Association between extradiol and progesterone levels and chronic endometritis in women with reproductive disorders
Ievleva K.D., Danusevich I.N., Sholokhov L.F., Suturina L.V., Kolesnikova L.I.
Objective: To identify between and progesterone levels and chronic endometritis (CE) in women with reproductive disorders.
Materials and methods: A retrospective analysis of data that were obtained in the previously conducted study involving the patients with miscarriage/infertility, who had CE (n=49) or had no CE (n=42), and 24 healthy women, was performed. All patients underwent general and gynecological examination, pelvic ultrasound, endometrial pipelle biopsy for pathomorphological assessment of the endometrial samples. The diagnostic tests for serum levels of estradiol and progesterone were performed. The levels of pro-inflammatory and anti-inflammatory cytokines in endometrial samples were detected.
Results: Serum estradiol concentrations and estradiol/progesterone (E/P) ratio were higher, and progesterone levels were lower, as well the levels of IL-1, IL-6, IL-4, IL-8, TNF-α, and IFN-γ in endometrial cells were higher in patients with CE and miscarriage/infertility compared with healthy women. In patients with miscarriage/infertility, and without CE, serum progesterone levels were lower, and the levels of IL-6, IL-8, IL-10 and TNF-α in endometrial cells were higher compared with healthy women. It was found that with increased E/P ratio, the risk of chronic endometritis was by 1,5 times higher in women with miscarriage/infertility compared with healthy women (OR 1.525; 95% CI 1.247–2.026).
Conclusion: Without regard to the presence or absence of CE, there was an imbalance in steroid hormone concentrations in the blood and cytokines in the endometrium in the patients with miscarriage/infertility, and it was severe in the presence of CE. The patients with miscarriage/infertility, and with a high E/P ratio, had a higher risk of developing CE.
Authors' contributions: Ievleva K.D. – the concept of the study, statistical data processing, manuscript writing; Danusevich I.N. – the concept of the study, manuscript; Sholokhov L.F., Suturina L.V., Kolesnikova L.I. – manuscript editing.
Conflicts of interest: The authors confirm that they have no conflict of interest to declare.
Funding: The study was conducted within the framework of the State budgetary task on the topic “Pathophysiological mechanisms and genetic and metabolic predictors for maintaining reproductive health and longevity in different age, gender, and ethnic groups” (FGMZ-2021-0002).
Ethical Approval: The study was approved by the local Ethics Committee of the Scientific Center of Family Health Problems and Human Reproduction.
Patient Consent for Publication: The patients have signed informed consent publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Ievleva K.D., Danusevich I.N., Sholokhov L.F., Suturina L.V., Kolesnikova L.I. Association
between extradiol and progesterone levels and chronic endometritis in women with reproductive disorders.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (12): 88-96 (in Russian)
https://dx.doi.org/10.18565/aig.2025.225
Keywords
Along with genetic, endocrine or hyperplastic disorders, pelvic inflammatory disease belongs to the group of diseases affecting female reproductive system, which includes chronic endometritis (CE) [1–3]. The meta-analysis of the data of 1038 women conducted by Ticconi C. et al. (2025) found that the prevalence of CE was higher in patients with infertility (19,46 %) compared with women without reproductive disorders (7.7%, p<0.05). Also, the prevalence of CE was higher in women with recurrent miscarriage (37,6%) compared with women without reproductive disorders (16.4%, p<0.001) [4]. The average prevalence of CE in women with infertility was found to be 2.8–56.8%, and 9.3–67.6% in women with miscarriage [5]. However, despite the fact that association between CE and occurrence of miscarriage and infertility was detected, there are currently no recommendations regarding the necessity to include the diagnostic tests for CE in the list of primary tests for the couples facing infertility. This may be due to diagnostic difficulties related to the ambiguous and non-specific clinical picture, on the one hand, and exclusively invasive diagnostic testing for the presence of CE, on the other hand [4, 6]. Furthermore, the disease is asymptomatic in 25% of patients. For this reason, the initial diagnosis of CE is often made, when the patients developed complications including miscarriage and/or infertility [6].
Chronic endometritis is a persistent inflammation of the endometrium, often due to intrauterine infection caused by Escherichia coli, Enterococcus faecalis, Streptococcus, Staphylococcus, Mycoplasma, and Ureaplasma [7]. However, development of complications (reproductive disorders) is primarily associated with impaired endometrial receptivity due to uncoupling of pro- and anti-inflammatory properties of cytokine production. In addition to epithelial and stromal cells, endometrial tissue is populated by immune cells (natural killer cells, macrophages, and T cells) [8]. Changes in composition and quantity of these cell populations in the endometrium occur during the menstrual cycle. Reproductive impairment in the presence of CE is associated with excessive production of pro-inflammatory mediators produced by both immune cells, stromal and epithelial cells – interleukins (IL-1β, IL-8), tumor necrosis factor α (TNF-α) and other [9]. These cytokines are involved in the cyclical changes in the endometrium, as well as participate in embryo implantation. Estradiol and progesterone are also involved in regulation of cytokine production in the endometrial cells. Progesterone suppresses IL-8 production, thereby preventing leukocyte infiltration [10]. On the other hand, in vitro studies have shown that estradiol can participate in stimulation of pro-inflammatory cytokine production. In addition, estradiol and IL-1β exert a synergistic effect on endometrial cells [11]. At the same time, there is an imbalance in the expression of estrogens and progesterone receptors in the endometrium in women with CE and in women with miscarriage and/or infertility [12]. Nevertheless, there is limited research on the levels of steroid hormones (estradiol and progesterone) in women with CE against the background of miscarriage and/or infertility [13].
