Синдром поликистозных яичников (СПКЯ) – наиболее распространенная эндокринопатия женщин репродуктивного возраста, его частота достигает 10–15% [1, 2]. СПКЯ характеризуется комплексом патофизиологических нарушений, основными среди них являются гиперандрогения (ГА) яичникового генеза, хроническая ановуляция и метаболическая дисфункция.
Согласно Роттердамским критериям (2003), диагноз СПКЯ устанавливается при наличии как минимум двух из трех ключевых симптомов: ГА, олигоменореи, поликистозной трансформации по данным ультразвукового исследования. В зависимости от комбинации клинико-лабораторных признаков выделяют четыре репродуктивных фенотипа СПКЯ: классический фенотип А (ГА+ОМ+ПКЯ) и 3 неполных фенотипа (В – неовуляторный, ГА+ОМ; С – овуляторный – ГА+ПКЯ; Д – неандрогенный – ПКЯ+ОМ). Среди них доминирует классический фенотип А, его частота составляет 48,4–52,9% [1, 2]. Гетерогенность клинических проявлений вызывает трудности в диагностике неполных фенотипов синдрома и обосновывает целесообразность оптимизации диагностических критериев. В качестве наиболее перспективного маркера СПКЯ в настоящее время рассматривают антимюллеров гормон (АМГ) – гликопротеин, член семейства трансформирующего фактора роста β. Продукция АМГ зависит от стадии развития фолликула. Экспрессия АМГ отсутствует в примордиальных фолликулах, обнаруживается в клетках гра n нулезы преантральных фолликулов, достигает пика в малых антральных фолликулах и прекращается в больших антральных фолликулах. АМГ участвует в регуляции фолликулогенеза как на ФСГ-независимой стадии путем подавления рекрутинга примордиальных фолликулов в преантральные, так и на ФСГ-зависимой стадии – подавляется селекция и созревание доминантного фолликула за счет снижения чувствительности к ФСГ [3]. АМГ ингибирует также ФСГ-зависимую экспрессию ароматазы в клетках гранулезы, снижает конверсию тестостерона в эстрадиол [4]. Поскольку АМГ продуцируется только клетками гранулезы, его концентрация в сыворотке крови находится в прямой зависимости от числа преантральных и малых антральных фолликулов. В связи с этим уровень АМГ используют как предиктор ответа на стимуляцию яичников, как маркер старения и ятрогенного поражения яичников [3, 5, 6].
 В начале 2000-х годов были опубликованы результаты первых исследований о повышении уровня АМГ при СПКЯ. По данным литературы, уровень АМГ в сыворотке крови женщин с СПКЯ в 2–4 раза превышает таковой у здоровых женщин репродуктивного возраста [7–9]. Предполагают, что продукция АМГ повышается не только за счет большого числа преантральных и малых антральных фолликулов, но также за счет усиления экспрессии гормона клетками гранулезы [10]. Данные об уровне АМГ при различных фенотипах СПКЯ достаточно противоречивы. Результаты большинства исследований указывают на более высокий уровень АМГ у женщин с классическим фенотипом А. Однако результаты других исследований свидетельствуют о сопоставимом среднем уровне АМГ при андрогенных и неандрогенном фенотипах СПКЯ [11–13].
В начале 2000-х годов были опубликованы результаты первых исследований о повышении уровня АМГ при СПКЯ. По данным литературы, уровень АМГ в сыворотке крови женщин с СПКЯ в 2–4 раза превышает таковой у здоровых женщин репродуктивного возраста [7–9]. Предполагают, что продукция АМГ повышается не только за счет большого числа преантральных и малых антральных фолликулов, но также за счет усиления экспрессии гормона клетками гранулезы [10]. Данные об уровне АМГ при различных фенотипах СПКЯ достаточно противоречивы. Результаты большинства исследований указывают на более высокий уровень АМГ у женщин с классическим фенотипом А. Однако результаты других исследований свидетельствуют о сопоставимом среднем уровне АМГ при андрогенных и неандрогенном фенотипах СПКЯ [11–13].
