Characteristics of blood supply to uterine fibroids after laparoscopic myomectomy in history
Dolgikh M.S., Polenov N.I., Yarmolinskaya M.I., Kogan I.Yu.
Modern ultrasound technologies, including dynamic perfusion assessment, can deepen our understanding of the mechanisms that activate growth of residual uterine fibroids after myomectomy.
Objective: To evaluate the dynamics of blood supply to uterine fibroids remaining after laparoscopic myomectomy during 6 months of follow-up.
Materials and methods: A prospective cohort longitudinal study included 30 patients with a single uterine fibroid of intramural, intramural-subserous localization (FIGO type 4–5) that remained after laparoscopic myomectomy. We conducted a dynamic monitoring at 1, 3, and 6 months postoperatively, with the ultrasound assessment of biometry parameters and vascular morphology, as well as Doppler blood flow velocimetry. Three-dimensional ultrasound reconstruction with the VOCAL software was used to evaluate fibroid volume, echogenicity, as well as its vascularization indices (VI), flow index (FI), and perfusion index (VFI).
Results: We observed a decrease in uterine volume and restoration of endometrial thickness, accompanied by changes in the vascular architecture of the remaining uterine fibroids. The resistance index of the central fibroid vessels remained stable, while that of the peripheral vessels gradually increased. Small fibroids showed signs of angiogenic activity, while larger ones featured reduced perfusion. The perfusion index of uterine fibroids, both intramural (FIGO type 4) and intramural-subserosal (FIGO type 5), increased toward the end of the follow-up period; in type 4 nodules the enhancement of perfusion and flow index occurred earlier and was more pronounced than in intramural-subserosal nodules. Surgical factors had an impact on the perfusion indices of the residual uterine fibroids after myomectomy.
Conclusion: Size, location and type of the fibroid according to FIGO, as well as its surgical characteristics (number of removed fibroids, opening of the uterine cavity) were associated with differences in the perfusion indices of the residual uterine fibroids in postoperative observation. These data confirm that the combination of ultrasound features can be used for more substantiated planning of the frequency and extent of postoperative monitoring, but their prognostic role requires further study.
Authors’ contributions: Dolgikh M.S., Polenov N.I., Yarmolinskaya M.I., Kogan I.Yu. – study conception and design; Dolgikh M.S., Polenov N.I. – data collection and processing; Dolgikh M.S. – manuscript composition; Kogan I.Yu., Yarmolinskaya M.I. – manuscript editing and critical revision. All authors have read and approved the final version of the manuscript.
Conflicts of interest: The authors declare no obvious or potential conflicts of interest regarding the publication of this article.
Funding: The study was supported by the research program "Development of a combined diagnostic method using optical biopsy in patients with benign tumors of the reproductive system (uterine fibroids) to enhance reproductive potential"
(No. 1022040700754-2-3.2.2-1-9).
Ethical Approval: The study was approved by the Local Ethics Committee of the D.O. Ott Research Institute for Obstetrics, Gynecology and Reproductology (Protocol No. 1496 dated 12.09.2025).
Patient Consent for Publication: The patients signed informed consent for the participation in the study and publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Dolgikh M.S., Polenov N.I., Yarmolinskaya M.I., Kogan I.Yu. Characteristics of
blood supply to uterine fibroids after laparoscopic myomectomy in history.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (12): 98-110 (in Russian)
https://dx.doi.org/10.18565/aig.2025.351
Keywords
Uterine fibroids are the most common benign neoplasms of the female reproductive system. According to various sources, they occur in 20–70% of women of reproductive age [1, 2]. The development of the disease can be accompanied by severe clinical symptoms – abnormal uterine bleeding, pain syndrome, and reproductive dysfunction [3–5]. Effective drug therapy for this condition has not yet been developed. Surgical methods, particularly myomectomy, remain the primary organ-sparing procedure to preserve the patient's reproductive potential [6, 7]. However, recurrence events after surgical treatment remain a pressing issue. According to large cohort studies, recurrence occurs in 15–30% of patients within 5 years of surgery [8, 9]. In this study, recurrence of uterine fibroids was defined as either the appearance of new fibroid nodes or regrowth of residual nodes after laparoscopic myomectomy. According to current research concepts, the growth and persistence of uterine fibroids are directly associated with their blood supply. It is known that myomas have a more enhanced and active vascular network compared to the intact myometrium [10]. Angiogenesis (the formation of new vessels) and vasculogenesis (the recruitment of endothelial precursors) play an important role in maintaining tumor growth and development [11, 12]. Modern ultrasound technologies made it possible not only to perform qualitative analysis but also to quantitatively assess tumor tissue perfusion characteristics. Color Doppler flow mapping (CDM) followed by spectral pulsed-wave Doppler analysis allows for recording linear blood flow velocity curves and calculating the resistance index (RI), enabling to assess central and peripheral intratumoral blood flow as well. VOCAL (Virtual Organ Computer-aided Analysis) technology is a three-dimensional ultrasound reconstruction method that provides highly accurate measurement of the uterine fibroid volume and its average echogenicity. Additionally, it automatically calculates quantitative vascularization indices: VI (vascularization index) reflects the degree of vascularization, defined as the ratio of vessel volume to the total volume of the node; FI (flow index) characterizes the intensity of intratumoral blood flow; VFI (vascularization flow index) is an integral parameter combining the degree of vascularization and blood flow intensity. The VOCAL includes a reproducible assessment of the vascular characteristics of myomatous nodes. It significantly enhances the possibilities of analyzing their hemodynamics and ongoing monitoring. However, there are currently no uniform standardized protocols for its use, which emphasizes the need for further research [13]. Some studies demonstrate that high VI and VFI volume values are associated with the growth of uterine myoma, and an increase in RI in the central and peripheral vessels was observed in patients with recurrent forms of the neoplasm after myomectomy [14–16]. However, changes in these indicators at different times after myomectomy have not been studied.
