Use of complex of natural antimicrobial peptides and cytokines for the treatment of patients with abnormal cervical screening results “atypical squamous cells of uncertain significance”
Dikke G.B., Sukhanov A.A., Kukarskaya I.I., Shilova N.V.
Objective: To evaluate the frequency of cervical lesions with atypical squamous cells of uncertain significance (ASCUS) in patients with chronic endometritis (CE) and the effectiveness of treatment using a complex of natural antimicrobial peptides and cytokines.
Materials and methods: A prospective open-label randomized controlled trial included 1,126 patients with CE. Group I (n=563) received a natural antimicrobial peptide and cytokine complex (Superlymph) at a dose of 25 U/day per vaginam for 20 days. Group II (n=563) did not receive the peptide-cytokine complex. Both groups underwent antimicrobial therapy. Examination methods included real-time polymerase chain reaction and Pap test.
Results: The patients' age ranged from 18 to 45 years: 36.0 (3.1) years in Group I and 35.9 (4.4) years in Group II (p=0.22). ASCUS was detected in 45.5% (256/563) and 42.6% (240/563) of patients (p=0.37), and human papillomavirus (HPV) was identified in 71.6% (403/563) and 71.8% (404/563), respectively (p=1.0). In patients who suffered from ASCUS before treatment, the condition improved in 85.6% (219/256) and 45.8% (110/240) in groups I and II, respectively. The potential for recovery was 2 times higher when using Superlymph (ОР=1,87, 95% ДИ 1,61; 2,16). HPV-negative status after treatment was observed in 85.4% (481/563) of patients in Group I versus 29.7% (167/563) in Group II (OR=0.21, 95% CI 0.17; 0.26, p<0.001).
Conclusion: Among patients with CE, the prevalence of ASCUS is 44.1%, and the prevalence of HPV-positive status is observed in 71.7% of women. Combination therapy with Superlymph promotes the resolution of ASCUS in 85.6% of patients (a 2-fold risk reduction compared to antibacterial/antimycotic therapy alone) with a negative HPV test rate in 85.4% of CE patients after treatment.
Authors' contribution: Dikke G.B. – study concept and design, analysis of the statistical processing of clinical material and its interpretation, data search, text composition and editing; Sukhanov A.A. – collection of clinical material, electronic database arrangement, text composition; Kukarskaya I.I. – clinical study site management, study supervision; Shilova N.V. – statistical analysis of the study results, text composition.
Conflicts of interest: The authors report no conflicts of interest and guarantee the original nature of the study.
Funding: The study had no financial support. Publication of the article was sponsored by OOO Biotechpharm.
Ethical Approval: The study was approved by the local Ethics Committee (final protocol version v3.0 dated July 20, 2019).
Patient Consent for Publication: The patients signed informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Dikke G.B., Sukhanov A.A., Kukarskaya I.I., Shilova N.V. Use of complex of
natural antimicrobial peptides and cytokines for the treatment of patients with
abnormal cervical screening results “atypical squamous cells of uncertain significance”.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (11): 130-140 (in Russian)
https://dx.doi.org/10.18565/aig.2025.331
Keywords
Cervical lesions characterized by the presence of atypical squamous cells of uncertain significance (ASCUS) represent changes that can be considered either the absence of intraepithelial atypical changes (Negative for Intraepithelial Lesion or Malignancy, NILM) or low-grade squamous intraepithelial lesions (LSIL). As cellular ASCUS changes can reflect both benign and potentially serious lesions, they are known as of "uncertain significance." The diagnosis of ASCUS is controversial not only because of the clinical significance of its subcategories (reactive or atypical), but also due to the ambiguity of the term itself [1].
The prevalence rate of ASCUS, according to foreign experts, varies from 2.5 to 19.1% [1]. In Russia, there are no statistical data on the prevalence of ASCUS, since this term refers to cytological screening, and not to registered cervical diseases. A cytological screening test of 800 women aged 30 to 78 years in Krasnodar showed that ASCUS was detected in 20% of patients under 30 years and in 32% of patients over 30 years [2]. In the study including 2,970 women, the incidence of ASCUS was only 0.2% and 3.6% in the same age categories, respectively [3]. In an article by Bebneva T.N. et al. among 330 pregnant women (aged 18–45) infected with the human papillomavirus (HPV), ASCUS occurred with a frequency of 19.8% [4].
The clinical significance of ASCUS is based on the fact that approximately 10–20% of patients with ASCUS have varying degrees of cervical intraepithelial neoplasia (CIN), which is a precursor to cervical squamous cell carcinoma [5].
ASCUS was originally described by Papanicolaou as an atypical pathology characterized by reactive changes of squamous cells caused by inflammation. It was defined as an inflammatory smear type (class II) [6]. Subsequently, a link between CIN severity and high microbiota diversity with low levels of Lactobacillus spp. (community state type - CST IV) was found, regardless of HPV status [7]. A study by Liu Y. et al. (2023) also detected a correlation between decreased Lactobacillus levels in the vagina and increased susceptibility to HPV infection and the presence of precancerous cervical lesions [8]. The authors noted a gradual increase in the proportion of certain genera, such as Gardnerella, Dialister, and Prevotella, as precancerous cervical lesions developed, with CST type IV, representative of the dominance of these bacteria, demonstrating an increased risk of more severe changes. Restoring the balance of vaginal microbiota, according to Liu Y. et al., can prevent disease progression [8].
However, the data by Long T. et al., based on the study conducted the same year, did not find an increase in the frequency of cervical cytological changes in the presence of bacterial vaginosis (BV) or vulvovaginal candida (VVC), with or without HPV [9]. However, Shen J. et al. (2024) stated in their review that the correlation between vaginal dysbiosis, HPV infection, and the progression of CIN to cancer had been widely documented [10], while emphasizing that there is still no evidence of a causal relationship and the mechanisms by which vaginal microbiota participates in disease progression are unknown.