The aim of the study was to identify association of estradiol and progesterone levels with chronic endometritis (CE) in women with reproductive disorders.
Material and methods
The study design
A retrospective analysis of data of the previously conducted cross-sectional study was performed [14, 15]. A total of 327 women of reproductive age participated in the primary questionnaire survey that covered complaints on reproductive disorders. Inclusion criteria in the study were the following: absence of pregnancy after a year or more of regular and unprotected sexual intercourse, or miscarriage within the past year, or failed attempts of assisted reproductive technology programs.
The second step was selection of women with suspected chronic endometritis. The inclusion criteria in the study were complaints on bleeding after periods and/ or intermenstrual bleeding; drawing pain in the lower abdomen; dyspareunia; serous or seropurulent vaginal discharge; infertility (primary or secondary); miscarriage or pregnancy loss in assisted reproductive technology programs; history of complications in the postpartum period, history of acute endometritis; history of intrauterine manipulations (therapeutic and diagnostic curettage, medical abortions); ultrasound signs of CE.
The patients, who had endocrine factors for infertility or miscarriage; congenital thrombophilia; antiphospholipid syndrome; acute pelvic inflammatory disease; sexually transmitted infections and those who used immunomodulators and combined oral contraceptives less than 6 months ago, were excluded from the study.
Healthy women were also the participants of the study. Inclusion criteria for healthy women were the following: regular periods, absence of neuroendocrine and severe somatic disorders; pregnancy history that ended in childbirth within the past year, as well as intrauterine interventions during the past year.
The study was approved by the local Ethics Committee of the Scientific Center of Family Health Problems and Human Reproduction. Each participant signed the informed consent form.
The clinical and instrumental methods used in the study
Medical documentation of all patients was analyzed. The patients underwent general gynecological examination, pelvic ultrasound, endometrial pipelle biopsy and blood sampling. The diagnosis of miscarriage or infertility was based on the data of medical documentation and anamnesis.
Pelvic ultrasound was performed to identify the signs of CE (thin endometrium, uterine synechiae) using the Aloka-5500 device with a 7MHz vaginal probe in two-dimensional visualization mode.
Endometrial aspiration biopsy (pipelle biopsy) was performed on days 4–9 of the menstrual cycle (mid-proliferative phase) using a disposable intrauterine probe (Taizhou Kechuang Medical Apparatus Co., Ltd., China).
Hormone tests
Blood samples for hormone level testing were collected from the cubital vein from 8 to 9 a.m., on an empty stomach, in phase 1 of the menstrual cycle (days 3–9) for estradiol, and in phase 2 (days 20–24) for progesterone. Enzyme-linked immunosorbent assay (ELISA) was performed on the Cobos analyzer (USA) to detect estradiol levels using Hema test system (Russia) and progesterone levels using AlcorBio test system.
Immunological and pathomorphological examination of endometrial biopsy samples
To detect the levels of cytokine production (IL-1β, interferon γ (IFN-γ), TNF-α, IL-4, IL-6, IL-8, IL-10) in endometrial samples, ELISA was performed on the Multiscan EX analyzer (Germany) using test systems that were developed at the State Research Institute of Highly Pure Biopreparations (St. Petersburg) and manufactured by the company “Protein Contour” (St. Petersburg). Immunoglobulin levels were measured in g/L.
Pathomorphological examination was performed using the standardized method. The obtained material was formalin-fixed, dehydrated with alcohol reagent and paraffin-embeded. Tissue sections of 6–9 μm thick were prepared and stained with hematoxylin and eosin. The following criteria for morphological verification of chronic endometritis were used: the presence of inflammatory infiltrates composed of plasma and lymphoid elements; sclerotic changes in the vessels; and focal stromal fibrosis.
Statistical analysis
Statistical analysis of the obtained data was performed using SPSS software program. Continuous variable distribution (age, hormone levels, hormone ratio and cytokine levels) was tested using the Shapiro–Wilk test. Given that the analyzed parameters had non-normal distribution, the values were presented as median and the upper and lower quartiles – Me (Q1; Q3). The values of continuous variables between the groups were compared using the Kruskal–Wallis test (to compare 3 groups) with Bonferroni adjusted p-value of 0.017. The Mann–Whitney U test was used for pairwise comparison. Qualitative parameters were compared using the Chi-square test and Fisher's exact test. Correlation analysis of hormonal and immune parameters was performed using Spearman’s rank correlation coefficient for nonparametric distribution. The odds ratio (OR) and 95% confidence interval (CI) were calculated to assess the impact of changes in serum hormone levels and/or their ratio on the presence of CE. The OR was calculated using univariate logistic regression. Missing data were not processed due to their absence. Outliers were identified using the ROUT method (Robust Regression and Outlier Removal). Statistical significance level of 0.05 was set for all types of data analysis.