Study objective: to evaluate the changes in biometric indicators and blood supply to uterine fibroids of various localization remaining after laparoscopic myomectomy, during 1, 3 and 6 months of follow-up.
Materials and methods
Study design
This prospective cohort longitudinal study was conducted in the Ist Gynecology Department with the operating unit of the D.O. Ott Research Institute for Obstetrics, Gynecology and Reproductology during the period of November 1, 2023 to July 30, 2025. Patients were enrolled among women admitted for laparoscopic myomectomy and who met the inclusion criteria.
The study description is presented in accordance with the main provisions of the STROBE guidelines for cohort studies.
Inclusion criteria: age 25–49 years; signed informed consent for participation in the study; a single uterine fibroid of intramural or intramural-subserous localization (FIGO type 4–5), remaining after laparoscopic myomectomy; myomectomy performed no more than 1 month after inclusion in the study.
Exclusion criteria: pregnancy, decompensation or presence of severe somatic symptom disorder, malignancy, pelvic inflammatory diseases, hormone therapy 3 months before the study, external genital endometriosis, adenomyosis, abnormal development of the genital organs.
The study included 30 patients with a single uterine fibroid remaining after laparoscopic myomectomy. All patients completed three-stage follow-up (1, 3, and 6 months post-surgery); no losses detected.
The sample size was identified by the number of patients undergoing surgery during the specified period and meeting the inclusion criteria. A separate statistical calculation of the required sample size was not conducted in advance due to the pilot nature of the study.
In accordance with STROBE guidelines for cohort studies, the outcomes and variables analyzed were pre-defined.
Major study results:
- Changes in uterine volume and endometrial thickness at 1, 3, and 6 months post-surgery;
- Changes in ultrasound morphology of radial or tortuous/branching vessels at 1, 3, and 6 months post-surgery;
- Changes in uterine fibroid volume and average echogenicity based on 3D reconstruction data in the VOCAL program at 1, 3, and 6 months post-surgery;
- Changes in Doppler ultrasound parameters (resistance index of central and peripheral vessels of the node) and quantitative vascular indices (VI, FI, VFI) at the same observation periods.
Analyzed factors included:
- fibroid volume 1 month after surgery, divided into three categories: <20 cm³, 20–40 cm³, and >40 cm³;
- fibroid location (anterior wall, posterior wall, or fundus of the uterus);
- fibroid type according to FIGO (type 4 or 5);
- Surgery details (number of removed fibroids ≤2 or ≥3; uterine cavity incision – yes/no).
Each participant was examined at fixed time points: 1, 3, and 6 months after a standardized laparoscopic myomectomy procedure with a unified pelvic ultrasound protocol [17, 18].
To minimize interdevice and interoperator variability, all studies were performed on a single W10-RUS ultrasound machine “Samsung Medison Co. LTD” using a transvaginal EV2-10A probe with a frequency of 2–10 MHz in real time, on the 5th–7th day of the menstrual cycle, by the same operator.
Uterine volume and endometrial thickness were assessed in two-dimensional mode. We also evaluated the location of uterine fibroids (anterior or posterior uterine wall, fundus) and the FIGO myoma type (type 4 or 5).
Central and peripheral intratumoral vessels were visualized in two-dimensional Color Doppler flow mapping, followed by a qualitative analysis of their ultrasound morphology. Depending on the number and nature of the vessels, two types of vascular architecture were detected: tortuous/branching architecture – multiple vessels inside the myoma with a tortuous course and/or the presence of clearly visualized branches (Fig. 1a); radial architecture – a single peripherally located vessel without signs of intratumor branching (Fig. 1b).
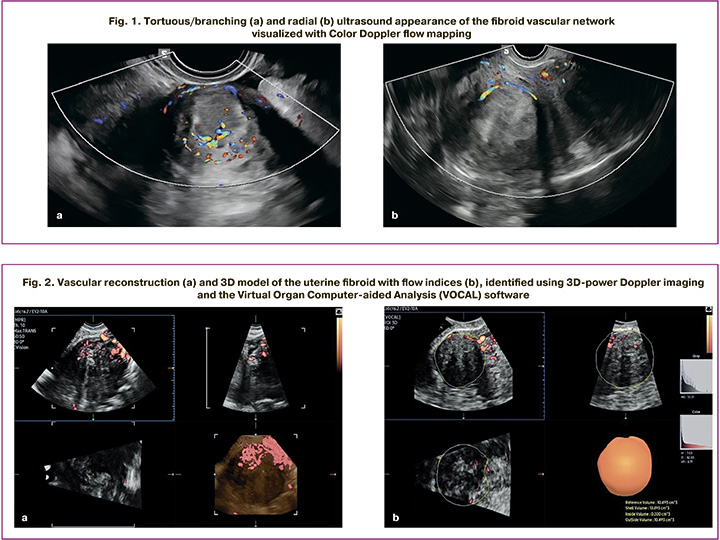
To quantitatively characterize the blood flow, we performed spectral pulse-wave Doppler ultrasound scan, with RI calculated in the central and peripheral vessels of the uterine fibroids [19].