The risk of ASCUS was 10 times higher in patients co-infected with high-risk HPV and herpes simplex virus (HSV), but there was no increase in the likelihood of developing high-grade squamous intraepithelial lesions (HSIL) [11]. Elevated risk of developing ASCUS in the presence of HSV-2, but not HSIL was demonstrated in another study which concluded that HSV-2 may be involved in the initial transformation of cells, but not in the progression of changes [11]. Furthermore, HSV has been shown to cause cervical inflammation, which may act as a cofactor in the progression of cervical cancer. 56.1% of women with a positive HSV-2 had inflammation compared to 49% of patients with a negative status. Abnormal oncocytological results occurred in 11.5% and 7.8% of patients, respectively [12]. However, a direct link has not yet been established, and other studies doubt the involvement of HSV in the pathogenesis of cervical cancer.
The expansion of cervical lesions depends on local immune responses, with both HPV and pathogenic/opportunistic microorganisms potentially accelerating disease progression by suppressing the immune response. On the other hand, HPV-induced immune changes create an environment causing the growth of opportunistic microorganisms, which further increases inflammation, creates a positive feedback loop, and ultimately contributes to the development and development of cervical lesions [13].
Given the close relationship between the vaginal microbiota and HPV infection, as well as cervical lesions, the recent attention was focused on modulation of the microbiota. Prevention and treatment strategies are mainly based on the maintenance or restoration of vaginal microecological homeostasis. Probiotics have been shown to reduce the risk of HPV infection and secondary infections, maintain a balanced vaginal microbiome, and resolve HPV-associated cervical lesions by enhancing the immune response [14, 15]. Both oral and vaginal administration of probiotics can significantly increase the rate of HPV clearance [16]. Chen T. et al. demonstrated that, in patients with vaginal dysbiosis, the use of a combination of probiotics from several Lactobacillus strains significantly reduced bacterial-induced inflammation, levels of proinflammatory cytokines (interleukin-1β and tumor necrosis factor-alpha), and immune cell infiltration (neutrophils, lymphocytes, and monocytes), which contributed to the restoration of the affected vaginal microbiota to normal levels [17].
Immunotherapy is currently considered as a promising option not only for the prevention but also for the treatment of cervical cancer [12].
Bayramova G.R. et al. conducted a comparative analysis of treatment outcomes for HPV-infected patients diagnosed with cervicitis associated with BV and/or VVC with frequent recurrences. Therapy combined with a combination of antimicrobial peptides and cytokines (25 U suppositories twice daily rectally for 20 days) yielded significantly better results compared to standard treatment: HPV clearance was achieved in 57.5% of women compared to 27.5% of women, respectively. Recurrences of BV and VVC 3 months after treatment were observed only in the standard treatment group (the incidence of cervical lesions and their dynamics were not reported) [18].
Women with chronic endometritis (CE) have an affected vaginal microbiome, with a relative abundance of bacteria such as Gardnerella, Streptococcus, and Ureaplasma, instead of the typical dominance of Lactobacillus [18, 19]. Bacterial infections and HSV-1 and HSV-2 were detected in 80% of CE patients, with 50% of these infections combined with HPV and accompanied by an imbalance of pro- and anti-inflammatory cytokines in the vagina [21]. The TULPAN study demonstrated disruptions in the vaginal microbiota in the majority of CE patients (90%) at baseline. After antimicrobial therapy and Superlymph intake they persisted in only a small number (18.6%, p<0.001) [22].
Basing on the above studies, we assumed that the persistence of opportunistic microorganisms and the presence of vaginal dysbiosis in patients with chronic endometriosis pose a high risk of HPV and ASCUS infection. However, the available studies do not indicate a specific frequency of ASCUS in patients with CE and do not suggest a relationship between them, which defines the relevance of this study.
The objective of the study: To evaluate the frequency of cervical lesions with atypical squamous cells of uncertain significance (ASCUS) in patients with chronic endometritis (CE) and the effectiveness of treatment using a complex of natural antimicrobial peptides and cytokines.
Materials and methods
Study design: Prospective, open-label, randomized, controlled, interventional clinical trial. The study protocol was conducted given the provisions of the Declaration of Helsinki of the World Medical Association (revised at the 64th WMA General Assembly, Fortaleza, Brazil, 2013), the International Ethical Guidelines for Biomedical Research Involving Human Subjects of the Council for the International Organization of Medical Sciences (2002 edition), the National Standard of the Russian Federation GOST R 52379-2005 "Good Clinical Practice" (2005), and other regulatory documents of the Russian Federation. The study received a positive opinion from the local Ethics Committee (final protocol version v3.0 dated July 20, 2019). All patients signed informed consent to participate in the study after receiving oral and written explanations regarding the parallel-group study.
The study included 1,126 patients who received medical care from September 2019 to June 2023 at the antenatal clinic of the Tyumen Perinatal Center (Tyumen, Russia) for female infertility of uterine origin, oocyte implantation defect (N97.2), and chronic inflammatory disease of the uterus (N71.1) (the diagnosis was confirmed histologically and immunohistochemically). Patients with the hypoplastic cervical endometritis, characterized by an M-echo thickness of 7 mm or less were not included in the study. Patients with oncocytological results of LSIL or higher were excluded from the study.
Inclusion criteria: Aged 18–45 years, uterine infertility (N97.2 Female infertility of uterine origin, ovum implantation defect) with chronic uterine inflammatory disease confirmed histologically and immunohistochemically (N71.1 Chronic inflammatory disease of the uterus), viral shedding (herpes simplex virus, human papillomavirus), patent fallopian tubes, absence of atrophic endometrium (M-echo greater than 8 mm in the first phase of the menstrual cycle), ovulatory menstrual cycle, blood isocoagulation, absence of nedication contraindications, patient’s informed consent.