Results
We used the data of the previously conducted study [14, 15] to perform the retrospective analysis, which according to inclusion and exclusion criteria involved 50 women with CE and 50 women without CE against the background of miscarriage/infertility, and 31 healthy women (the control group). In the process of analysis, the data of 1 patient with CE, 8 patients without CE, and 5 healthy women were excluded from analysis due to the absence of the results of histomorphological evaluation of the endometrium. In addition, two healthy women were excluded from analysis due to dystrophic changes in the endometrium or the signs of endometrial regression. The groups of patients and healthy women were age-matched. Furthermore, no significant differences were found in the incidence of infertility and miscarriage between the groups with or without CE (Table 1).
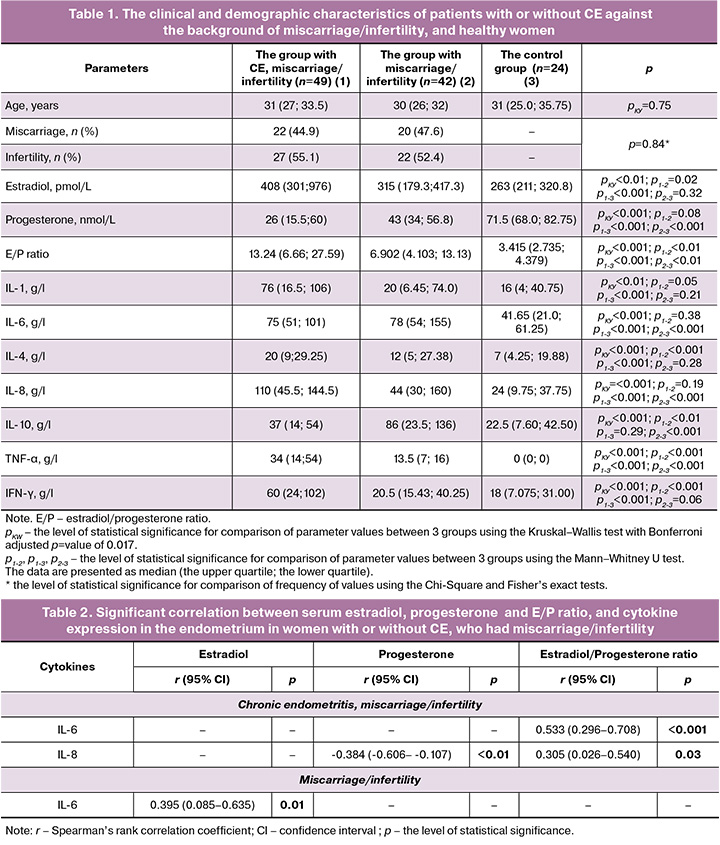
Serum estradiol analysis showed that hormone levels were higher in women with CE, miscarriage and infertility, compared with women without CE, miscarriage and infertility, and healthy women. Serum progesterone test results showed statistically significant differences in hormone concentrations between healthy women and patients with miscarriage/infertility, regardless of the presence of CE. At the same time, no differences in progesterone levels were found between the groups of women with or without CE, who had miscarriage/infertility.
Assessment of cytokine levels in the endometrium in the examined patients and healthy women showed significantly higher levels of IL-1, IL-4, Il-6, and IL-8, as well as higher levels of TNF-α and INF-γ in patients with CE, who had miscarriage or infertility compared with healthy women. In patients with miscarriage or infertility, the levels of IL-6, IL-8, and IL-10, as well as TNF-α, were higher compared with healthy women. Furthermore, against the background of miscarriage or infertility we found significantly higher levels of IL-4, IL-8, TNF-α, and INF-γ, as well as lower levels of IL-10 in women with CE compared with women without CE.
The next step was correlation analysis of serum estradiol and progesterone levels, as well as estradiol-to-progesterone ratio, and cytokine levels in the endometrium in women with or without CE, who had miscarriage or infertility, and in healthy women (Table 2).
The results of correlation analysis showed a negative correlation between IL-8 levels in the endometrium and serum progesterone concentrations or serum estradiol-to-progesterone ratio in women with chronic endometritis, miscarriage or infertility. In addition, we found a positive correlation between IL-6 levels in the endometrium and serum E/P ratio. The correlation between serum estradiol concentration and IL-6 level in the endometrium was found in patients with miscarriage or infertility and without CE. In the group of healthy women, no statistically significant correlation was found between serum hormone concentrations and cytokine levels in the endometrium.
