAS function [23]. A decrease in miRNA-181a expression correlates with increased renin levels in the kidneys. In experimental animals modeling a hereditary form of arterial hypertension, reduced concentrations of this miRNA were observed in both blood plasma and kidney tissue [24], and similar results have been reported in human studies [25].
In a study by Hromadnikova I. et al. (2022), which explored the pathogenetic significance of dysregulation of cardiac-specific miRNAs in blood plasma during the first trimester, an increase in miRNA-181a was observed in women with CAH and in pregnant women who subsequently developed preeclampsia [26], contradicting our findings. However, this study did not conduct a differentiated analysis of preeclampsia cases concerning the presence or absence of concomitant CAH. Conversely, Lin W. et al. (2022) reported a decrease in miRNA-181a expression specifically in CAP [27]. Notably, during early gestation, miRNA-181a expression levels decreased, whereas they significantly increased later during pregnancy.
A study by Nikitina N.A. et al. (2025) also found decreased miRNA-181a expression, although it was conducted after 20 weeks of pregnancy [28].
The role of miRNA-20a in the pathogenesis of preeclampsia is not well understood. Wang Y. et al. (2014) demonstrated that miRNA-20a-5p reduces trophoblast cell proliferation and invasiveness by regulating the transcription factor FOXA1 [29]. Hromadnikova I. et al. (2022) found increased expression of miRNA-20a-5p in the first trimester in women with CAH and those who subsequently developed preeclampsia, with or without fetal growth restriction [26]. Interestingly, another study reported a decrease in miRNA-20a expression under similar conditions, which is consistent with our findings [30]. This likely reflects a compensatory response to the placental dysfunction.
In summary, the literature indicates that plasma miRNAs play a role in modulating various biological processes that are critical in the pathogenesis of preeclampsia. This suggests that miRNAs could serve as effective risk stratifiers for the development of pregnancy complications. Further in-depth studies are necessary to fully understand the mechanisms by which miRNAs influence preeclampsia development in patients with CAH.
Conclusion
Our study revealed statistically significant changes in the expression levels of miRNAs 221, 146a, 210, 20a, and 181a in preeclampsia associated with CAH. Aberrant expression of these miRNAs disrupts key biological processes, including trophoblast differentiation and invasion, cell migration, vasculogenesis, angiogenesis, and cellular metabolism regulation, thereby directly contributing to the pathogenesis of CAH. Further studies are needed to assess the potential of using circulating miRNAs as biomarkers for predicting the development of preeclampsia in patients with CAH.
References
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Преэклампсия. Эклампсия. Отеки, протеинурия и гипертензивные расстройства во время беременности, в родах и послеродовом периоде. 2024. [Ministry of Health of the Russian Federation. Clinical guidelines. Preeclampsia. Eclampsia. Edema, proteinuria, and hypertensive disorders during pregnancy, childbirth, and the postpartum period. 2024. (in Russian)].