Exclusion criteria: Male factor infertility (grade III-IV teratozoospermia), gynecological diseases (present or a history of uterine fibroids, endometriosis, ovarian cysts, cervical intraepithelial lesions), congenital malformations, other causes of infertility, sexually transmitted infections (except viral) and/or acute infectious and inflammatory diseases of the genital tract within the past 6 months, pregnancy and breastfeeding, severe somatic diseases, Rh-negative blood, hypersensitivity to the components of the medications used.
Hypothesis. Complex treatment of patients with chronic endometritis, including the use of Superlymph and an antimicrobial drug, is more effective in ASCUS resolution than antimicrobial therapy alone.
Sample size calculation was performed using G*Power software (Heinrich-Heine-Universität Düsseldorf, Germany). The calculation included the research data on the ASCUS resolution rate in HPV-positive patients with empirical antimicrobial therapy of 54% [23] and an expected 30% superiority of treatment efficacy using a combination of antimicrobial peptides and cytokines. With an expected model accuracy of 95%, type I and type II error rates of α=0.05 and β=0.2, respectively, a power of 1-β=0.95, and a case-to-control ratio of 1:1, the required sample size was 54 patients for each group. Considering that not all patients with CE had ASCUS at baseline, the number of patients included in the analysis was 10-fold higher in order to strengthen the statistical methods of the study.
Randomization to two groups was performed using a computer program to generate a random sequence and a random number table with a 50% probability of inclusion into a group.
Interventions. The database consisted of two parts, randomized into two groups (563 patients each). In both groups patients received one course of antibacterial therapy with doxycycline (100 mg once daily, 10–14 days), while for vaginal dysbiosis, they took a combination of antimicrobial agent with antibacterial/antimycotic action (vaginally once daily at night, 10 days), followed by a course of topical probiotic administration (14 days). In Group I (n=563) patients received a complex of natural antimicrobial peptides and cytokines (Superlymph, INN: protein-peptide complex from porcine blood leukocytes, Altpharm LLC, Russia), 25 U suppositories once a day vaginally in the evening for 20 days. In Group II (n=563) no therapy was conducted.
The study design flow chart is presented at Figure.
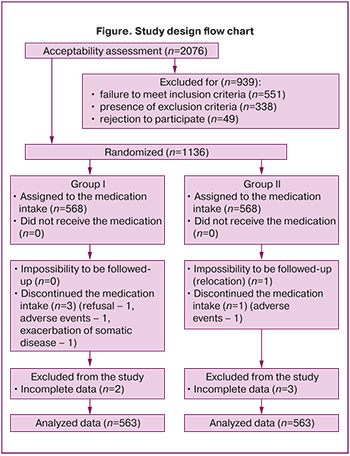
Examination methods. A comprehensive examination of patients was performed using clinical and laboratory methods. To detect viruses (qualitatively) and opportunistic microorganisms (quantitatively) we used a real-time polymerase chain reaction (PCR-test). Cell scrapings from the vagina (posterolateral fornix) served as the analysis material. Oncocytological examination of smears from the exocervix and endocervix was performed using microscopy after Pap-test and assessment according to the Bethesda Terminology System (2014 edition). Laboratory processing was conducted before treatment and 30 days after its completion.
Outcomes assessment. The primary outcome described the ASCUS resolution rate. Secondary outcomes identified the proportion of patients with HPV and HSV elimination and normalization of vaginal flora in patients with ASCUS. The analysis was conducted based on the electronic database after the results verification. Patients with missing data were excluded from the analysis. The final analysis was conducted using the primary outcome (ASCUS resolution rate) among 563 patients in each group.
Statistical analysis
Statistical analysis was performed using the Statistica software for Windows 10.0 (StatSoft Inc., USA). To assess the distribution of features we used the Kolmogorov–Smirnov test. Normally distributed continuous variables were described as mean values (M) with standard deviations (SD). Qualitative features were expressed as absolute numbers (n) and relative values (%). Differences in independent samples were assessed using the Mann–Whitney U-test, and differences between qualitative features were analyzed using the χ2 test. The significance level was set at p<0.05, with type I and type II error rates of α=5% and β=20%, respectively.
Treatment effectiveness analysis was performed on an intention-to-treat basis. To assess the association between the studied factor and the outcome the following statistical indicators were used: relative risk (RR) and risk difference (RD) with 95% confidence intervals (CI). The relative risk and its CI were calculated using the epi.2by2 function from the epiR package (version 2.0.88), which is designed for analyzing data presented in 2x2 tables. This function calculates measures of the association between the exposure and outcome, including RR, using standard methods for cohort study data. The risk difference and its CI were analyzed using the risk difference function from the fmsb package (version 0.7.6). This function presents the number of cases and the total number of individuals at risk as input data in the two comparison groups, and then enables to calculate the absolute difference in risk and the corresponding confidence interval, given the correction data (CRC option).
To ensure an independent assessment of the study results, the analysis was conducted by a statistician and an expert who had no contact with the patients, the researchers, or the laboratory diagnostic specialists.
Results
General information about the patients. Patients’ age ranged from 18 to 45 years, with a mean of 36.0 (3.1) years in Group I and 35.9 (4.4) in Group II, p=0.22. The patients' social and clinical status is presented in Table 1.