Comparison between serum hormone levels in women with CH against the background of miscarriage or infertility and in healthy women showed statistically significant differences only in estradiol levels or estradiol-to-progesterone ratio. Due to this, logistic regression was used and the odds ratio was calculated only for these parameters. The presence or absence of CE was considered as a dependent variable, and serum estradiol level or estradiol-to-progesterone ratio was considered as an independent variable (Table 3).
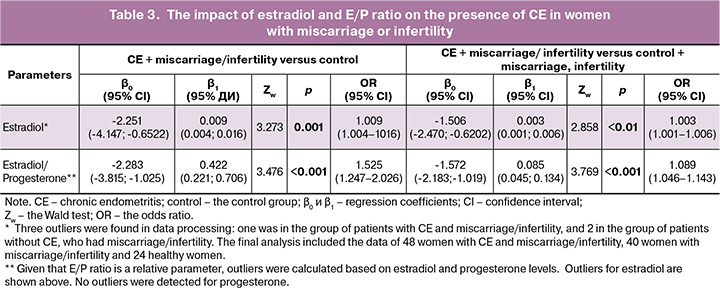
Thus, we found that high estradiol levels or estradiol-to-progesterone ratios significantly contribute to the risk of CE in women with recurrent miscarriage and infertility. Moreover, high E/P ratio (OR 1.525; 95% CI 1.247–2.026) was associated with a higher risk of CE in women with miscarriage or infertility compared with healthy women.
Discussion
Our retrospective analysis determined significantly high serum estradiol levels and low progesterone levels in women with miscarriage or infertility compared with healthy women. At the same time in patients with CE, miscarriage and infertility, estradiol concentrations and estradiol-to-progesterone ratios were significantly higher than in patients without CE. In addition, significantly high levels of pro-inflammatory cytokine levels in the endometrium were found in women with CE, miscarriage or infertility, compared with healthy women. It should be noted that, less severe, but significant increase in the levels of pro-inflammatory cytokines was found in women with miscarriage or infertility without CE. Moreover, in this group of patients IL-10 levels were significantly low. Correlation analysis showed a negative correlation between serum progesterone level and the expression of IL-8 in the endometrium in women with CE against the background of miscarriage or infertility, as well as showed a positive correlation between estradiol-to-progesterone ratio and IL-6, IL-8 in this group of women. In women without CE, who had miscarriage and infertility, a positive correlation was found between serum estradiol level and expression of IL-6 in the endometrium. Logistic regression results showed that elevated levels of serum estradiol or high estradiol-to-progesterone ratio contributed to the presence of chronic endometritis. At the same time, the leading factor was increased estradiol-to-progesterone ratio in women with miscarriage or infertility compared with healthy women.
Estradiol and progesterone play an important part in regulation of endometrial function in the menstrual cycle. Increased serum estradiol concentrations are observed during the growth of the dominant follicles. In the proliferative phase, estradiol is an important factor in endometrial proliferation and provides normal endometrial thickness, that positively influences embryo implantation. Progesterone stimulates decidualization of endometrial cells and is responsible for regulation of the implantation window. Progesterone deficiency and a shortened luteal phase lead to impaired endometrial maturation, which is associated with recurrent miscarriage. Moreover, a normal estradiol-to-progesterone ratio is necessary for embryo implantation. Low levels of both estradiol and progesterone are associated with occurrence of miscarriage in the first trimester of pregnancy [16]. At the same time, there are few studies that reported elevated basal estradiol levels in women with recurrent miscarriage [17]. In addition, the study by Aganezova N.V. et al. (2022) found no significant differences in serum concentrations of estradiol and progesterone, as well as estradiol-to-progesterone ratio in women with thin endometrium experiencing miscarriage compared with women with normal endometrial thickness and miscarriage, or with healthy women [13]. The meta-analysis of 41 studies related to female patients with infertility of unknown origin (n=4023) found no significant changes in serum progesterone levels compared with those in healthy women [18]. Our study found statistically significant increase in serum estradiol level and reduced progesterone level in patients with recurrent miscarriage or infertility, who had CE, and significant reduction in progesterone level in patients with recurrent miscarriage or infertility, who had no CE compared with healthy women.
Chronic endometriris can be one of the causes of recurrent miscarriage or infertility [7]. Increased expression of estrogen and progesterone receptors in endometrial stromal and epithelial cells occurs in the presence of local inflammation of the endometrium, that along with increased expression of the antiapoptotic genes (BCL2 and BAX) may reflect the changes in the proliferative phenotype and decidualization of the endometrium [6]. In vivo studies have shown that low progesterone level contributes to leukocyte infiltration in uterine and cervical tissues both in pregnant and non-pregnant animals. IL-8 stimulates leukocyte (neutrophil) infiltration. This pro-inflammatory cytokine induces neutrophil chemotaxis and degranulation in tissues, the growth and differentiation of monocytes and macrophages, and promotes endothelial cell survival and proliferation, as well as angiogenesis. [16]. IL-8 also contributes to endometrial receptivity, ensuring normal interaction between embryonic and endometrial tissues during implantation [19]. At the same time, an in vitro study found that progesterone inhibits IL-8 production in the endometrial cells both in the absence and presence of lipopolysaccharides. Our study found not only significantly increased IL-8 expression in the endometrium, but also a negative correlation between this indicator and serum progesterone level in women with CE against the background of miscarriage or infertility. Moreover, we found increased IL-6 expression in women with CE, who had miscarriage or infertility. This is consistent with the results of the studies involving patients with CE [9, 20]. Correlation analysis also showed a positive relationship between estradiol-to-progesterone ratio and the expression of IL-6 and IL-8 in the endometrium. This indirectly indicates that steroid hormone imbalance may also contribute to the development of CE.