- Kametas N.A., Nzelu D., Nicolaides K.H. Chronic hypertension and superimposed preeclampsia: screening and diagnosis. Am. J. Obstet. Gynecol. 2022; 226(2S): S1182-95. https://dx.doi.org/10.1016/j.ajog.2020.11.029
- DeSisto C.L., Robbins C.L., Ritchey M.D., Ewing A.C., Ko J.Y., Kuklina E.V. Hypertension at delivery hospitalization – United States, 2016–2017. Pregnancy Hypertens. 2021; 26: 65-8. https://dx.doi.org/10.1016/j.preghy.2021.09.002
- Greenberg V.R., Silasi M., Lundsberg L.S., Culhane J.F., Reddy U.M., Partridge C. et al. Perinatal outcomes in women with elevated blood pressure and stage 1 hypertension. Am. J. Obstet. Gynecol. 2021; 224(5): 521.e1-11. https://dx.doi.org/10.1016/j.ajog.2020.10.049
- Rezk M., Gamal A., Emara M. Maternal and fetal outcome in de novo preeclampsia in comparison to superimposed preeclampsia: a two-year observational study. Hypertens. Pregnancy. 2015; 34(2): 137-44. https://dx.doi.org/10.3109/10641955.2014.982329
- Leimena C., Qiu H. Non-coding RNA in the pathogenesis, progression and treatment of hypertension. Int. J. Mol. Sci. 2018; 19(4): 927. https://dx.doi.org/10.3390/ijms19040927
- Lopes A.C.S., Macedo A.A., Mendes F.S., Costa I.M., Dusse L.M.S., Alpoim P.N. Changes in microRNA expression associated with preeclampsia: a systematic review. Braz. J. Med. Biol. Res. 2025; 58: e13988. https://dx.doi.org/10.1590/1414-431X2025e13988
- Никитина Н.А., Сидорова И.С., Райгородская М.П., Морозова Е.А., Тимофеев С.А., Агеев М.Б., Амирасланова Н.И. Эпигенетические механизмы развития преэклампсии: роль плазменных микроРНК. Архив акушерства и гинекологии им. В.Ф. Снегирёва. 2024; 11(2): 179-92. [Nikitina N.А., Sidorova I.S., Raygorodskaya M.Р., Morozova E.А., Timofeev S.А., Ageev M.В., Amiraslanova N.I. Epigenetic mechanisms of preeclampsia: Role of plasma microRNAs. V.F. Snegirev Archives of Obstetrics and Gynecology. 2024; 11(2): 179-92 (in Russian)]. https://dx.doi.org/10.17816/aog623622
- Семенов Ю.А., Казачков Е.Л., Веряскина Ю.А., Чижовская А.В. Профиль экспрессии микроРНК в плазме крови беременных женщин с высоким и низким риском спонтанных преждевременных родов и перинатальных потерь. Южно-Уральский медицинский журнал. 2021; 2: 18-23. [Semyonov Yu.A., Kazachkov E.L., Veryaskina Yu.A., Chizhovskaya A.V. MicroRNA expression profile in the blood plasma of pregnant women with high and low risk of spontaneous preterm birth and perinatal loss. South Ural Medical Journal. 2021; 2: 18-23 (in Russian)].
- Борис Д.А., Шмаков Р.Г. Преэклампсия: современные концепции патогенеза. Акушерство и гинекология. 2022; 12: 12-7. [Boris D.A., Shmakov R.G. Preeclampsia: modern concepts of pathogenesis. Obstetrics and Gynecology. 2022; (12): 12-7 (in Russian)]. https://dx.doi.org/10.18565/aig.2022.213
- Hayder H., O'Brien J., Nadeem U., Peng C. MicroRNAs: crucial regulators of placental development. Reproduction. 2018; 155(6): R259-71. https://dx.doi.org/10.1530/REP-17-0603
- Luizon M.R., Conceição I.M.C.A., Viana-Mattioli S., Caldeira-Dias M., Cavalli R.C., Sandrim V.C. Circulating MicroRNAs in the second trimester from pregnant women who subsequently developed preeclampsia: potential candidates as predictive biomarkers and pathway analysis for target genes of miR-204-5p. Front. Physiol. 2021; 12: 678184. https://dx.doi.org/10.3389/fphys.2021.678184
- Celic T., Metzinger-Le Meuth V., Six I., Massy ZA., Metzinger L. The mir-221/222 cluster is a key player in vascular biology via the fine-tuning of endothelial cell physiology. Curr. Vasc. Pharmacol. 2017; 15(1): 40-6. https://dx.doi.org/10.2174/1570161114666160914175149