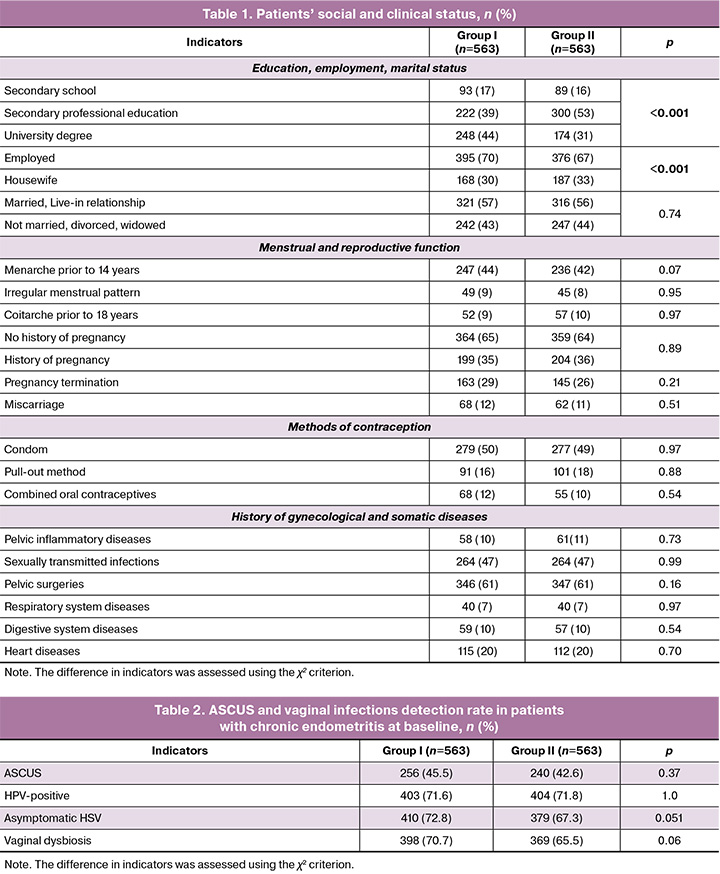
The analysis of Table 1 revealed statistically significant differences between the groups only in education and employment history; there were no differences between Groups I and II for any other indicators. Individual physical examination indicators in patients in both groups were comparable.
The frequency of ASCUS and vaginal infections at baseline in patients with CE is presented in Table 2.
Thus, the incidence of ASCUS and vaginal infections in the examined patients did not show statistically significant differences between the groups.
Therapy effectiveness assessment
The primary outcomes are presented in Table 3, which shows that the use of Superlymph drug reduced the incidence of ASCUS from 45.5% (256/563) to 6.6% (37/563), and in patients receiving antimicrobial therapy – from 42.6% (240/563) to 23.1% (130/563), with a statistically significant difference between the studied groups (p<0.001).
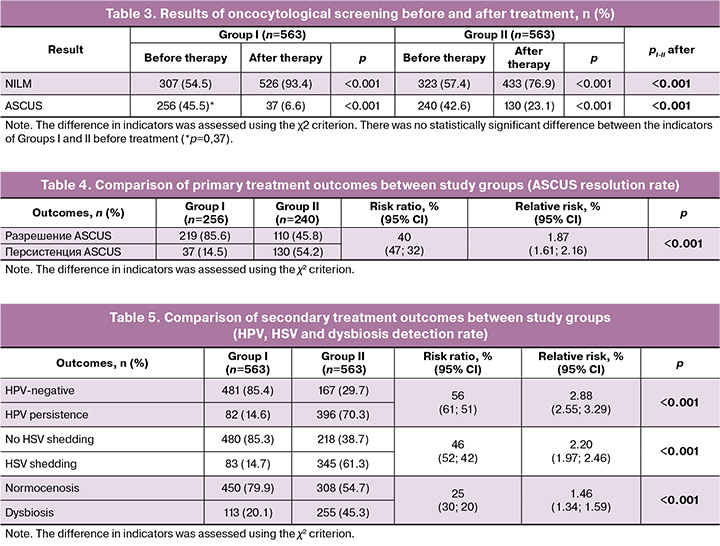
The results of group comparison by ASCUS resolution rate after treatment are presented in Table 4. In patients with ASCUS before treatment, its resolution in Group I occurred in 85.6% versus 45.8% in Group II with 2 times lower ASCUS detection, respectively (OR=1.87, 95% CI 1.61; 2.16; p<0.001).
Thus, ASCUS resolution in patients receiving Superlymph drug was 85.6% (OR=1.87, 95% CI 1.61; 2.16, p<0.001), indicating the high effectiveness of combination treatment using Superlymph, which has a significantly greater impact on ASCUS compared to antibacterial/antimycotic therapy alone.
Secondary outcomes. The rate of vaginal microflora normalization after treatment is presented in Table 5.
The number of HPV-negative patients after treatment was 85.4% in Group I versus 29.7% in Group II, p<0.001, and the risk of detecting HPV was 3 times lower in Group I compared to Group II. The number of patients with no asymptomatic HSV shedding after treatment was 85.3% versus 38.7%, respectively, p<0.001, with a 2-fold reduction in the risk of HSV detection after treatment. The percentage of patients with normal vaginal microbiota after treatment in Group I was 79.9% and Group I it accounted for 54.7%, p<0.001, and the risk of dysbiosis was 1.5 times lower when using Superlymph compared to patients who received only antimicrobial therapy.
Accordingly, secondary outcomes indicate high efficacy of immunomodulatory therapy when using Superlymph as part of combination therapy with antimicrobial drugs - absence of HPV in 85.4% (OR=2.88, 95% CI 2.55; 3.29), asymptomatic shedding of HSV in 85.3% (OR=2.20, 95% CI 1.97; 2.46) and achievement of vaginal normocenosis in 79.9% of patients (OR=1.46, 95% CI 1.34; 1.59).
Discussion
As ASCUS can be the result of inflammation, patients are prescribed antibiotic treatment on a routine basis. However, several studies have shown that antibiotic use has low efficacy, leading to unnecessary costs and unreasonable antibiotic use in an era of growing antibiotic resistance [13]. This is supported by the results of the current study: antimicrobial therapy resulted in ASCUS resolution in less than half of cases (45.8%), whereas a comprehensive approach using Superlymph was effective in 85.6% of patients and contributed to a 2-fold reduction in the risk of ASCUS detection compared to antimicrobial therapy alone. This is likely due to its greater effectiveness in HPV elimination, HSV shedding arrest, and normalization of vaginal microbiota.