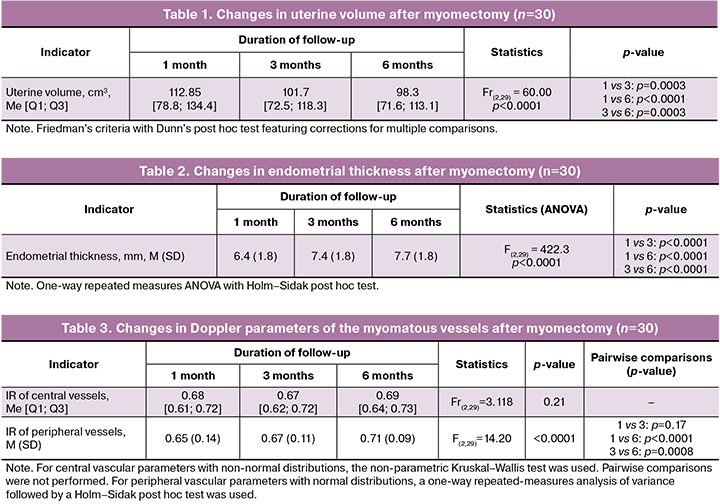
All patients also underwent a 3D ultrasound examination using power Doppler and specialized VOCAL technology. A 3D-image of each uterine fibroid was obtained using a minimum acquisition angle of 90° and maximum inclusion of the node in the scan volume. The data was then analyzed in VOCAL mode using sequential rotation in 15° increments (12 planes, total angle of 180°). The fibroid contours were sequentially manually traced in the selected plane on each slice, automatically generated by the software during 15° volume rotation around a fixed axis. After contouring on all slices, the software calculated the volume of the fibroid (cm³) and the average echogenicity of the tumor tissue (MG). Additionally, a three-dimensional reconstruction of the fibroid’s vascular network was performed using power Doppler imaging (Fig. 2a). The histogram function was then activated, allowing the program to calculate vascular indices in a semi-automatical mode: vascularization index (%), flow index (relative units), and perfusion index (relative units) (Fig. 2b).
To reduce systematic measurement errors, all studies were performed by the same operator on the same ultrasound machine, on the 5th–7th day of the menstrual cycle, using the same examination scheme (two-dimensional scanning, color Doppler imaging, pulsed-wave Doppler, then 3D reconstruction in VOCAL mode).
Informed consent was obtained from each patient prior to inclusion in the study. The study was approved by the local ethics committee of the D.O. Ott Research Institute for Obstetrics, Gynecology and Reproductology (Protocol No. 1496, dated September 12, 2025).
Statistical analysis
Statistical data processing was performed using standard Microsoft Excel spreadsheets and the GraphPad Prism software package. If the data conformed to a normal distribution, parametric methods were used and the data was presented as mean ± standard deviation. If the data did not conform to a normal distribution, nonparametric methods were used and the data was presented as median (25th–75th quartile). Normality of distribution was carried out using the Shapiro – Wilk test. Student's t-test or its nonparametric analogue, the Mann–Whitney U-test, were applied to compare two groups. Changes in the parameters were assessed using ANOVA analysis for repeated measures with Tukey's post hoc test if normal distribution correlated; otherwise, the Friedman test with Dunn's post hoc method were used. Pearson's chi-square test (χ²) was applied to compare binary (categorical) variables. Statistical significance was detected at p<0.05.
Adjustment for potential confounders (age, body mass index, comorbidities) was not performed due to the small sample size and pilot nature of the study; results are presented as unadjusted estimates. There were no missing values for key ultrasound variables; all 30 patients completed follow-up for up to 6 months, with no dropouts. Separate sensitivity analyses were not performed.
Results
The study included 30 patients with a single uterine fibroid of intramural or intramural-subserous localization (FIGO type 4–5), remaining after laparoscopic myomectomy.
The patients' ages ranged 29–49 years: the mean value was 41.3 (6.37) years.
Analysis of changes in uterine ultrasound characteristics revealed a consistent decrease in uterine volume over the observation period. The data are presented in Table 1.
Endometrial thickness, on the other hand, showed a gradual increase over time (Table 2). Despite the fact that the patients aged 45–49 were in the late reproductive and perimenopausal periods, they also showed a restoration of endometrial thickness, indicating the preservation of its functional potential.
Analysis of the changes in ultrasound vascular morphology revealed differences in the structure of the fibroid tissue vascular bed.
One month after surgery, the majority of uterine fibroids retained a radial vascular organization of 20/30 (66.6%), reflecting a mature and relatively stable network. Six months after surgery, the McNemar test revealed a statistically significant increase in the frequency of transition from radial to tortuous vascular network (χ²=0.640, p=0.042).
During ongoing monitoring of uterine fibroids of various localizations, the RI in the central and peripheral vessels remained stable, while vascularization indicators gradually increased.
The changes in Doppler parameters of the myomatous vessels are reflected in Table 3. Statistical analysis revealed fundamental differences: while the resistance index of the central vessels remained stable throughout the observation period, the resistance index of the peripheral vessels of the fibroid demonstrated a statistically significant progressive increase with maximum values 6 months after myomectomy.
An increase in the VOCAL volume of the myomatous node was observed during the 6 months following surgery. The volume increased from 8.25 cm³ at 1 month to 9.3 cm³ at 3 months and 11.0 cm³ at 6 months after myomectomy. All comparisons between time points showed statistically significant differences (Table 4).
The mean echogenicity of uterine fibroids gradually decreased over the 6 months after surgery (Table 5). The greatest reduction was noted at 6 months (67.6 U) compared with the baseline value at 1 month after myomectomy (72.2 U, p<0.05).
After evaluation of perfusion as an integral indicator of blood flow, differences were found between the study groups, depending on their volume (Table 6).
Small fibroids (<20 cm³) showed a gradual increase in the perfusion during the period of 1 month to 3 and 6 months of observation. The values increased sequentially.
In medium-sized fibroids (20–40 cm³), the perfusion index changed slightly: a small increase was noted by the 3rd month, followed by a decrease at the 6th month of continuous monitoring, in general remaining at a comparable level.
In large fibroids (>40 cm³), the perfusion index increased moderately from 1 to 3 and 6 months postoperatively; however, the differences did not reach statistical significance, with the greatest increase occurring in the first 3 months. Given the small sample size, these changes can be interpreted as a trend.
During the follow-up, a gradual restoration of vascular activity was noted in the residual uterine fibroids (Table 7).
The vascularization index increased consistently throughout the entire observation period, reaching its maximum value by month 6.
The flow index also elevated, but less significantly, with a marked raise only by month 6 compared to month 1.
The perfusion index (a combined indicator) showed prominent improvement, with a consistent increase at each follow-up stage.
All three indicators demonstrated a trend toward improved blood flow to the myomatous nodes over the 6 months post-surgery.
When comparing the changes of vascular indices depending on the node type, the following differences were identified.