В связи с существующими методологическими проблемами до настоящего времени нет единого мнения относительно универсального диагностического порога уровня АМГ, который служил бы предиктором овариальной дисфункции при СПКЯ. Ввиду отсутствия стандартизации методов определения АМГ Общество гиперандрогенных состояний и СПКЯ (2014) рекомендует использовать собственные пороговые значения, при условии, что при их определении отбор контрольной популяции будет проводиться согласно строгим критериям включения и исключения [14]. Эта рекомендация послужила поводом для планирования данного исследования.
Цель исследования: оценить значение АМГ для диагностики СПКЯ и возможные особенности его секреции при различных фенотипах синдрома.
Материал и методы исследования
Исследование, одобренное этическим комитетом, проводилось на базе отделения гинекологической эндокринологии и научно-диагностической лаборатории ФГБУ НЦАГиП им. В.И. Кулакова с января 2014 по апрель 2016 года. Критериями включения были: возраст женщин от 18 до 35 лет, наличие СПКЯ (по Роттердамским критериям 2003 г.), отсутствие сопутствующей эндокринной и некомпенсированной экстрагенитальной патологии, а также гормональной терапии в течение не менее трех месяцев до вступления в исследование.
В исследование включены 502 женщины: 250 – с СПКЯ и 252 – соматически здоровые женщины без нарушения функции репродуктивной системы и ГА, которые составили группу контроля. Пациентки были сопоставимы по возрасту и индексу массы тела (ИМТ) (группа СПКЯ – средний возраст 25,4±4,2 года, средний ИМТ – 24,2±5,6 кг/м2; группа контроля средний возраст 25,2±3,8 года, средний ИМТ – 24,2±5,6 кг/м2, p>0,05). Избыточная масса тела выявлена у 41 (16,4%) пациентки, ожирение – у 29 (11,6%). Признаки гирсутизма диагностированы у 164 (65,6%) больных. Из 95 женщин, заинтересованных в наступлении беременности, 85 (89,5%) предъявляли жалобы на бесплодие, средняя длительность которого составила 2,7±0,9 года. Нарушения менструального цикла по типу олигоменореи имели 200 (80,0%) больных, по типу первичной или вторичной аменореи – 49 (19,6%).
На основании результатов клинико-лабораторного обследования проведена стратификация пациенток по фенотипам и разделение на подгруппы. Фенотип А имели 145 (58%) женщин, фенотип В – 26 (10,4%), фенотип С – 16 женщин (6,4%), фенотип Д – 63 женщины (25,2%). Для женщин с фенотипом А характерны более длительные задержки менструаций по сравнению с пациентками, имеющими фенотипы В, С и Д (p<0,01). Так, у 87 (60%) пациенток с фенотипом А интервалы между менструациями были более 3 месяцев, при фенотипах В, С и Д этот показатель составил 38,4, 18,8 и 42,9% соответственно.
Помимо клинико-анамнестического обследования всем пациенткам на 5–7-й день менструального цикла проведено ультразвуковое исследование на аппарате 2000 Toshiba SSA-240 (Япония) трансвагинальным конвексным датчиком частотой 7,5 Мгц, с определением количества фолликулов и объема яичников.
В обеих группа произведено определение уровня АМГ в сыворотке крови методом ELISA с использованием тест-систем AMH GenII ELISA (Beckman Coulter, USA).
Для диагностики СПКЯ, выделения фенотипов и дифференциальной диагностики с другими эндокринопатиями на 2–3-й день собственного или индуцированного приемом гестагенов менструального цикла проводилось исследование гормонального профиля (ЛГ, ФСГ, общего и свободного тестостерона, андростендиона, глобулина, связывающего половые стероиды, пролактина, ТТГ) иммунохемилюминесцентным методом на автоматическом анализаторе Immulite 2000 (Siemens, USA).
Для статистического анализа материала использовалась программа SPSS (IBM Statistical Package for the Social Sciences, 21-я версия). Все данные представлены как средние ± стандартное отклонение. Сравнение проводилось с помощью U-теста Манна–Уитни, метод Спирмена был использован для выявления корреляций. Cтатистически значимыми считали результаты при достижении уровня ошибки p<0,05. Для расчета порогового уровня АМГ, специфичности и чувствительности метода проведен ROC-анализ.
Результаты исследования
Согласно полученным данным уровень АМГ в сыворотке крови здоровых женщин репродуктивного возраста варьировал от 1,0 нг/мл до 10,9 нг/мл и в среднем составил 3,3±1,9 нг/мл. В группе женщин той же возрастной категории с СПКЯ уровень АМГ колебался от 1,9 нг/мл до 66,4 нг/мл, в среднем составил 15,8±10,2 нг/мл, что превышало средний уровень АМГ у женщин группы контроля в 4,8 раза (р<0,05).