- Naderi-Meshkin H., Setyaningsih W.A.W. Endothelial cell dysfunction: onset, progression, and consequences. Front. Biosci. (Landmark Ed). 2024; 29(6): 223. https://dx.doi.org/10.31083/j.fbl2906223
- Alexandru N., Constantin A., Nemecz M., Comariţa IK., Vîlcu A., Procopciuc A. et al. Hypertension associated with hyperlipidemia induced different microRNA expression profiles in plasma, platelets, and platelet-derived microvesicles; effects of endothelial progenitor cell therapy. Front. Med. (Lausanne). 2019; 6: 280. https://dx.doi.org/10.3389/fmed.2019.00280
- Yang Y., Li H., Ma Y., Zhu X., Zhang S., Li J. MiR-221-3p is down-regulated in preeclampsia and affects trophoblast growth, invasion and migration partly via targeting thrombospondin 2. Biomed. Pharmacother. 2019; 109: 127-34. https://dx.doi.org/10.1016/j.biopha.2018.10.009
- Щербаков В.И., Поздняков И.М., Ширинская А.В. Уровень фактора-1α, индуцируемого гипоксией, и ассоциированных с ним молекул при преэклампсии. Российский вестник акушера-гинеколога. 2025; 25(1): 5-10. [Shcherbakov V.I., Pozdnyakov I.M., Shirinskaya A.V. Level of hypoxia-inducible factor-1α and associated molecules in preeclampsia. Russian Bulletin of Obstetrician-Gynecologist. 2025; 25(1): 5-10 (in Russian)]. https://dx.doi.org/10.17116/rosakush2025250115
- Jaszczuk I., Koczkodaj D., Kondracka A., Kwaśniewska A., Winkler I., Filip A. The role of miRNA-210 in pre-eclampsia development. Ann. Med. 2022; 54(1): 1350-6. https://dx.doi.org/10.1080/07853890.2022.2071459
- Youssef H.M.G., Marei E.S. Association of MicroRNA-210 and MicroRNA-155 with severity of preeclampsia. Pregnancy Hypertens. 2019; 17: 49-53. https://doi.org/10.1016/j.preghy.2019.05.010
- Lumbers E.R., Delforce S.J., Arthurs A.L., Pringle K.G. Causes and consequences of the dysregulated maternal renin-angiotensin system in preeclampsia. Front. Endocrinol. (Lausanne). 2019; 10: 563. https://dx.doi.org/10.3389/fendo.2019.00563
- Takeda Y., Demura M., Yoneda T., Takeda Y. Epigenetic regulation of the renin-angiotensin-aldosterone system in hypertension. Int. J. Mol. Sci. 2024; 25(15): 8099. https://doi.org/10.3390/ijms25158099
- Артемьева К.А., Низяева Н.В., Баев О.Р., Романов А.Ю., Хлестова Г.В., Болтовская М.Н., Щеголев А.И., Кактурский Л.В. Регуляция ренин-ангиотензин-альдостероновой системы плаценты при ранней и поздней преэклампсии. Доклады Российской академии наук. Науки о жизни. 2022; 507(1): 475-82. [Artemieva K.A., Nizyaeva N.V., Baev O.R., Romanov A.Yu., Khlestova G.V., Boltovskaya M.N., Shchegolev A.I., Kakturskiy L.V. Regulation of the placental renin-angiotensin-aldosterone system in early and late onset preeclampsia. Reports of the Russian Academy of Sciences. Life sciences. 2022; 507(1): 475-82 (in Russian)]. https://dx.doi.org/10.31857/S2686738922060026
- Lv B., He S., Li P. Jiang S., Li D., Lin J. et al. MicroRNA-181 in cardiovascular disease: emerging biomarkers and therapeutic targets. FASEB J. 2024; 38(9): e23635. https://doi.org/10.1096/fj.202400306R
- Jackson K.L., Gueguen C., Lim K., Eikelis N., Stevenson E.R., Charchar F.J. et al. Neural suppression of miRNA-181a in the kidney elevates renin expression and exacerbates hypertension in Schlager mice. Hypertens. Res. 2020; 43(11): 1152-64. https://doi.org/10.1038/s41440-020-0453-x
- Marques F.Z., Romaine S.P., Denniff M., Eales J., Dormer J., Garrelds I.M. et al. Signatures of miR-181a on the renal transcriptome and blood pressure. Mol. Med. 2015; 21(1): 739-48. https://dx.doi.org/10.2119/molmed.2015.00096