Increased expression of other pro-inflammatory cytokines (IL-1β, IL-6, IL-8, TNF-α, IFN-γ and other) was also found in endometrial samples from women with chronic endometritis [9, 20]. These cytokines are important in the normal endometrial function, including at the stage of embryo implantation [16]. We also found that in women with CE against the background of miscarriage and infertility, the levels of these pro-inflammatory cytokines in the endometrium were high. However, less severe, but statistically significant cytokine imbalance was found in women with miscarriage and infertility, who had no chronic endometritis. It has been previously shown that excessively high or low levels of IL-6 and IL-8 in the endometrium can lead to abnormal trophoblast invasion and spiral artery remodeling, that increases the risk of early miscarriage [21]. At the same elevated levels of IL-6 and IL-8 are associated with recurrent miscarriage [22]. Thus, cytokine shift in the endometrium to pro-inflammatory state is most common both in women with CE, and in women with miscarriage and without CE.
By now numerous studies have shown that CE significantly reduces the success rates of in vitro fertilization in women with infertility or miscarriage. However, accurate diagnosis of chronic endometritis remains difficult, in some cases due to conflicting results of various diagnostic tests (hysteroscopy and immunohistochemical examination of endometrial biopsy samples) [23, 24]. Therefore, it is important to have additional tolls for chronic endometritis risk assessment. In recent years, there is an active search for serum markers of this pathological condition. However, these studies were conducted without determining the presence or absence of reproductive disorders in patients [25, 26]. Our study found that elevated estradiol levels and estradiol-to-progesterone ratio significantly contribute to the presence of CE in women with miscarriage or infertility. At the same time, elevated estradiol-to-progesterone ratio was associated with a high risk of CE. However, further research is needed to confirm the feasibility of using this criterion to determine the risk of CE in a larger sample size, as well as in a group of patients with CE, and without miscarriage or infertility.
The advantage of this study is that it compared estradiol and progesterone levels in women with or without CE against the background of reproductive disorders. This made it possible to evaluate the differences in the hormonal status of patients with miscarriage or infertility depending on the presence or absence of CE. In addition, the levels of the main pro-inflammatory and anti-inflammatory cytokines in the endometrium were assessed, that helped to confirm the presence of inflammatory imbalance in the endometrium not only in patients with chronic endometritis against the background of miscarriage or infertility, but also in patients with miscarriage or infertility, and without chronic endometritis. Another advantage of this study is that it included women with non-specific chronic endometritis, which was not complicated by concomitant endocrine pathology. Another advantage of this study is that analysis involved women with non-specific chronic endometrities, which was not accompanied by concomitant endocrine pathology.
The limitations of our study include relatively small sample size, absence of comparison group of women with CE and without miscarriage or infertility, as well as the use of standardized pathomorphological examination without assessment of the expression of CD4+, CD8+ CD20+ and CD138+ cells in the endometrial stroma to verify the diagnosis of CE [27]. These limitations could influence the results of correlation analysis of serum estradiol and progesterone concentrations, and cytokine levels in the endometrium. Moreover, the use of standardized pathomorphological examination as a method of verification of CE could lead to false-negative or false-positive test results. Due to this fact, some patients with CE could have been included in the group without CE or vice versa. Furthermore, another limitation of this study is a lack of data on the expression of estradiol and progesterone receptors in the endometrium in the study participants, that could have made it possible to assess the relationship between serum estradiol and progesterone levels and the local state of the receptor system.
Conclusion
Thus, our study identified an imbalance both of endometrial cytokines and steroid hormones (estradiol, progesterone) in serum of women with miscarriage or infertility, regardless of the presence of CE. Furthermore, we found indirect relationship between serum progesterone level and estradiol-to-progesterone ratio, and the expression of pro-inflammatory cytokines in the endometrium. It was also found that elevated estradiol-to-progesterone ratio in women with miscarriage or infertility is a risk factor for the presence of chronic endometritis.
Further research involving a larger number of patients and healthy women, including patients with CE, who have no miscarriage or infertility, will make it possible to confirm feasibility of the clinical use of elevated serum estradiol-to-progesterone ratio as a risk marker for the presence of chronic endometritis. Moreover, simultaneous analysis of estradiol and progesterone levels in serum and in the endometrium, as well as the expression of estrogen and progesterone receptors and cytokines in the endometrium in women with or without CH against the background of miscarriage or infertility will help determine the relationship between the changes in steroid hormone levels and endometrial function in patients with concomitant pathology associated with reproductive disorders.