In intramural myomatous nodes (FIGO type 4, n=17), vascular indices increased over the 6 months following surgery (Table 8).
The vascularization index elevated by the 3rd month of follow-up and continued to raise by the 6th month.
The flow index raised less significantly, reaching statistically significant growth only by the 6th month post-surgery.
The perfusion index showed the most pronounced changes, with a consistent increase at each follow-up stage, particularly noticeable by the 6th month after the intervention.
A statistically significant increase in vascular indices of intramural-subserosal myomatous nodes was observed 6 months post-surgery (FIGO type 5, n=13).
The vascularization index increased at both the 3rd and 6th months of follow-up compared to baseline.
The perfusion index also increased by the 6th month of follow-up (p<0.05). The flow index showed positive trend, although pairwise comparisons did not reach statistical significance (Table 9).
Changes in the vascular indices of myomatous nodes were also found depending on their location in relation to the uterine wall.
Within 6 months after myomectomy, patients with fibroids in the anterior uterine wall showed statistically significant improvements in all vascularization indices (Table 10).
All three indices consistently increased over the follow-up period, demonstrating a sustained positive trend in the blood supply to the uterine fibroids.
For uterine fibroids located on the posterior uterine wall, improvements in vascular indices were also observed in the postoperative period (Table 11).
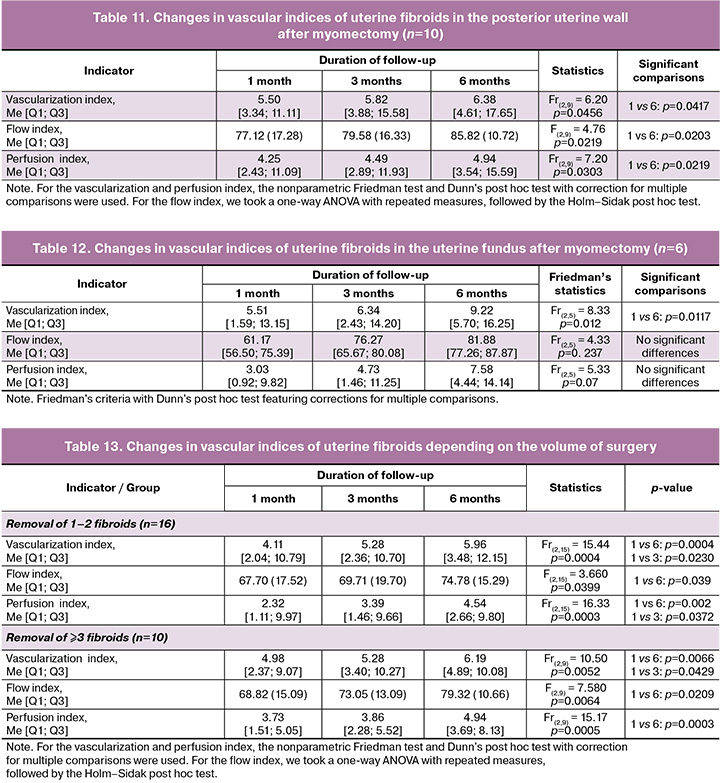
All three indices were significantly higher by the 6th month of the follow-up compared to the first month after myomectomy.
For uterine fibroids located in the fundus, vascular indices also changed during the postoperative period (Table 12).
A statistically significant increase in the vascularization index was observed at month 6 compared both to month 1 and month 3 post-surgery. However, the flow index and perfusion index did not show significant changes throughout the entire observation period.
Analysis of vascular indices and the characteristics of the myomectomy history revealed the following characteristics. The median number of fibroids removed was 2 [1; 3].
The changes in vascular indices of the residual myomatous fibroids did not depend on the extent of the surgery (Table 13). In both groups (with 1–2 fibroids removed and with 3 or more fibroids removed) a statistically significant increase in all three vascularization indices was identified over the 6-month follow-up period. Moreover, patients with more extensive procedures demonstrated a more pronounced increase in the flow index by the 6th month.
Uterine cavity opening was observed in 8/30 (26.7%) patients and was accompanied by the following index changes.
Uterine cavity opening during surgery significantly impacted the changes in vascular indices of the residual myomatous nodes (Table 14).
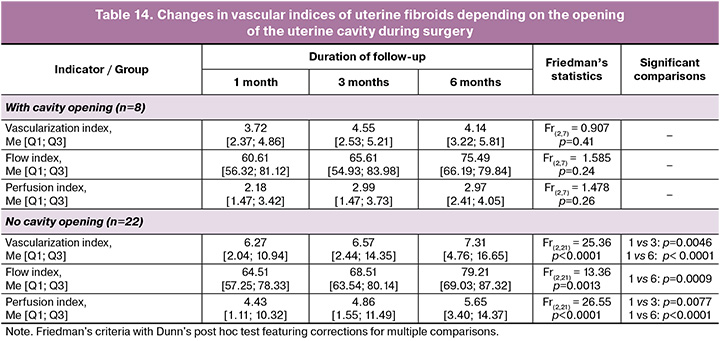
In cases where the uterine cavity was not incised, no significant changes in vascular indices were observed over the 6-month follow-up period. In contrast, uterine cavity opening resulted in statistically significant increases in all three indices. The most pronounced trend was manifested by the 6th month of follow-up, when all indices reached their maximum values.
Discussion
The results of this study confirm that changes in blood supply to uterine fibroids after laparoscopic myomectomy reflect complex pathophysiological processes, including remodeling of the vascular network, alterations in the density of the extracellular matrix and adaptation to hypoxia.
The significant reduction in the uterine volume observed in the first months reflects the regression of the hypertrophied myometrium and connective tissue structures previously involved in the formation of fibroids and compensatory remodeling of the organ. By 3–6 months uterine volume stabilized, which is consistent with research data on the cessation of active myometrial remodeling processes after restoration of anatomical integrity [20, 21, 23].