It is known that the majority of primary HPV infections (up to 80–90%) resolve spontaneously within 12–24 months. For persistent forms of infections (more than 24 months) data on subsequent spontaneous resolution is not available, but it is stated that the likelihood of HPV elimination decreases with the increase in infection duration [24]. As expected, the frequency of HPV detection in Group II did not change significantly under the influence of antimicrobial therapy (71.8% before treatment, 70.3% after, p=0.30), whereas in Group I, just one month after treatment, the number of HPV-positive patients decreased from 71.6% to 14.6%, p<0.001, and the risk of HPV detection was 3 times lower compared to Group II.
Asymptomatic HSV shedding is analyzed mostly in terms of the risk of infecting sexual partners, and it is also indicated that at least 70% of people can shed HSV having no symptoms at least once a month [25], which is consistent with the results obtained in our study (80%). However, the impact of viral activity during asymptomatic shedding on the vaginal biocenosis and immune defense is discussed quite rarely. Nevertheless, activation of the herpes virus infection, even in the absence of symptoms, is manifested by an inflammatory response in vaginal tissues, as evidenced by the presence of CD8+ T cells and their interaction with epithelial cells. This leads to the activation of innate antiviral defense mechanisms, the activation of interferon-γ-related responses, and cytokine-mediated communication [26]. Data on the major role of HSV in the pathogenesis of cervical cancer suggest that the most probable mechanism is the "hit and run" hypothesis (by Sausen D.G.), according to which HSV infection is involved in the initiation of atypical changes but does not play any role in their progression in later stages [12]. The following is of fundamental importance: transforming HSV activity and transition of the infection to a latent form can reduce the risk of cervical lesions in the early stages and prevent transition of epithelial cells to more severe forms. In patients with chronic endometritis, combination treatment with Superlymph resulted in a reduction in asymptomatic HSV shedding from 72.8% to 14.7% (in Group II, from 67.3% to 61.3%), with a probability twice as high as with antimicrobial therapy alone. These results are consistent with the study by Dobrokhotova Yu.E. et al. (2021), in which the number of cells with HSV twice decreased [27]. While a number of studies indicate that HPV/HSV coinfection leads to a higher risk of developing cervical cancer, the results of other scientific works doubt the feasibility of this association [12].
There is evidence that HSV infection is associated with bacterial vaginosis (OR=1.73), which disrupts the epithelial barrier function and triggers inflammatory response, which is facilitated by the production of proinflammatory cytokines and the recruitment of immune cells to the site of inflammation [28]. Some authors, on the contrary, point to BV as a protective factor against cytological abnormalities in HPV-infected patients (OR=0.17; p<0.05) [9]. Similarly, the presence of Candida was not found to be associated with an increased risk of squamous cell abnormalities (OR=0.73 for LSIL and OR=0.65 for HSIL) [29]. This suggests a subadditive effect, where the combined activity of two infections is less than their individual effects.
In some publications it is claimed that vaginal dysbiosis, characterized by a decrease in the number of Lactobacillus species, increases susceptibility to HPV infection and promotes HPV persistence, but a causal relationship with the progression of cervical lesions has not been proven [30]. According to Cui M. et al. (2025), the progression of cervical lesions depends on local immune responses, with HPV infection potentially accelerating disease progression by suppressing local immunity [31]. When considering these data given HPV prevalence rate, it is notable that HPV was detected at a much higher rate in patients with CE (71.7%), whose average age was 35 years, compared to population-based studies among women of a similar age (from 15 to 20%, depending on the region) [32]. Ağar M. et al. (2022) also indicate a high rate of HPV detection in patients with CE (86.7%, which is 2 times higher than in women without CE) [33]. Since CE is the result of long-term persistence of viral and opportunistic microorganisms in both the endometrium and vagina [34, 35], this conforms to the statement of greater susceptibility to HPV infection and a higher frequency rate of ASCUS detection – in 44.1% of patients with CE, which was 10 times higher compared to women over 30 years in general population (without information on the presence of CE) in three studies – 3.6% [3], 4.4% [36], and 5.0% [37].
According to the world data, the effectiveness of treating vaginal dysbiosis with antibacterial/antimycotic agents ranges from 55 to 90% and is determined by three factors: the characteristics of the microbiome itself (mono- or combined infections, resistant forms), integrity disruption of tissue barriers, and changes in the host's immune defense [38]. In patients with CE and bacterial/fungal infections, the effectiveness of treatment – normalization of the vaginal microbiota – was 79.9% versus 54.7% (p<0.001) in both study groups, and the likelihood of achieving normocenosis was 1.5 times higher with Superlymph. These results are similar to those obtained by Tapilskaya N.I. et al.: the use of local cytokine therapy in a combined form (Superlymph + antimicrobial therapy) resulted in the elimination of 84.0% of identified microorganisms (versus 59.1% in the placebo group, p<0.001) [39].
The antimicrobial peptides and cytokines in Superlymph enhance the effects of antibiotics/antimycotics and have antiviral, anti-inflammatory, regenerative, and immunomodulatory effects, which confirms its high effectiveness in eliminating viral and opportunistic microorganisms, as well as in ASCUS resolution [40].
Conclusion
Among female patients with chronic endometritis aged 18–45 the prevalence of ASCUS accounts for 44.1%, and the prevalence of HPV-positive status is 71.7%. Combined treatment with Superlymph facilitates ASCUS resolution in 85.6% of patients (a 2-fold reduction in risk compared to antibacterial/antimycotic therapy alone), with a negative HPV test rate in 85.4% of patients with chronic endometritis after treatment.