References
- Bala R., Singh V., Rajender S., Singh K. Environment, lifestyle, and female infertility. Reprod. Sci. 2021; 28(3): 617-38. https://dx.doi.org/10.1007/s43032-020-00279-3
- Пестрикова Т.Ю., Хамроева У.Ж., Колоусова А.Л. Хронический эндометрит как базовый фактор бесплодия у женщин (обзор литературы). Дальневосточный медицинский журнал. 2024; 3: 100-6. [Pestrikova T.Yu., Kamroeva U.Zh., Kolousova A.L. Chronic endometritis as a basic factor of female infertility (literature review). Far Eastern Medical Journal. 2024; 3: 100-6 (in Russian)]. https://dx.doi.org/10.35177/1994-5191-2024-3-17
- Данусевич И.Н. Цитокино-гормональные взаимодействия при хроническом эндометрите у женщин с репродуктивными нарушениями. Вопросы гинекологии, акушерства и перинатологии. 2015; 14(4): 42-8. [Danusevich I.N. Cytokine-hormone interactions in chronic endometritis in women with reproductive disorders. Problems of gynecology, obstetrics and perinatology. 2015; 14(4): 42-48 (in Russian)].
- Ticconi C., Inversetti A., Marraffa S., Campagnolo L., Arthur J., Zambella E. et al. Chronic endometritis and recurrent reproductive failure: a systematic review and meta-analysis. Front. Immunol. 2024; 15: 1427454. https://dx.doi.org/10.3389/fimmu.2024.1427454
- Kimura F., Takebayashi A., Ishida M., Nakamura A., Kitazawa J., Morimune A. et al. Review: chronic endometritis and its effect on reproduction. J. Obstet. Gynaecol. Res. 2019; 45(5): 951-60. https://dx.doi.org/10.1111/jog.13937
- Espinós J.J., Fabregues F., Fontes J., Garcıa-Velasco J.A., Llacer J., Requena A. et al. Impact of chronic endometritis in infertility: a SWOT analysis. Reprod. Biomed. Online. 2021; 42(5): 939-51. https://dx.doi.org/10.1016/j.rbmo.2021.02.003
- Cicinelli E., Matteo M., Tinelli R., Pinto V., Marinaccio M., Indraccolo U. et al. Chronic endometritis due to common bacteria is prevalent in women with recurrent miscarriage as confirmed by improved pregnancy outcome after antibiotic treatment. Reprod. Sci. 2014; 21(5): 640-7. https://dx.doi.org/10.1177/1933719113508817
- Kim C.J., Kim D.J., Kang J.H. Immune cells in the female reproductive tract. Immune Netw. 2015; 15(1): 16-26. https://dx.doi.org/10.4110/in.2015.15.1.16
- Ткаченко Л.В., Свиридова Н.И., Жаркин Н.А., Бурова Н.А., Белан Э.Б. Оценка цитокинового статуса у пациенток с хроническим эндометритом в сочетании с гиперпластическими процессами эндометрия в репродуктивном периоде. Инфекция и иммунитет. 2020; 10(4): 762-8. [Tkachenko L.V., Sviridova N.I., Zharkin N.A., Burova N.A., Belan E.B. Assessing cytokine status of patients with chronic endometritis combined with endometrial hyperplastic processes in reproductive period. Russian Journal of Infection and Immunity. 2020; 10(4): 762-8 (in Russian)]. https://dx.doi.org/10.15789/2220-7619-AOC-1357
- Kelly R.W., Illingworth P., Baldie G., Leask R., Brouwer S., Calder A.A. Progesterone control of interleukin-8 production in endometrium and chorio-decidual cells underlines the role of the neutrophil in menstruation and parturition. Hum. Reprod. 1994; 9(2): 253-8. https://dx.doi.org/10.1093/oxfordjournals.humrep.a138491
- Arlıer S, Kayışlı ÜA, Arıcı A. Tumor necrosis factor alfa and interleukin 1 alfa induced phosphorylation and degradation of inhibitory kappa B alpha are regulated by estradiol in endometrial cells. Turk. J. Obstet. Gynecol. 2018; 15(1): 50-9. https://dx.doi.org/10.4274/tjod.47700
- Ticconi C., Di Simone N., Campagnolo L., Fazleabas A. Clinical consequences of defective decidualization. Tissue Cell. 2021; 72: 101586. https://dx.doi.org/10.1016/j.tice.2021.101586
- Аганезова Н.В., Аганезов С.С., Гогичашвили К.Э. Характеристики рецептивности эндометрия у женщин с различной толщиной эндометрия. Акушерство, гинекология и репродукция. 2022; 16(2): 108-21. [Aganezova N.V., Aganezov S.S., Gogichashvili K.E. Characteristics of endometrial receptivity in women with different endometrial thickness. Obstetrics, Gynecology and Reproduction. 2022; 16(2): 108-21 (in Russian)]. https://dx.doi.org/10.17749/2313-7347/ob.gyn.rep.2022.303
- Данусевич И.Н. Частота встречаемости хронического эндометрита у женщин с различными вариантами репродуктивных нарушений. Бюллетень ВСНЦ СО РАМН. 2013; 4(92): 18-20. [Danusevich I.N. The frequency of occurrence of chronic endometritis in women with different variants of reproductive disorders. Bulletin of the All-Russian Scientific Center for Cardiovascular Surgery of the Russian Academy of Medical Sciences. 2013; 4(92): 18-20 (in Russian)].
