Регулярный менструальный цикл свидетельствует о скоординированной работе всех уровней репродуктивной системы, является показателем фертильности, а также служит признаком нормального функционирования большинства других эндокринных органов женского организма. С другой стороны, нарушение ритма менструаций может быть следствием не только гинекологических заболеваний, но и симптомом целого ряда эндокринопатий, которые нуждаются в распознавании и дифференцированном подходе к лечению.
К самым частым вариантам нарушений менструального цикла относят олигоменорею, под которой понимают увеличение продолжительности цикла свыше 35 дней (или менее 9 циклов в год), и аменорею – отсутствие менструаций более 3 месяцев у женщин, которые ранее имели регулярные менструальные циклы, или более 6 месяцев у женщин с нерегулярными менструациями [1, 2]. Во взрослой гинекологической практике наиболее распространены вторичные формы олиго- и аменореи, частота встречаемости которых достигает, по некоторым данным, 4% [3, 4].
Порой неудавшиеся попытки поиска причин олиго- или аменореи заканчиваются установлением диагнозов в виде кодов по МКБ, таких как «дисфункция яичников», «вторичная аменорея», «отсутствие менструаций, скудные и редкие менструации», или диагнозов с традиционными формулировками – «гипоменструальный синдром», «нарушение менструального цикла по типу олиго/аменореи», которые лишь указывают на факт нарушения цикла, но не являются отдельной нозологией и, вероятно, имеют право лишь на временное существование до момента верификации окончательного клинического диагноза.
Несмотря на то что в распоряжении современных врачей имеются протоколы профессиональных международных организаций и сообществ, а также клинические рекомендации, разработанные экспертами Российского общества акушеров-гинекологов [1, 2, 4–10], в реальной клинической практике нередко можно наблюдать «шаблонный» подход к пациенткам с нарушениями менструального цикла, который заключается в назначении комбинированных гормональных контрацептивов (КГК). Безусловно, если причиной нерегулярных менструаций является синдром поликистозных яичников (СПКЯ), и женщина заинтересована в надежной контрацепции, то подобная терапевтическая стратегия может считаться обоснованной [5, 6]. В иных клинических ситуациях КГК, к сожалению, могут лишь завуалировать некоторые заболевания (например, преждевременную недостаточность яичников (ПНЯ) [8, 9] или функциональную гипоталамическую аменорею (ФГА)) [7], и под «маской» регулярных менструальноподобных реакций на фоне приема КГК неидентифицированные эндокринопатии могут прогрессировать до серьезных и необратимых последствий, проявляясь, например, малотравматичными переломами или полным истощением овуляторного резерва у женщины с нереализованными репродуктивными планами.
В этой связи план лечебных мероприятий, в том числе с применением КГК, у женщин с нарушениями менструального цикла целесообразно разрабатывать после установления диагноза, симптомом которого является олиго- или аменорея, а также с учетом репродуктивных планов и предпочтений пациентки по режимам терапии.
При этом однократное нарушение ритма менструаций может не требовать тщательной клинической оценки и углубленного обследования (после исключения беременности), поскольку спорадические и редкие сбои в функционировании гипоталамо-гипофизарно-яичниковой (ГГЯ) оси, как правило, не имеют серьезных последствий.
При олиго- и аменорее огромное значение имеет тщательный сбор дополнительных жалоб и анамнестических данных, позволяющий значительно сузить круг предполагаемых причин [1, 2]. Прежде всего необходимо выяснить, какие лекарственные препараты, способные вызвать аменорею, принимает пациентка (нейролептики, селективные ингибиторы обратного захвата серотонина, метоклопрамид, внутриматочная левоноргестрел-содержащая система, КГК в пролонгированном режиме и другие). Наличие у женщины в анамнезе или в момент обращения гирсутизма, акне, алопеции направят диагностический вектор в сторону поиска эндокринопатий, сопровождающихся избыточной продукцией андрогенов, таких как СПКЯ, неклассическая форма врожденной гиперплазии коры надпочечников (нВГКН) или андроген-продуцирующие опухоли, а галакторея – в сторону гиперпролактинемии. Постоянная или преходящая головная боль, выпадение полей зрения и другая очаговая неврологическая симптоматика в сочетании с нарушениями менструального цикла потребует исключения опухолей центральной нервной системы. Аносмия или гипоосмия у женщин с отсутствием менструаций может быть признаком наследственного гипогонадотропного гипогонадизма, связанного с врожденной недостаточной секрецией гонадотропин-рилизинг-гормона (ГнРГ). Симптомы эстрогенодефицита (приливы, нарушения сна, депрессия, вагинальная атрофия и связанная с ней диспареуния) на фоне олиго- и аменореи у женщины до 40 лет с высокой долей вероятности будут указывать на ПНЯ, а перенесенный стресс, изменения веса, связанные с диетой (в том числе с расстройством пищевого поведения) или занятиями спортом, – на ФГА. Внутриматочные манипуляции и операции, особенно осложнившиеся острым воспалительным процессом, могут быть причиной развития внутриматочных синехий или синдрома Ашермана [1, 2, 5–10].