References
- Al-Eyd G., Mikes B.A. Atypical squamous cells of undetermined significance. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025. URL: https://www.ncbi.nlm.nih.gov/books/NBK557739/
- Мингалева Н.В., Дегтярева О.Г., Абрамашвили Ю.Г., Метелева Н.С. Тяжесть цервикальных поражений по данным цитологии и их взаимосвязь с выявлением вируса папилломы человека высокого онкогенного риска у женщин до 30 лет и старше. Кубанский научный медицинский вестник. 2016; 1(156): 88-95. [Mingaleva N.V., Degtyareva O.G., Abramashvili Yu.G., Meteleva N.S. The severity of cervical cytology of lesions according to their relationship to the detection of human papillomavirus risk High risk in women under 30 years of age or older. Kuban Scientific Medical Bulletin. 2016; 1(156): 88-94 (in Russian)].
- Будыкина Т.С., Краснопольский В.И., Зароченцева Н.В., Джиджихия Л.К., Меньшикова Н.С., Трищенкова О.В., Минина М.Н., Пельше Э.В. Опыт и результаты ВПЧ-тестирования женщин Московской области. Вопросы практической кольпоскопии. Генитальные инфекции. 2022; (1): 28-31. [Budykina T.S., Krasnopolsky V.I., Zarochentseva N.V., Dzhidzhikhiya L.K., Menshikova N.S., Trishchenkova O.V., Minina M.N., Pelshe E.V. Experience and results of HPV-testing of women in the Moscow region. Colposcopy Issues & Genital Infections. 2022; (1): 28-31 (in Russian)]. https://dx.doi.org/10.46393/27826392_2022_1_28
- Бебнева Т.Н., Дикке Г.Б. Папилломавирусная инфекция и заболевания шейки матки у беременных женщин. Факторы риска социального статуса, репродуктивного и контрацептивного поведения. Гинекология. 2020; 22(6): 74-9. [Bebneva T.N., Dikke G.B. Papillomaviral infection and cervical diseases in pregnant women. Risk factors of social status, reproductive and contraceptive behavior. Gynecology. 2020; 22(6): 74-9 (in Russian)]. https://dx.doi.org/1026442/20795696.2020.6.200460
- Alrajjal A., Pansare V., Choudhury M.S.R., Khan M.Y.A., Shidham V.B. Squamous intraepithelial lesions (SIL: LSIL, HSIL, ASCUS, ASC-H, LSIL-H) of uterine cervix and Bethesda system. Cytojournal. 2021; 18: 16. https://dx.doi.org/0.25259/Cytojournal_24_2021
- Kamal M.M. The Pap smear in inflammation and repair. Cytojournal. 2022; 19: 29. https://dx.doi.org/10.25259/CMAS_03_08_2021
- Wu S., Ding X., Kong Y., Acharya S., Wu H., Huang C. et al. The feature of cervical microbiota associated with the progression of cervical cancer among reproductive females. Gynecol. Oncol. 2021 Nov; 163(2): 348-57. https://dx.doi.org/10.1016/j.ygyno.2021.08.016
- Liu Y., Wang S., Liu J., Su M., Diao X., Liang X. et al. Characteristics of vaginal microbiota in various cervical intraepithelial neoplasia: a cross-sectional study. J. Transl. Med. 2023; 21(1): 816. https://dx.doi.org/10.1186/s12967-023-04676-5
- Long T., Zhang C., He G., Hu Y., Lin Z., Long L. Bacterial vaginosis decreases the risk of cervical cytological abnormalities. Cancer Prev. Res (Phila). 2023; 16(2): 109-17. https://dx.doi.org/10.1158/1940-6207.CAPR-22-0288
- Shen J., Sun H., Chu J., Gong X., Liu X. Cervicovaginal microbiota: a promising direction for prevention and treatment in cervical cancer. Infect. Agent. Cancer. 2024; 19(1): 13. https://dx.doi.org/10.1186/s13027-024-00573-8
- Bahena-Roman M., Sanchez-Aleman M.A., Contreras-Ochoa C.O., Lagunas-Martinez A., Olamendi-Portugal M., Lopez-Estrada G. et al. Prevalence of active infection by herpes simplex virus type 2 in patients with high-risk human papillomavirus infection: a cross-sectional study. J. Med. Virol. 2020; 92:1246-52. https://dx.doi.org/10.1002/jmv.25668
- Sausen D.G., Shechter O., Gallo E.S., Dahari H., Borenstein R. Herpes simplex virus, human papillomavirus, and cervical cancer: overview, relationship, and treatment implications. Cancers (Basel). 2023; 15(14): 3692. https://dx.doi.org/10.3390/cancers15143692
- Schellekens H.C.J., Schmidt L.M.S., Morré S.A., van Esch E.M.G., de Vos van Steenwijk P.J. Vaginal microbiota and local immunity in HPV-induced high-grade cervical dysplasia: a narrative review. Int. J. Mol. Sci. 2025; 26(9): 3954. https://dx.doi.org/10.3390/ijms26093954
- Gutiérrez Salmeán G., Delgadillo González M., Rueda Escalona A.A., Leyva Islas J.A., Castro-Eguiluz D. Effects of prebiotics, probiotics, and synbiotics on the prevention and treatment of cervical cancer: Mexican consensus and recommendations. Front. Oncol. 2024; 14: 1383258. https://dx.doi.org/10.3389/fonc.2024.1383258
- Supriya Y., Sivamalar S., Nallusamy D., Sureka V., Arunagirinathan N., Saravanan S. et al. Application of probiotics in cervical cancer infections to enhance the immune response. Microbial. Pathogenesis. 2024; 1936: 10676. https://dx.doi.org/10.1016/j.micpath.2024.106764
- Huang R., Wu F., Zhou Q., Wei W., Yue J., Xiao B. et al. Lactobacillus and intestinal diseases: mechanisms of action and clinical applications. Microbiol. Res. 2022; 260: 127019. https://dx.doi.org/10.1016/j.micres.2022.127019