- Данусевич И.Н. Факторы риска развития хронического эндометрита у женщин с репродуктивными нарушениями. Бюллетень ВСНЦ СО РАМН. 2013; 4(92): 111-4. [Danusevich I.N. Risk factors for development of chronic endometritis in women with reproductive disorders. Bulletin of the All-Russian Scientific Center for Cardiovascular Surgery of the Russian Academy of Medical Sciences. 2013; 4(92): 111-4 (in Russian)].
- Günther V., Allahqoli L., Deenadayal-Mettler A., Maass N., Mettler L., Gitas G. et al. Molecular determinants of uterine receptivity: comparison of successful implantation, recurrent miscarriage, and recurrent implantation failure. Int. J. Mol. Sci. 2023; 24(24): 17616. https://dx.doi.org/10.3390/ijms242417616
- Gürbüz B., Yalti S., Ozden S., Ficicioglu C. High basal estradiol level and FSH/LH ratio in unexplained recurrent pregnancy loss. Arch. Gynecol. Obstet. 2004; 270(1): 37-9. https://dx.doi.org/10.1007/s00404-003-0490-0
- Raperport C., Chronopoulou E., Homburg R., Khan K., Bhide P. Endogenous progesterone in unexplained infertility: a systematic review and meta-analysis. J. Assist. Reprod. Genet. 2023; 40(3): 509-24. https://dx.doi.org/10.1007/s10815-022-02689-5
- Caballero-Campo P., Dominguez F., Coloma J., Meseguer M., Remohi J., Pellicer A., Simon C. Hormonal and embryonic regulation of chemokines IL-8, MCP-1 and RANTES in the human endometrium during the window of implantation. Mol. Hum. Reprod. 2002; 8(4): 375-84. https://dx.doi.org/10.1093/molehr/8.4.375
- Полина М.Л., Витязева М.М., Ордиянц И.М., Лебедева М.Г., Шеленина Л.А., Захарова П.Н., Дуглас Н.И. Микросреда имплантации при хроническом эндометрите. Пермский медицинский журнал. 2023; 40(3): 10-9. [Polina M.L., Vityazeva I.I., Ordiyants I.M., Lebedeva M.G., Shelenina L.A., Zakharova P.N., Douglas N.I. Implantation microenvironment in chronic endometritis. Perm Medical Journal. 2023; 40(3): 10-9 (in Russian)]. https://dx.doi.org/10.17816/pmj40310-19
- Pitman H., Innes B.A., Robson S.C., Bulmer J.N., Lash G.E. Altered expression of interleukin-6, interleukin-8 and their receptors in decidua of women with sporadic miscarriage. Hum. Reprod. 2013; 28(8): 2075-86. https://dx.doi.org/10.1093/humrep/det233
- Zhao L., Han L., Hei G., Wei R., Zhang Z., Zhu X. et al. Diminished miR-374c-5p negatively regulates IL (interleukin)-6 in unexplained recurrent spontaneous abortion. J. Mol. Med. 2022; 100(7): 1043-56. https://dx.doi.org/10.1007/s00109-022-02178-3
- Локшин В.Н., Куценко И.И., Боровиков И.О., Булгакова В.П., Кравцова Е.И., Бирюкова М.И., Боровикова О.И., Никогда Ю.В. Хронический эндометрит и инфертильность – исходы экстракорпорального оплодотворения (систематический обзор и мета-анализ). Кубанский научный медицинский вестник. 2023; 30(5): 15-40. [Lokshin V.N., Kutsenko I.I., Borovikov I.O., Kravtsova E.I., Biryukova M.I., Borovikova O.I., Nikogda Ju.V. Chronic endometritis and infertility – in vitro fertilization outcomes: systematic review and meta-analysis. Kuban Scientific Medical Bulletin. 2023; 30(5): 15-40 (in Russian)]. https://dx.doi.org/10.25207/1608-6228-2023-30-5-15-40
- Арутюнян Н.А., Кацалап С.Н., Акатьева А.С., Хмелевская В.Ф., Алёхин А.И. Фотодинамическая терапия при синдроме «тонкого» эндометрия у пациенток с бесплодием. Радиация и риск. 2023; 32(2): 56-65. [Arutyunyan N.A., Katsalap S.N., Akatieva A.S., Khmelevskaya V.F., Alekhin A.I. Photodynamic therapy for thin endometrium in patients with infertility. Radiation and risk. 2023; 32(2): 56-65 (in Russian)]. https://dx.doi.org/10.21870/0131-3878-2023-32-2-56-65