- Hromadnikova I., Kotlabova K., Krofta L. Cardiovascular disease-associated MicroRNA dysregulation during the first trimester of gestation in women with chronic hypertension and normotensive women subsequently developing gestational hypertension or preeclampsia with or without fetal growth restriction. Biomedicines. 2022; 10(2): 256. https://dx.doi.org/10.3390/biomedicines10020256
- Lin W., Teng S.W., Lin T.Y. Lovel R., Sung H.Y., Chang W.Y. et al. Combinatorial analysis of circulating biomarkers and maternal characteristics for preeclampsia prediction in the first and third trimesters in Asia. Diagnostics (Basel). 2022; 12(7): 1533. https://dx.doi.org/10.3390/diagnostics12071533
- Никитина Н.А., Сидорова И.С., Райгородская М.П., Морозова Е.А., Тимофеев С.А., Агеев М.Б., Амирасланова Н.И. Роль микроРНК, ассоциированных с кардиоваскулярными заболеваниями и плацентарными нарушениями, в развитии преэклампсии. Вопросы гинекологии, акушерства и перинатологии. 2025; 24(2): 5-19. [Nikitina N.A., Sidorova I.S., Raigorodskaya M.P., Morozova E.A., Timofeev S.A., Ageev M.B., Amiraslanova N.I. Role of microRNAs associated with cardiovascular disease and placental abnormalities in the development of pre-eclampsia. Gynecology, Obstetrics and Perinatology. 2025; 24(2): 5-19 (in Russian)]. https://dx.doi.org/10.20953/1726-1678-2025-2-5-19
- Wang Y., Zhang Y., Wang H., Wang J., Zhang Y., Wang Y. et al. Aberrantly up-regulated miR-20a in pre-eclampsic placenta compromised the proliferative and invasive behaviors of trophoblast cells by targeting forkhead box protein A1. Int. J. Biol. Sci. 2014; 10(9): 973-82. https://dx.doi.org/10.7150/ijbs.9088
- Hu T.X., Wang G., Guo X.J., Sun Q.Q., He P., Gu H. et al. MiR 20a, -20b and -200c are involved in hydrogen sulfide stimulation of VEGF production in human placental trophoblasts. Placenta. 2016; 39: 101-10. https://dx.doi.org/10.1016/j.placenta.2016.01.019
Received 12.08.2025
Accepted 01.09.2025
About the Authors
Yurii A. Semenov, Dr. Med. Sci., Associate Professor, Director, Ural Research Institute for Maternal and Child Health, Ministry of Health of the Russian Federation,620028, Russia, Yekaterinburg, Repin str., 1, +7(904)815-62-46, u-sirius@mail.ru, https://orcid.org/0000-0002-3268-7981
Vladimir N. Antonov, Dr. Med. Sci., Associate Professor, Professor at the Therapy Department of the Institute of Continuing Professional Education,
South Ural State Medical University, Ministry of Health of the Russian Federation, 454092, Russia, Chelyabinsk, Vorovskogo str., 64, +7(919)357-04-73, ant-vn@yandex.ru, https://orcid.org/0000-0002-3531-3491
Evgeniia A. Azarenkova, Head of the Educational and Methodological Department, Ural Research Institute for Maternal and Child Health, Ministry of Health of the Russian Federation, 620028 Russia, Yekaterinburg, Repin str., 1, +7(908)581-51-86, jennysplean@mail.ru, https://orcid.org/0000-0001-9656-350X
Anna V. Chizhovskaya, Deputy Director for Organizational and Methodological Work and Public Issues, Ural Research Institute for Maternal and Child Health,
Ministry of Health of the Russian Federation, 620028 Russia, Yekaterinburg, Repin str., 1, +7(951)124-60-13, ms.chizhovskaya@mail.ru, https://orcid.org/0000-0003-1574-1613
Yuliya A. Veryaskina, PhD, Researcher at the Laboratory of Molecular Genetics, Department of Chromosome Structure and Function, Institute of Molecular and
Cellular Biology of the Siberian Branch of the Russian Academy of Sciences, 630090, Russia, Novosibirsk, Lavrentiev Ave., 8/2, +7(383)363-90-42, microrna@inbox.ru,
https://orcid.org/0000-0002-3799-9407
Corresponding author: Evgeniia A. Azarenkova, jennysplean@mail.ru