- Chen T., Xia C., Hu H., Wang H., Tan B., Tian P. et al. Dysbiosis of the rat vagina is efficiently rescued by vaginal microbiota transplantation or probiotic combination. Int. J. Antimicrob. Agents. 2021; 57: 106277. https://dx.doi.org/10.1016/j.ijantimicag.2021.106277
- Байрамова Г.Р., Андреев А.О., Кечерукова И.Б. Современные возможности комплексной терапии инфекционно-воспалительных заболеваний шейки матки и влагалища в практике акушера-гинеколога. Акушерство и гинекология. 2024; 8: 127-32. [Bayramova G.R., Andreev A.O., Kecherukova I.B. Modern possibilities for complex therapy of infectious and inflammatory diseases of the cervix and vagina in the practice of an obstetrician-gynecologist. Obstetrics and Gynecology. 2024; (8): 127-32 (in Russian)]. https://dx.doi.org/10.18565/aig.2024.190
- Lozano F.M., Bernabeu A., Lledo B., Morales R., Diaz M., Aranda F.I. et al. Characterization of the vaginal and endometrial microbiome in patients with chronic endometritis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021; 263: 25-32. https://dx.doi.org/10.1016/j.ejogrb.2021.05.045
- Суханов А.А., Дикке Г.Б., Кукарская И.И. Проблемы диагностики и лечения хронического эндометрита у женщин репродуктивного возраста. Женское здоровье и репродукция. 2024; 1(62): 20-40 [Sukhanov A.A., Dikke G.B., Kukarskaya I.I. Problems of diagnosis and treatment of chronic endometritis in women of reproductive age. Women's Health and Reproduction. 2024; 1(62): 20-40 (in Russian)]. https://dx.doi.org/10.31550/2712-8598-2024-1-2-ZhZiR
- Дикке Г.Б., Суханов А.А., Кукарская И.И., Остроменский В.В. Цитокиновый профиль пациенток с хроническим эндометритом и нарушением репродуктивной функции. Вопросы гинекологии, акушерства и перинатологии. 2021; 20(6): 82-91. [Dikke G.B., Sukhanov A.A., Kukarskaya I.I., Ostromensky V.V. Cytokine profile of patients with chronic endometritis and reproductive dysfunction. Gynecology, Obstetrics and Perinatology. 2021; 20(6): 82-91 (in Russian)]. https://dx.doi.org/10.20953/1726-1678-2021-6-82-91
- Дикке Г.Б., Суханов А.А., Остроменский В.В., Кукарекая И.И. Течение и исходы беременности у пациенток с хроническим эндометритом и нарушением репродуктивной функции, получавших комплексное лечение с использованием препарата «Суперлимф» (рандомизированное контролируемое испытание в параллельных группах «ТЮЛЬПАН»). Акушерство и гинекология. 2023; 4: 132-44. [Dikke G.B., Sukhanov A.A., Ostromensky V.V., Kukarskaya I.I. Course and outcomes of pregnancy in patients with chronic endometritis and impaired reproductive function after receiving complex treatment with drug Superlymph: randomized control trial in parallel groups “TULIP”. Obstetrics and Gynecology. 2023; (4): 132-44 (in Russian)]. https://dx.doi.org/10.18565/aig.2023.74
- Connor J.P., Elam G., Goldberg J.M. Empiric vaginal metronidazole in the management of the ASCUS Papanicolaou smear: a randomized controlled trial. Obstet. Gynecol. 2002; 99(2): 183-7. https://dx.doi.org/10.1016/s0029-7844(01)01725-2
- Tuerxun G., Abudurexiti G., Abulizi G. Prevalence, persistence, clearance and risk factors for HPV infection in rural Uyghur women in China. BMC Womens Health. 2023; 23(1): 433. https://dx.doi.org/10.1186/s12905-023-02558-y
- Sabater Cabrera E., Trennery C., Jones A.M., Griffiths N., Foley E., Hamill M.M. et al. Real-life experiences of herpes simplex virus type 2 genital herpes in the United States: a structured literature review and qualitative concept elicitation study. Infect. Dis. Ther. 2025; 14(6): 1239-64. https://dx.doi.org/10.1007/s40121-025-01118-1
- Zhu J., Miner M.D. Local power: the role of tissue-resident immunity in human genital herpes simplex virus reactivation. Viruses. 2024; 16(7): 1019. https://dx.doi.org/10.3390/v16071019
- Доброхотова Ю.Э., Ганковская Л.В., Боровкова Е.И., Нугуманова О.Р. Экзогенная цитокинотерапия в лечении пациенток с хроническим эндометритом. Акушерство и гинекология. 2021; 2: 119-26. [Dobrokhotova Yu.E., Gankovskaya L.V., Borovkova E.I., Nugumanova O.R. Exogenous cytokine therapy in the treatment of patients with chronic endometritis. Obstetrics and Gynecology. 2021; (2): 119-26 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.2.119-126
- Segui-Perez C., van Smoorenburg M.Y., Maranus A.E., Geijtenbeek T.B.H., Strijbis K. Impact of bacterial vaginosis on sexually transmitted viral infections: a bacterial point of view. FEMS Microbiol. Rev. 2025; 49: fuaf039. https://dx.doi.org/10.1093/femsre/fuaf039
- Zhang Y., Chen L., Li H., Zhuang Y., Xie Q., Li W. et al. Unveiling the hidden link: fungi and HPV in cervical lesions. Front. Microbiol. 2024; 15: 1400947. https://dx.doi.org/10.3389/fmicb.2024.1400947
- Ye J., Qi X. Vaginal microecology and its role in human papillomavirus infection and human papillomavirus associated cervical lesions. APMIS. 2024; 132(12): 928-47. https://dx.doi.org/10.1111/apm.13356