The endometrium thickness in the postoperative period gradually increased, and by the 6th month of the follow-up period exceeded the values recorded at the 1st month after the surgery, which can be regarded as the restoration and maintenance of its function capability. This phenomenon is paramount, as it demonstrates the endometrial capacity for repair and confirms the possibility of preserving fertility after myomectomy. Similar data are presented in studies by Pritts E.A. et al., which demonstrate that endometrial restoration is finished within 3–6 months and is associated with normalization of uterine blood flow [21].
Although the 45–49-year age group is related to the late reproductive and perimenopausal periods, patients in this group also showed restoration of endometrial thickness. This fact reflects the preservation of endometrial functional capability. Although in clinical practice, the interpretation of such changes requires caution and ongoing monitoring due to the increased possibility of hyperplastic processes in older age groups.
It should be noted that endometrium restoration and decrease in uterine volume occurred simultaneously featuring changes in the vascularization indices of uterine fibroids. Our study showed that decrease in uterine volume was associated with gradual volume enlargement of the residual uterine fibroid from the 1st to the 3rd and 6th month of observation, accompanied by an increase in the vascularization and perfusion indices. Foreign research data, including studies by Elkattan E. et al. [22] and Stępniak A. et al. [23] demonstrate that high and increasing values of VI and VFI reflect the maintenance of vascular activity of an uterine fibroid and are associated with the risk of its further growth.
When analyzing ultrasound morphology in 1-month post-surgery period, radial vessel architecture predominated in most cases, corresponding to a relatively mature and stable vascular network. By the 6th month, some lesions were covered with branching and tortuous vessels with a more chaotic dissemination, reflecting the activation of neoangiogenesis and the formation of a less mature, chaotically organized vascular network. Similar features of this network in uterine myoma have been described in morphological and ultrasound studies demonstrating a connection between pronounced vascularization and abnormal vessel architecture with the growth and high cellular activity of fibroids [23–26].
Morphological changes are closely related to the molecular mechanisms of angiogenesis. Hypoxia in myomatous tissue activates HIF-1α, inducing the expression of VEGF, FGF, and other growth factors, which triggers the formation of new vessels. These vessels are characterized by a tortuous course, multiple branches, and an irregular lumen, which explains the chaotic ultrasound image [27, 28]. At the same time, TGF-β and the MMP/TIMP system are involved in the remodeling of the extracellular matrix, facilitating the growth of new vessels and the formation of an unstable network [29].
Thus, changes in the vascular morphology after myomectomy can be considered as an ultrasound marker of angiogenesis activity, allowing differentiation between nodes with a stable and potentially progressive course.
During ongoing follow-up, we managed to reveal differences between the RI values of the central and peripheral vessels of uterine fibroids. The RI value of the central vessels remained relatively stable throughout the entire observation period, reflecting the stable hemodynamics of the main vessels and lower sensitivity to local angiogenic changes. At the same time, peripheral vascular RI values gradually increased by the sixth month of observation. This value elevation indicates the increase in vascular resistance in the peripheral network, which can be explained as a consequence of microvascular remodeling and perfusion restriction. Our results are consistent with the data from clinical studies, which indicate that the Doppler ultrasound parameters of peripheral vessels are more variable and better reflect the characteristics of the blood supply to uterine fibroids. In particular, a study by Idowu V.M. et al. demonstrated that in patients with recurrent uterine fibroids after myomectomy, the RI and pulsatility indices in peripheral vessels were significantly higher than in patients without recurrence, while central vessels demonstrated less variability. These data suggest that peripheral vessel RI is a more informative indicator for assessing angiogenic activity and the risk of further nodule growth [14, 30].
A gradual decrease in the average echogenicity of myomatous nodes was noted during follow-up. This decrease with simultaneous enlargement in the node volume and an increase in vascularization and perfusion indices, can be interpreted as a relative predominance of cellular and vascular components over the dense fibrous matrix, consistent with the echographic appearance of a more active, proliferating myoma [31]. It should be noted that the average echogenicity of uterine fibroids can vary depending on their size: differences in volume influence the severity of fibrotic changes and the degree of vascularization. Therefore, this indicator serves as an indirect marker of tissue remodeling and indicates that fibroids differ in their growth potential and the degree of vascular rearrangement.
Continuous observation revealed a gradual restoration of vascular activity in the fibroids remaining after myomectomy. The vascularization index increased by the sixth month of follow-up. Similar changes were noted for the perfusion index. The flow index changed less significantly, indicating relative stability of blood flow intensity, which depends not only on the morphology of the vascular network but also on systemic factors [29].
Differences in vascular indices depended on the location of the node in the uterine wall. In our study, for fibroids located on the anterior uterine wall, a significant increase in the above-mentioned indices was detected by the third month post-surgery, with a continued upward trend till the sixth month of observation. For posterior wall uterine fibroids, more pronounced changes in VI and VFI were observed predominantly by the 6th month post-surgery, whereas an increase in VI and VFI was described for fibroids with localization in the fundus of the uterus in the absence of significant FI shifts. The obtained distribution of VI, FI, and VFI patterns are consistent with the data on differences in regional uterine and fibroid hemodynamics, in particular with the results of studies on intratumor and peripheral blood flow in intramural uterine myoma, demonstrating more intense intratumor blood flow compared to peripheral [31, 32].
It should be noted that the fibroids varied in initial size, and this feature largely determines the nature of vascular changes: some myomatous nodules tend to stabilize, while others retain the potential for growth. We found that vascularization indicators are size-dependent. When assessing size-dependent perfusion characteristics, we observed the greatest and statistically significant increase in the perfusion index in small-volume nodules (<20 cm³), while medium-sized nodules (20–40 cm³) were characterized by high VFI variability without significant changes over time. Large fibroids (>40 cm³) also showed raise in the perfusion index by the 6th month of monitoring, compared with the first month, though with relatively low absolute values of this indicator. This distribution may reflect the limited angiogenic potential of large myomas, as opposed to small formations [33].