- Иевлева К.Д., Данусевич И.Н., Аталян А.В., Шарифулин Э.М., Лазарева Л.М., Наделяева Я.Г., Рашидова М.А., Ахмедзянова М.Р., Беленькая Л.В., Шолохов Л.Ф., Сутурина Л.В. Уровень адипокинов и их ассоциация с хроническим эндометритом у женщин репродуктивного возраста. Вопросы гинекологии, акушерства и перинатологии. 2023; 22(5): 60-8. [Ievleva K.D., Danusevich I.N., Atalyan A.V., Sharifulin E.M., Lazareva L.M., Nadelyaeva Ya.G., Rashidova M.A., Akhmedzyanova M.R., Belenkaya L.V., Sholokhov L.F., Suturina L.V. Adipokine levels and their association with chronic endometritis in reproductive-aged women. Gynecology, Obstetrics and Perinatology. 2023; 22(5): 60-8 (in Russian)]. https://dx.doi.org/10.20953/1726-1678-2023-5-60-68
- Иевлева К.Д., Данусевич И.Н., Аталян А.В., Егорова И.Ю., Бабаева Н.И., Рашидова М.А., Ахмедзянова М.Р., Шолохов Л.Ф., Наделяева Я.Г., Лазарева Л.М., Сутурина Л.В. Диагностическая значимость уровней интерлейкинов в сыворотке крови у женщин репродуктивного возраста с хроническим эндометритом и нормальной или избыточной массой тела. Acta Biomedica Scientifica. 2024; 9(3): 38-48. Ievleva K.D., Danusevich I.N., Atalyan A.V., Egorova I.Yu., Babaeva N.I., Rashidova M.A., Akhmedzyanova M.R., Sholokhov L.F., Nadeliaeva I.G., Lazareva L.M., Suturina L.V. Diagnostic significance of interleukin levels in blood serum in premenopausal women with chronic endometritis and normal weight or overweight. Acta Biomedica Scientifica. 2024; 9(3): 38-48. (in Russian)]. https://dx.doi.org/10.29413/ABS.2024-9.3.4
- Толибова Г.Х., Траль Т.Г., Клещёв М.А., Кветной И.М., Айламазян Э.К. Эндометриальная дисфункция: алгоритм гистологического и иммуногистохимического исследования. Журнал акушерства и женских болезней. 2015; 64(4): 69-77. [Tolibova G.H., Tral´ T.G., Kleshchov M.A., Kvetnoy I.M., Aylamazyan E.K. Endometrial dysfunction: an algorithm for histological and immunohistochemical studies. Journal of Obstetrics and Women's Diseases. 2015; 64(4): 69-77 (in Russian)]. https://dx.doi.org/10.17816/JOWD64469-77
Received 21.08.2025
Accepted 26.11.2025
About the Authors
Kseniia D. Ievleva, PhD, Researcher at the Laboratory of Gynecological Endocrinology, Scientific Center for Family Health and Human Reproduction Problems,664003, Russia, Irkutsk, Timiryazev str., 16, +7(3952)20-76-36, asiy91@mail.ru, https://orcid.org/0000-0002-0177-234X
Irina N. Danusevich, Dr. Med. Sci., Head of the Laboratory of Gynecological Endocrinology, Scientific Center for Family Health and Human Reproduction Problems,
664003, Russia, Irkutsk, Timiryazev str., 16, +7(3952)20-76-36, irinaemails@gmail.com, https://orcid.org/0000-0002-8862-5771
Leonid F. Sholokhov, Dr. Med. Sci., Professor, Head of the Laboratory of Physiology and Pathology of Endocrine System, Scientific Center for Family Health and Human Reproduction Problems, 664003, Russia, Irkutsk, Timiryazev str., 16, +7(3952)20-76-36, lfshol@mail.ru, https://orcid.org/0000-0003-3588-6545
Larisa V. Suturina, Dr. Med. Sci., Professor, Head of the Department of Reproductive Health Protection, Scientific Center for Family Health and Human Reproduction Problems, 664003, Russia, Irkutsk, Timiryazev str., 16, +7(3952)20-76-36, lsuturina@mail.ru, https://orcid.org/0000-0002-6271-7803
Lubov I. Kolesnikova, Academician of the RAS, Dr. Med. Sci., Professor, Scientific Supervisor, Scientific Center for Family Health and Human Reproduction Problems,
664003, Russia, Irkutsk, Timiryazev str., 16; Professor at the Department of Physiology and Psychophysiology, Irkutsk State University, +7(3952)20-76-36, iphr@sbamsr.irk.ru, https://orcid.org/0000-0003-3354-2992
Corresponding author: Kseniia D. Ievleva, asiy91@mail.ru