- Smith J.S., Melendy A., Rana R.K., Pimenta J.M. Age-specific prevalence of infection with human papillomavirus in females: a global review. J. Adolesc. Health. 2008; 43(4 Suppl): S5-25, S25.e1–41. https://dx.doi.org/10.1016/j.jadohealth.2008.07.009
- Cui M., Wu Y., Liu Z., Liu Y., Fan L. Advances in the interrelated nature of vaginal microecology, HPV infection, and cervical lesions. Front. Cell. Infect. Microbiol. 2025; 15: 1608195. https://dx.doi.org/10.3389/fcimb.2025.1608195
- Ağar M., Ayar Madenli A., Gürbüz T. Human papillomavirus prevalence in unexplained infertile women with chronic endometritis. J. Health Sci. Med. 2022; 5(4): 1124-27. https://dx.doi.org/10.32322/jhsm.1111517
- Yan X., Jiao J., Wang X. The pathogenesis, diagnosis, and treatment of chronic endometritis: a comprehensive review. Front. Endocrinol. (Lausanne). 2025; 16: 1603570. https://dx.doi.org/10.3389/fendo.2025.160357
- Тапильская Н.И., Будиловская О.В., Крысанова А.А., Толибова Г.Х., Копылова А.А., Цыпурдеева Н.Д., Гзгзян А.М., Савичева А.М., Коган И.Ю. Микробиота эндометрия женщин с хроническим эндометритом и идиопатическим бесплодием. Акушерство и гинекология. 2020; 4: 72-81. [Tapilskaya N.I., Budilovskaya O.V., Krysanova A.A., Tolibova G.Kh., Kopylova A.A., Tsypurdeeva N.D., Gzgzyan A.M., Savicheva A.M., Kogan I.Yu. Еndometrial microbiota of women with chronic endometritis and idiopathic infertility. Obstetrics and Gynecology. 2020; (4): 72-81 (in Russian)]. https://dx.doi.org/10.18565/aig.2020.4.72-81
- d'Enfert C., Kaune A.K., Alaban L.R., Chakraborty S., Cole N., Delavy M. et al. The impact of the fungus-host-microbiota interplay upon Candida albicans infections: current knowledge and new perspectives. FEMS Microbiol. Rev. 2021; 45(3): fuaa060. https://dx.doi.org/10.1093/femsre/fuaa060
- Li B., Dong L., Wang C., Li J., Zhao X., Dong M. et al. Analysis of the related factors of atypical squamous cells of undetermined significance (ASC-US) in cervical cytology of post-menopausal women. Front. Cell. Infect. Microbiol. 2023; 13: 1123260. https://dx.doi.org/10.3389/fcimb.2023.1123260
- Mishra J., Kalantri S., Raphael V., Dey B., Khonglah Y., Das A. Prevalence of human papillomavirus infection in abnormal pap smears. Cytojournal. 2023; 20: 21. https://dx.doi.org/10.25259/Cytojournal_8_2021
- Тапильская Н.И., Толибова Г.Х., Савичева А.М., Копылова А.А., Глушаков Р.И., Будиловская О.В., Крысанова А.А., Горский А.Г., Гзгзян А.М., Коган И.Ю. Эффективность локальной цитокинотерапии хронического эндометрита пациенток с бесплодием. Акушерство и гинекология. 2022; 2: 91-100. [Tapilskaya N.I., Tolibova G.Kh., Savicheva A.M., Kopylova A.A., Glushakov R.I., Budilovskaya O.V., Krysanova A.A., Gorskii A.G., Gzgzyan A.M., Kogan I.Yu. The effectiveness of local cytokine therapy for chronic endometritis in patients with infertility. Obstetrics and Gynecology. 2022; (2): 91-100 (in Russian)]. https://dx.doi.org/10.18565/aig.2022.2.91-100
- Дикке Г.Б., Бебнева Т.Н. Современные подходы к прогнозированию и лечению рецидивирующего бактериального вагиноза и сочетанных дисбиозов влагалища и мочевыводящих путей. Акушерство и гинекология. 2025; 9: 56-68. [Dikke G.B., Bebneva T.N. Modern approaches to prediction and treatment of recurrent bacterial vaginosis and combined dysbiosis of the vagina and urinary tract. Obstetrics and Gynecology. 2025; (9): 56-68 (in Russian)]. https://dx.doi.org/10.18565/aig.2025.250
Received 14.11.2025
Accepted 27.11.2025
About the Authors
Galina B. Dikke, Dr. Med. Sci., Professor, Department of Obstetrics and Gynecology with a Course of Reproductive Medicine, F.I. Inozemtsev Academy of Medical Education, 22 Liter M, Moskovskiy Ave., St. Petersburg, 190013, Russia, galadikke@yandex.ru, https://orcid.org/0000-0001-9524-8962Anton A. Sukhanov, PhD, Head of the Department of Family Planning and Reproduction, Tyumen Perinatal Center, 1 Daudelnaya str., Tyumen, 625002, Russia; Associate Professor, Department of Obstetrics and Gynecology, Tyumen State Medical University, Ministry of Health of Russia, 10 Permyakov str., Tyumen, 625013, Russia, saa2505anton@yandex.ru, https://orcid.org/0000-0001-9092-9136
Irina I. Kukarskaya, Dr. Med. Sci., Professor at the Department of Obstetrics, Gynecology and Reanimatology with a Course of Clinical Laboratory Diagnostics, Tyumen State Medical University, Ministry of Health of Russia, 10 Permyakov str., Tyumen, 625013, Russia; Chief Physician, Tyumen Region Perinatal Center, 1 Daudelnaya str., Tyumen, 625002, Russia; Chief Specialist in Obstetrics and Gynecology, Department of Health of the Tyumen Region, https://orcid.org/0000-0002-8275-3553
Natalya V. Shilova, PhD, Associate Professor, Department of Industrial Pharmacy, National Research Nuclear University MEPhI (Moscow Engineering Physics Institute), 31 Kashirskoe shosse, Moscow, 115409, Russia, nvshilova@gmail.com, https://orcid.org/0000-0001-6734-0147
Corresponding author: Galina B. Dikke, galadikke@yandex.ru