Объективный осмотр женщин с вторичной олиго- и аменореей позволит выявить стрии, acanthosis nigricans, галакторею, вульвовагинальную атрофию, в ряде случаев, если женщина не пользуется косметическими способами ухода, – признаки гиперандрогении (гирсутизм, акне). У девушек и молодых женщин, страдающих расстройствами пищевого поведения (РПП) по типу нервной булимии, акушеры-гинекологи могут обнаружить отечность околоушных слюнных желез, эрозии зубной эмали, формирующиеся вследствие многократно индуцированной рвоты [1, 2, 4, 7].
Лабораторная диагностика вторичной олиго- и аменореи может осуществляться по алгоритму (рисунок), в основе которого лежит определение сывороточных концентраций пролактина, тиреотропного гормона (ТТГ), гонадотропных гормонов – фолликулостимулирующего гормона (ФСГ) и лютеинизирующего гормона (ЛГ) и эстрадиола (E2). Первоочередной задачей является исключение физиологических форм аменореи, связанных с беременностью и лактацией. Для подтверждения беременности необходимо определить уровень хорионического гонадотропина человека (ХГЧ) в сыворотке крови [1, 2, 4].
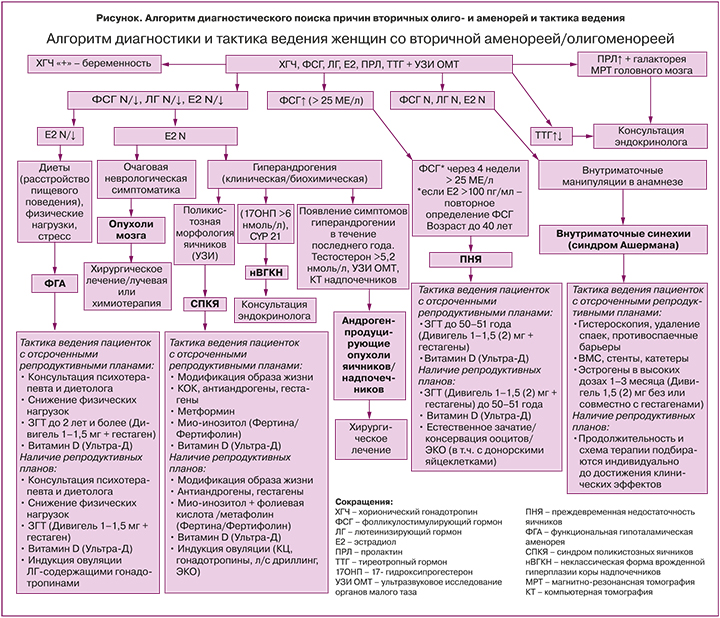
На фоне лактации требования, предъявляемые к нормальному менструальному циклу, безусловно, теряют свою актуальность. По этой причине лактационная аменорея, как и беременность, могут явиться причиной отсрочки распознавания ПНЯ.
Повышение плазменной концентрации пролактина, помимо физиологической и индуцированной лекарственными препаратами гиперпролактинемии, позволит верифицировать функциональную гиперпролактинемию или аденому гипофиза, которые требуют совместного динамического наблюдения с эндокринологом [11]. Лечение пациенток с олиго- и аменореей, обусловленной гипо- или гиперфункцией щитовидной железы, также находится в компетенции врача-эндокринолога [1]. У части пациенток с гипотиреозом наблюдается повышенная секреция пролактина, которая нормализуется после коррекции гипотиреоза. Хотя патофизиологические механизмы развития гиперпролактинемии при гипотиреозе неизвестны, предполагается, что тиреотропин-рилизинг-гормон стимулирует выработку пролактина гипофизом [12, 13].
После исключения преимущественно эндокринологических заболеваний и беременности/лактации по сывороточным уровням пролактина, ТТГ и ХГЧ «управление» ходом диагностического поиска будет осуществляться уровнями гонадотропных гормонов ФСГ и ЛГ и Е2 [2]. В зависимости от концентраций ФСГ, ЛГ и Е2 в сыворотке крови в сочетании с жалобами, анамнезом и данными объективного осмотра, лабораторного и инструментального обследований поиск причин аменореи будет происходить в направлении эндокринопатий, проявляющихся гипер-, нормо- и гипогонадотропными олиго- или аменореей соответственно. К таким нозологиям, симптомом которых являются олиго- и аменорея, относят наиболее часто встречающиеся в гинекологической практике ПНЯ, СПКЯ и ФГА (рисунок).