The contrast between the FIGO node types was also marked. In our study, the perfusion index of intramural myomatous nodes (FIGO type 4) increased by the 3rd and 6th months after surgery, while the vascularization and flow indices retained pronounced upward trend by the 6th month, indicating increase both in the volume of the vascular network and in the average intensity of intratumoral blood flow. For intramural-subserosal nodes (FIGO type 5), a significant elevation in vascularization and perfusion indices was observed primarily by the sixth month after myomectomy, with no change in the flow index. This may reflect an increase in the number of functioning vessels without large changes in blood flow velocity. Thus, the FIGO node type largely influences the rate of increase in vascular network volume and perfusion. The obtained patterns are consistent with the anatomical features of the blood supply: the subserous component, as a rule, is supplied mainly from the superficial vascular plexuses and is less connected with the deep myometrial vascular tree, which may limit the possibilities of vascular remodeling and slow down the increase in perfusion compared to intramural nodes [14, 34].
The results of the analysis of the perfusion dependence on the characteristics of the previous myomectomy proved to be largely intriguing. In our sample the median number of removed nodes was 2 [1; 3]. When comparing subgroups, it was discovered that perfusion (VFI) was partially dependent on surgical factors. In patients with ≤2 fibroids removed, the residual myomatous tissue demonstrated a moderate but statistically significant increase in the vascularization, flow, and perfusion indices by the 6th month of continuous follow-up. In contrast, the removal of ≥3 fibroids was associated with higher values of these parameters at early stages of follow-up, which may indicate a compensatory increase in the angiogenic activity of the remaining fibroid tissue.
Uterine cavity opening, noted in 26.7% of patients also showed no less importance for our study. In cases with the cavity not incised, no significant changes in vascularization, flow, or perfusion indices were observed over time. In contrast, the opening of the uterine cavity demonstrated a significant increase in VI, FI, and VFI at the 3rd and 6th months of ongoing monitoring. It is likely that more traumatic uterine interventions lead to changes in regional blood flow and activation of neoangiogenesis in residual myomatous nodes and adjacent myometrium.
Drawing the conclusion, the combination of the obtained results supports the idea that uterine fibroids represent a heterogeneous population with varying vascular and growth potential. As a result of myomectomy, some nodules demonstrate paramount perfusion restoration and volume increase, while others are characterized by more stable or lower blood flow rates. The clinical significance of these data indicates that uterine myoma is a dynamic entity capable of further growth and remodeling of its own vascular network.
Study limitations. This study has several limitations that must be considered when interpreting the results. Firstly, the sample size is relatively small, which limits the applicability of the obtained data to a wider patient population. Secondly, the cohort design without randomization does not allow for the complete elimination of potential confounding factors (age, body mass index, concomitant somatic diseases, and postoperative therapy characteristics) that may influence the blood supply of the residual uterine fibroids and changes in its volume. Thirdly, the analysis included only intramural and intramural-subserosal uterine fibroids (FIGO grades 4–5), so the findings primarily characterize this group of myomas. Finally, the observation period was only 6 months post-surgery. A longer follow-up period is required to evaluate the relationship between perfusion parameters and long-term changes in the volume and vascularization values of the fibroids.
Conclusion
The present study demonstrated that uterine fibroids retained after laparoscopic myomectomy exhibit heterogeneous trend associated with their size, vascular network morphology, and perfusion parameters.
During the first 6 months after surgery, a decrease in uterine volume and gradual restoration of endometrial thickness were observed, accompanied by changes in the vascular architecture of residual uterine fibroids, with an elevation in vascularization and perfusion indices featuring stable RI of central vessels and an upward trend in RI of peripheral vessels. Small myomatous nodes demonstrated a more pronounced raise in perfusion index, while large ones maintained relatively low values of this indicator.
The size, location, and type of the uterine fibroid according to FIGO, as well as surgical intervention characteristics (number of fibroids removed, opening of the uterine cavity) were associated with contrast in perfusion parameters of myomatous nodules remaining after myomectomy during postoperative follow-up period.
The combination of these ultrasound characteristics can serve as a basis for more well-reasoned decision on the frequency and extent of follow-up monitoring, though their prognostic value requires further research.
References
- Аганезова Н.В., Аганезов С.С., Шило М.М. Миома матки: современные практические аспекты заболевания. Проблемы репродукции. 2022; 28(4): 97-105. [Aganezova N.V., Aganezov S.S., Shilo M.M. Uterine fibroids: modern practical aspects of the disease. Russian Journal of Human Reproduction. 2022; 28(4): 97-105 (in Russian)]. https://dx.doi.org/10.17116/repro20222804197