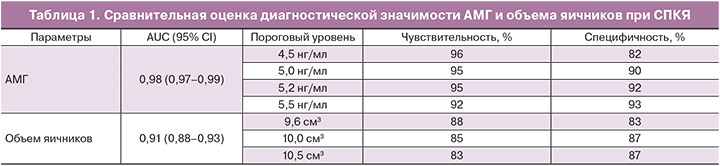
С целью определения нормативных значений АМГ и последующей оценки его информативности в диагностике СПКЯ был проведен расчет чувствительности и специфичности метода с использованием ROC-анализа. Площадь под кривой (AUC) для АМГ составила 0,98 (чувствительность и специфичность метода 95% и 92%, доверительный интервал, CI 95%, рис. 1).
Как видно из данных, представленных в табл. 1, наиболее высокая чувствительность и специфичность метода имела место при уровне АМГ 5,2 нг/мл, при более низких значениях АМГ снижалась специфичность, при более высоких – чувствительность.

Одним из ключевых диагностических критериев СПКЯ является объем яичников, в связи с этим была проведена оценка его диагностической значимости в сравнении с АМГ. При объеме яичников 10,0 см3, установленном в качестве порогового значения по Роттердамским критериям, площадь под кривой составила 0,91 (чувствительность и специфичность метода 85% и 87%, доверительный интервал, CI 95%). Результаты ROC-анализа, представленные на рис. 1 и в табл. 1, наглядно демонстрируют преимущества АМГ в диагностике СПКЯ.
Нормальные значения уровня АМГ в сыворотке крови (менее 5,2 нг/мл) были определены лишь у 11 (4,4%) пациенток с СПКЯ. У 72 (28,8%) пациенток уровень АМГ варьировал от 5,2 до 10,0 нг/мл, у 59 (23,6%) – от 10,1 до 15,0 нг/мл, у 49 (19,6%) – от 15,1 до 20,0 нг/мл и у 59 (23,6%) – превышал 20,0 нг/мл.
В последующем был проведен анализ уровней АМГ в основной и контрольной группе по возрастным категориям: до 20 лет, 21–25 лет, 26–30 лет, 31–35 лет (табл. 2). В контрольной группе средний уровень АМГ у женщин до 20 лет составил 5,2 нг/мл, его значения превышали пороговые у 15 из 44 (34%) женщин. В возрастных группах 21–25 лет и 26–30 лет средние уровни АМГ были достоверно более низкими (р<0,05), частота превышения пороговых значений снизилась до 8,7 и 2,4% соответственно. В возрастной категории 31–35 лет средний уровень АМГ был в 2 раза ниже, чем у женщин до 20 лет, его значения ни в одном случае не превышали пороговые. В контрольной группе выявлена отрицательная корреляция между возрастом пациенток и АМГ (r=-0,467, p<0,05), подтверждающая полученные закономерности.
При СПКЯ существенных различий по возрастным группам не было найдено (p>0,05) (табл. 2). Не установлено также корреляционной зависимости между АМГ и возрастом (r=0,054, р>0,05). Хотя в возрастной категории 30–35 лет в 10,8% случаев уровень АМГ был ниже порогового уровня (5,2 нг/мл).
В основной группе корреляционный анализ не позволил установить зависимости уровня АМГ с ИМТ с длительностью задержек менструаций (р>0,05). Выявлена прямая зависимость с объемом яичников (p<0,01), с уровнем ЛГ (r=0,196, р<0,05), общего тестостерона (r=0,196, р<0,05), андростендиона (r=0,219, р<0,05).
В рамках настоящего исследования была проведена сравнительная оценка уровня АМГ при различных фенотипах СПКЯ. Средний уровень АМГ был наиболее высоким в сыворотке крови пациенток с фенотипом А (17,9±11,6 нг/мл). Существенных различий в среднем уровне АМГ среди фенотипов В, С и Д, который составил соответственно 11,1±5,6 нг/мл, 14,2±10,6 нг/мл и 12,6±5,7 нг/мл, не было выявлено. Однако определены достоверные различия фенотипа А с фенотипами В и Д (p<0,05). Из 11 больных с СПКЯ, имеющих нормальные показатели АМГ, 3 (2,1%) принадлежали к фенотипу А, 2 (7,7%) – к фенотипу В, 1 (7,9%) – к фенотипу С и 5 (6,2%) – к фенотипу Д.