- Ящук А.Г., Мусин И.И., Гумерова И.А. Современные аспекты в изучении этиологии миомы матки. Российский вестник акушера-гинеколога. 2019; 19(3): 49-56. [Iashchuk A.G., Musin I.I., Gumerova I.A. Current aspects of the study of uterine myoma etiology. Russian Bulletin of Obstetrician-Gynecologist. 2019; 19(3): 49-56 (in Russian)]. https://dx.doi.org/10.17116/rosakush20191903149
- Беженарь В.Ф., Линде В.А., Аракелян Б.В., Садыхова Э.Э., Резник М.В., Тарасенкова В.А. Миома матки и фертильность: современный взгляд на проблему (обзор литературы). Журнал акушерства и женских болезней. 2022; 71(2): 79-86. [Bezhenar V.F., Linda V.A., Arakelyan B.V., Sadykhova E.E., Reznik M.V., Tarasenkova V.A. Uterine fibroids and fertility: a modern view of the problem (literature review). Journal of Obstetrics and Women's Diseases. 2022; 71(2): 79-86 (in Russian)]. https://dx.doi.org/10.17816/JOWD78831
- Yang Q., Ciebiera M., Bariani M.V., Ali M., Elkafas H., Boyer T.G. et al. Comprehensive review of uterine fibroids: developmental origin, pathogenesis, and treatment. Endocr. Rev. 2022; 43(4): 678-719. https://dx.doi.org/10.1210/endrev/bnab039
- Donnez J., Dolmans M.M. Uterine fibroid management: from the present to the future. Hum. Reprod. Update. 2016; 22(6): 665-86. https://dx.doi.org/10.1093/humupd/dmw023
- Li B., Wang F., Chen L., Tong H. Global epidemiological characteristics of uterine fibroids. Arch. Med. Sci. 2023; 19(6): 1802-10. https://dx.doi.org/10.5114/aoms/171786
- Stewart E.A. Uterine fibroids. Lancet. 2001; 357(9252): 293-8. https://dx.doi.org/10.1016/S0140-6736(00)03622-9
- Bulun S.E. Uterine fibroids. N. Engl. J. Med. 2013; 369(14): 1344-55. https://dx.doi.org/10.1056/NEJMra1209993
- Giuliani E., As-Sanie S., Marsh E.E. Epidemiology and management of uterine fibroids. Int. J. Gynaecol. Obstet. 2020; 149(1): 3-9. https://dx.doi.org/10.1002/ijgo.13102
- Kirschen G.W., AlAshqar A., Miyashita-Ishiwata M., Reschke L., El Sabeh M., Borahay M.A. Vascular biology of uterine fibroids: connecting fibroids and vascular disorders. Reproduction. 2021; 162(2): R1-18. https://dx.doi.org/10.1530/REP-21-0087
- Dolmans M.M., Petraglia F., Catherino W.H., Donnez J. Pathogenesis of uterine fibroids: current understanding and future directions. Fertil. Steril. 2024; 122(1): 6-11. https://dx.doi.org/10.1016/j.fertnstert.2024.02.048
- Testa A.C., Di Legge A., Bonatti M., Manfredi R., Scambia G. Imaging techniques for evaluation of uterine myomas. Best Pract. Res. Clin. Obstet. Gynaecol. 2016; 34: 37-53. https://dx.doi.org/10.1016/j.bpobgyn.2015.11.014
- Frijlingh M., Juffermans L., de Leeuw R., de Bruyn C., Timmerman D., van den Bosch T. et al. How to use power Doppler ultrasound in transvaginal assessment of uterine fibroids. Ultrasound Obstet. Gynecol. 2022; 60(2): 277-83. https://dx.doi.org/10.1002/uog.24879
- Idowu B.M., Ibitoye B.O. Doppler sonography of perifibroid and intrafibroid arteries of uterine leiomyomas. Obstet. Gynecol. Sci. 2018; 61(3): 395-403. https://dx.doi.org/10.5468/ogs.2018.61.3.395
- Nieuwenhuis L.L., Keizer A.L., Stoelinga B., Twisk J., Hehenkamp W., Brölmann H. et al. Fibroid vascularisation assessed with three-dimensional power Doppler ultrasound is a predictor for uterine fibroid growth: a prospective cohort study. BJOG. 2018; 125(5): 577-584. https://dx.doi.org/10.1111/1471-0528.14608
- Mavrelos D., Ben-Nagi J., Holland T., Hoo W., Naftalin J., Jurkovic D. The natural history of fibroids. Ultrasound Obstet. Gynecol. 2010; 35(2): 238-42. https://dx.doi.org/10.1002/uog.7482
- Беженарь В.Ф., Цыпурдеева А.А., Долинский А.К., Поленов Н.И., Байлюк Е.Н., Русина Е.И., Кахиани М.И. Опыт применения стандартизированной методики лапароскопической миомэктомии. Журнал акушерства и женских болезней. 2012; 61(4): 23-32. [Bezhenar V.F., Tsypurdeeva A.A., Dolinskiy A.K., Polenov N.I., Bayluk E.N., Rusina E.I., Kakhiani M.I. The experience of a standardized technique of laparoscopic myomectomy. Journal of Obstetrics and Women's Diseases. 2012; 61(4): 23-32 (in Russian)]. https://dx.doi.org/10.17816/JOWD61423-32
- Поленов Н.И., Долгих М.С., Ярмолинская М.И. Анализ эффективности миомэктомии лапароскопическим доступом при использовании стандартизированной методики. Журнал акушерства и женских болезней. 2024; 73(5): 76-83. [Polenov N.I., Dolgikh M.S., Yarmolinskaya M.I. Analysis of the effectiveness of myomectomy performed by laparoscopic access using a standardized technique. Journal of Obstetrics and Women's Diseases. 2024; 73(5): 76-83 (in Russian)]. https://dx.doi.org/10.17816/JOWD634591
- Долгих М.С., Поленов Н.И., Ярмолинская М.И., Коган И.Ю. Описание особенностей кровоснабжения интрамуральных и интрамурально-субсерозных миоматозных узлов различных размеров с применением Virtual Organ Computer-aided Analysis (VOCAL). Журнал акушерства и женских болезней. 2024; 73(6): 44-52. [Dolgikh M.S., Polenov N.I., Yarmolinskaya M.I., Kogan I.Yu. Description of the features of blood supply to intramural and intramural-subserous myomatous nodes of various sizes using Virtual Organ Computer-aided Analysis (VOCAL). Journal of Obstetrics and Women's Diseases. 2024; 73(6): 44-52 (in Russian)]. https://dx.doi.org/10.17816/jowd636413
- Malik N., Ranjan R., Khatri R., Kumari S., Malik V., Singh U.K. et al. A study of fibroid vascularization and vascular indices with three-dimensional power Doppler and superb microvascular imaging and the correlation with heavy menstrual bleeding. Cureus. 2024; 16(10): e71246. https://dx.doi.org/10.7759/cureus.71246
- Pritts E.A., Parker W.H., Olive D.L. Fibroids and infertility: an updated systematic review of the evidence. Fertil. Steril. 2009; 91(4): 1215-23. https://dx.doi.org/10.1016/j.fertnstert.2008.01.051
- Elkattan E., Kamel R., Elghazaly H., ElAriki E. Can three-dimensional power Doppler and uterine artery Doppler differentiate between fibroids and adenomyomas? Middle East Fertil. Soc. J. 2016; 21(1): 4651. https://dx.doi.org/10.1016/j.mefs.2015.07.004
- Stępniak A., Czuczwar P. 3D power Doppler vascular indices as a novel technique in assessing the outcome of minimally invasive techniques in uterine fibroids treatment. Prz. Menopauzalny. 2017; 16(4): 118121. https://dx.doi.org/10.5114/pm.2017.72755
- Ciarmela P., Delli Carpini G., Greco S., Zannotti A., Montik N., Giannella L. et al. Uterine fibroid vascularization: from morphological evidence to clinical implications. Reprod. Biomed. Online. 2022; 44(2): 281-94. https://dx.doi.org/10.1016/j.rbmo.2021.09.005
- Miyashita-Ishiwata M., El Sabeh M., Afrin S., Borahay M.A., Hopkins J. Hypoxia induces angiogenesis and proliferation in uterine fibroid cells. Fertil. Steril. 2021; 116(3): 740-8. https://dx.doi.org/10.1016/j.fertnstert.2021.07.041
- Olson S.L., Akbar R.J., Gorniak A., Fuhr L.I., Borahay M.A. Hypoxia in uterine fibroids: role in pathobiology and therapeutic opportunities. Oxygen. 2024; 4(2): 236-52. https://dx.doi.org/10.3390/oxygen4020013
- Fedotova M., Barysheva E., Bushueva O. Pathways of hypoxia-inducible factor (HIF) in the orchestration of uterine fibroids development. Life (Basel). 2023; 13(8): 1740. https://dx.doi.org/10.3390/life13081740
- AlAshqar A., Lulseged B., Mason-Otey A., Liang J., Begum U.A.M., Afrin S. et al. Oxidative stress and antioxidants in uterine fibroids: pathophysiology and clinical implications. Antioxidants (Basel). 2023; 12(4): 807. https://dx.doi.org/10.3390/antiox12040807
- Alcázar J.L. Three-dimensional power Doppler-derived vascular indices: what are we measuring and how are we doing it? Ultrasound Obstet. Gynecol. 2008; 32(4): 485-7. https://dx.doi.org/10.1002/uog.6144
- Russo C., Camilli S., Martire F.G., Di Giovanni A., Lazzeri L., Malzoni M. et al. Ultrasound features of highly vascularized uterine myomas (uterine smooth muscle tumors) and correlation with histopathology. Ultrasound Obstet. Gynecol. 2022; 60(2): 269-76. https://dx.doi.org/10.1002/uog.24855
- Хворостухина Н.Ф., Островская А.Е., Рогожина И.Е., Новичков Д.А., Степанова Н.Н. Особенности маточной гемодинамики и системы гемостаза при миоме, осложненной геморрагическим синдромом. Акушерство и гинекология. 2016; 6: 87-93. [Khvorostukhina N.F., Ostrovskaya A.E., Rogozhina I.E., Novichkov D.A., Stepanova N.N. The specific features of uterine hemodynamics and hemostatic system in myoma complicated by hemorrhagic syndrome. Obstetrics and Gynecology. 2016; (6): 87-93 (in Russian)]. https://dx.doi.org/10.18565/aig.2016.6.87-93
- Буянoва С.Н., Щукина Н.А., Чечнева М.А., Бабунашвили Е.Л. Ультразвуковая диагностика при планировании органосберегающих операций по поводу миомы матки. Российский вестник акушера-гинеколога. 2018; 18(6): 83-7. [Buianova S.N., Shchukina N.A., Chechneva M.A., Babunashvili E.L. Ultrasound diagnosis in the planning of organ-sparing surgery for uterine myoma. Russian Bulletin of Obstetrician-Gynecologist. 2018; 18(6): 83-7 (in Russian)]. https://dx.doi.org/10.17116/rosakush20181806183
- Khan A.T., Shehmar M., Gupta J.K. Uterine fibroids: current perspectives. Int. J. Womens Health. 2014; 6: 95-114. https://dx.doi.org/10.2147/IJWH.S51083
- Munro M.G., Critchley H.O.D., Fraser I.S. The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertil. Steril. 2011; 95(7): 2204-8. https://dx.doi.org/10.1016/j.fertnstert.2011.03.079
Received 03.12.2025
Accepted 12.12.2025
About the Authors
Maria S. Dolgikh, MD, Junior Researcher at the Department of Gynecology and Endocrinology, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, 199034, Russia, St. Petersburg, Mendeleyevskaya Line, 3, mariadolgikh1@gmail.com, eLibrary SPIN: 3879-7773,https://orcid.org/0000-0002-9910-9668
Nikolay I. Polenov, MD, PhD, Senior Researcher at the Department of Gynecology and Endocrinology, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, 199034, Russia, St. Petersburg, Mendeleyevskaya Line, 3, polenovdoc@mail.ru, eLibrary SPIN: 9387-1703, https://orcid.org/0000-0001-8575-7026
Maria I. Yarmolinskaya, Honored Scientist of the Russian Federation, Dr. Med. Sci., Professor of the Russian Academy of Sciences, Head of the Department of Gynecology and Endocrinology, Head of the Center of Diagnostics and Treatment of Endometriosis, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, 199034, Russia, St. Petersburg, Mendeleyevskaya Line, 3, m.yarmolinskaya@gmail.com, eLibrary SPIN: 3686-3605, Researcher ID: P-2183-2014, Scopus Author ID: 7801562649,
https://orcid.org/0000-0002-6551-4147
Igor Yu. Kogan, Dr. Med. Sci., Professor, Corresponding Member of the Russian Academy of Sciences, Director, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, 199034, Russia, St. Petersburg, Mendeleyevskaya Line, 3, ikogan@mail.ru, eLibrary SPIN: 6572-6450, Scopus Author ID: 56895765600,
Researcher ID: P-4357-2017, https://orcid.org/0000-0002-7351-6900
Corresponding author: Maria S. Dolgikh, mariadolgikh1@gmail.com