Результаты ROC-анализа свидетельствуют о высокой диагностической значимости АМГ при всех формах СПКЯ. Площадь под кривой при фенотипе А составила 0,97 (чувствительность и специфичность 97% и 92% соответственно, CI 95%); при фенотипе В – AUC=0,96, (чувствительность 92% и специфичность 92%, CI 95%), фенотипе Д – AUC=0,97, чувствительность и специфичность по 92%, CI 95%, рис. 2).
Произвести расчеты для овуляторного фенотипа С не представилось возможным в связи с малым числом больных в этой подгруппе. Достоверных различий в среднем объеме яичников между фенотипами А (17,2±6,0 см3), С (13,0±4,4 см3) и Д (16,1±5,1 см3) не установлено, при фенотипе В этот показатель был закономерно более низким (8,5±1,9 см3). Как видно из данных, представленных на рис. 2, определение уровня АМГ при фенотипах А и Д имеет более высокую диагностическую значимость по сравнению с объемом яичников. При фенотипе В сравнительный анализ не проводился в связи с отсутствием ультразвуковых признаков поликистозных яичников у пациенток этой категории.
Обсуждение
Фенотипическая вариабельность СПКЯ существенно затрудняет его диагностику. Традиционно используемые Роттердамские критерии СПКЯ в настоящее время нельзя считать абсолютно надежными ввиду недостаточной чувствительности и специфичности каждого признака, взятого в отдельности, а также отсутствия четких эхографических критериев поликистозной морфологии яичников и методологических проблем в оценке уровней андрогенов. Особые трудности возникают при постановке диагноза у больных с олигоменорей, ассоциированной с поликистозной морфологией яичников и отсутствием ГА. Такие больные могут быть отнесены как к СПКЯ, так и к гипоталамической ановуляции, подразумевающей иные подходы к терапии [15]. Трудности в диагностике делают актуальным поиск новых диагностических критериев СПКЯ.
С момента публикации первых исследований о высоких уровнях АМГ у больных с СПКЯ научный интерес к АМГ как к возможному диагностическому критерию СПКЯ неуклонно растет. Несмотря на более чем десятилетнюю историю изучения роли АМГ при СПКЯ, на сегодняшний день нет не только единых рекомендаций о диагностическом пороговом уровне АМГ, но и общепринятых нормативных значений у здоровых женщин. Согласно полученным данным, средний уровень АМГ при СПКЯ составил 15,8±10,2 нг/мл, что в 4,8 раза превышало значения аналогичного показателя у здоровых женщин репродуктивного возраста (3,3±1,9 нг/мл).
По данным ROC-анализа, проведенного на выборке из 502 женщин, уровень АМГ, равный 5,2 нг/мл, показал наиболее высокую чувствительность и специфичность (95% и 92% соответственно, CI 95%). Это позволило рассматривать его в качестве порогового значения при диагностике СПКЯ. Полученные данные оказались схожими с результатами, приведенными в работах Dewailly (2011) и H. Li (2011), где пороговыми уровнями были значения АМГ, равные 4,9 нг/мл и 5,9 нг/мл
[16, 17]. Хотя по данным литературы значения уровня АМГ, используемые в качестве пороговых при подозрении на СПКЯ, варьируют в широких пределах – от 2,8 нг/мл до 8,4 нг/мл [18, 19]. Разноречивость приведенных данных можно объяснить использованием разных тест-систем и критериев отбора пациенток для исследования. Так, например, Rosenfield и соавт. пишут о том, что пороговый уровень АМГ при использовании критериев National Institute of Health (NIH) составляет 6,2 нг/мл, однако для дифференциальной диагностики пациенток со «стертыми» формами СПКЯ от здоровых женщин с поликистозной морфологией яичников, по их мнению, целесообразно использовать порог отсечки 10,7 нг/мл [19].
Результаты ROC-анализа продемонстрировали также преимущества определения уровня АМГ в сыворотке крови по сравнению с оценкой объема яичников при диагностике СПКЯ. Так, площадь под кривой (AUC) для АМГ составила 0,97 при чувствительности и специфичности метода 95% и 92%, для объема яичников – 0,91 при чувствительности и специфичности метода 85% и 87%. Полученные данные подтверждают современную позицию о целесообразности совершенствования эхографических критериев СПКЯ, поскольку измерение объема яичников и подсчет числа антральных фолликулов в срезе яичника не являются высокоинформативными показателями. Современные ультразвуковые технологии позволяют производить подсчет числа фолликулов в объеме яичника, что по данным ряда исследований более информативно [20–22]. Однако широкое применение данной методики ограничено необходимостью использования высокочастотных датчиков с длиной волны более 8 мГц, отсутствием стандартизации методов подсчета фолликулов и общепринятых нормативных значений.
В группе женщин с СПКЯ достоверной взаимосвязи между уровнем АМГ и возрастом не установлено, несмотря на то что у каждой десятой пациентки в возрасте от 30 до 35 лет уровень АМГ был ниже пороговых значений. Возможно, это связано с более медленным старением яичников, которое, по мнению профессора В. Fauser, наблюдается у женщин с нормогонадотропным ановуляторным бесплодием [15]. Иная ситуация прослеживалась в группе здоровых женщин, где средний уровень АМГ в возрасте 30–35 лет был в 2 раза ниже, чем в возрасте до 20 лет. Следует отметить, что в группе до 20 лет значения АМГ превышали установленный пороговый уровень у каждой третьей женщины. В группе 26–30 лет подобная ситуация наблюдалась лишь у 2,4% женщин, после 30 лет ни в одном случае значений АМГ выше пороговых не было выявлено. Вопрос о целесообразности градации порогового уровня АМГ с учетом возрастных особенностей женщин до настоящего времени остается спорным. Однако, исходя из полученных данных, такой подход представляется достаточно перспективным [18, 23–26]. Высокий уровень АМГ, вероятно, можно рассматривать в качестве основного диагностического критерия СПКЯ у пациенток лишь старше 20 лет. В более молодом возрасте следует ориентироваться на результаты комплексного обследования, принимать во внимание наличие ГА, олигоменореи, поликистозной трансформации яичников.
Важным с клинической точки зрения является вопрос об уровнях АМГ при разных фенотипах СПКЯ. Данные литературы на эту тему немногочисленные и достаточно противоречивые. По выводам Piouka и соавт., уровень АМГ прогрессивно снижается от фенотипа А к фенотипу Д [11], по сведениям S. Sahmay и D. Romualdi, наиболее высокие уровни АМГ были обнаружены при фенотипах А и Д [12, 13]. По результатам проведенного исследования более высокий уровень АМГ также диагностируется при классическом фенотипе А. Его средние значения составляют 17,9±11,6 нг/мл и достоверно превышают аналогичные при фенотипе В и Д. Отсутствие статистически значимой разницы с фенотипом С можно объяснить малочисленностью подгруппы больных с овуляторной формой синдрома в связи с ее низкой представленностью в популяции. Хотелось бы обратить внимание на трехкратное повышение среднего уровня АМГ при фенотипе В, несмотря на характерные для него нормальные размеры яичников. Это может быть связано с повышением функциональной активности гранулезных клеток, описанной при СПКЯ [27, 28]. Результаты ROC-анализа указывают на высокий диагностический потенциал метода вне зависимости от фенотипа синдрома. Таким образом, можно полагать, что введение АМГ в число диагностических критериев поможет решить проблему диагностики неполных форм СПКЯ, характеризующихся отсутствием ГА или овуляторной дисфункции, а также, вероятно, может стать критерием выбора оптимальной тактики терапии [5].
Заключение
Уровень АМГ в сыворотке крови является более специфичным и чувствительным диагностическим критерием СПКЯ, чем объем яичников. Независимо от фенотипа синдрома, повышение уровня АМГ в сыворотке крови более 5,2 нг/мл можно рассматривать как диагностический маркер СПКЯ. Полученные результаты дают основание сделать заключение, что определение уровня АМГ целесообразно внести в перечень необходимых гормональных обследований при подозрении на СПКЯ. Для определения дифференцированных пороговых значений АМГ с учетом возраста пациенток необходимы дальнейшие исследования.