Преждевременная недостаточность яичников
При обнаружении повышенных значений ФСГ у женщин в возрасте до 40 лет с аменореей длительностью более 4 месяцев обязательно требуется повторное определение ФСГ через 4–6 недель для подтверждения диагноза ПНЯ [1, 8, 9], на долю которой приходится 10% в структуре причин вторичной аменореи [14]. ФСГ необходимо исследовать на 2–3-й день менструального цикла при сохраненной менструальной функции или в любой день на фоне аменореи [4, 5]. Несмотря на то что рекомендации различных профессиональных организаций несколько отличаются, диагностически значимым для ПНЯ уровнем ФСГ в Европе и в РФ считается его увеличение свыше 25 МЕ/л [1, 9]. Необходимо принимать во внимание, что спонтанные овуляции при ПНЯ, сопровождающиеся нарастанием сывороточной концентрации Е2, могут по механизму «отрицательной обратной связи» снижать уровень ФСГ, что является своеобразной диагностической «ловушкой». В этой связи при наличии менструаций у женщин с ПНЯ целесообразно одновременное определение E2, превышение концентрации которого свыше 100 пг/мл потребует повторного определения ФСГ [9]. Подтвержденный синдром Шерешевского–Тернера у женщин с нарушениями менструального цикла, скорее всего, указывает на манифестацию ПНЯ, свойственную этому генетическому заболеванию [1, 8, 9, 15]. Важно отметить, что наличие аменореи в момент обращения не является обязательным требованием для постановки диагноза ПНЯ, поскольку при ПНЯ возможно спонтанное восстановление активности яичников и менструальной функции даже по истечении длительного периода после появления первых симптомов заболевания [8, 9, 15]. По этой же причине считается необязательным присутствие постоянных жалоб на приливы, сухость во влагалище и других симптомов эстрогенодефицита (они могут быть эпизодическими). Но наличие перечисленных признаков с большей долей вероятности указывает на ПНЯ, поскольку симптомы дефицита эстрогенов нехарактерны для других эндокринопатий, сопровождающихся нарушением менструального цикла. В периоды спонтанного восстановления эстрогенпродуцирующей функции яичников у женщин с ПНЯ прогестероновая проба часто сопровождается кровотечением отмены, что снижает диагностическую ценность данного теста на эстрогенную насыщенность организма при данном заболевании [15, 16]. Вероятно, из-за непостоянства регистрируемых клинических и лабораторных признаков вследствие волнообразного течения заболевания у 25% женщин диагноз ПНЯ устанавливают с 5-летней отсрочкой после появления первых эпизодов нарушения менструального цикла [17]. В этой связи женщины в возрасте до 40 лет с нарушениями менструального цикла в течение трех или более месяцев подряд (особенно пациентки с нереализованными репродуктивными задачами) должны быть тщательно обследованы для исключения ПНЯ. Кроме того, назначение КГК с целью «нормализации менструального цикла», беременность, лактация, гиперпролактинемия могут маскировать развитие ПНЯ, что способствует существенному снижению шансов на установление диагноза на раннем этапе развития (так называемая «оккультная стадия») и, как следствие, уменьшению вероятности рождения детей с использованием собственных яйцеклеток [8, 15].
Вне зависимости от репродуктивных планов пациенткам с ПНЯ при отсутствии абсолютных противопоказаний к терапии эстрогенами рекомендована заместительная гормональная терапия (ЗГТ) эстрогенами и прогестинами до возраста естественной менопаузы [1, 8, 9]. Важно отметить, что показаниями для назначения ЗГТ при ПНЯ не являются восстановление овуляций, нормального ритма менструаций или наступление беременности. Результаты исследования показали, что использование физиологических доз эстрогенов при ПНЯ, по-видимому, не увеличивает частоту спонтанной овуляции, не влияет на средний объем яичников, количество или размер фолликулов [1, 8, 9, 15, 16]. Эстрогены при раннем снижении эстрогенпродуцирующей активности яичников необходимы для сокращения у молодых женщин рисков патологических состояний, связанных с дефицитом эстрогенов, таких как приливы, нарушение сна, депрессия, вагинальная сухость, а также остеопороз, сердечно-сосудистые заболевания, когнитивные нарушения [1, 8, 9, 15]. ЗГТ при ПНЯ также требуется для поддержания/восстановления сексуального здоровья и качества жизни [8, 9]. Необходимо корректно донести до пациентки с ПНЯ информацию о том, что между ПНЯ и физиологической менопаузой нельзя поставить знак равенства в отношении риска/пользы гормональной терапии эстрогенами. Распространенным заблуждением является экстраполирование данных о рисках, связанных с назначением менопаузальной гормональной терапии, полученных в исследованиях с участием женщин в постменопаузе, на молодых пациенток с ПНЯ [15]. Учитывая вышесказанное, отсутствует необходимость длительного и избыточного обследования как при первичном назначении ЗГТ, так и при дальнейшем наблюдении [1, 8, 9, 15].
ЗГТ для женщин репродуктивного возраста с ПНЯ должна максимально приближенно имитировать нормальную функцию яичников, что наиболее эффективно достигается назначением комбинации биоидентичного эстрадиола (17β-эстрадиол) и гестагенов в различных режимах: циклическом или непрерывном [1, 8, 9, 15]. Женщины с ПНЯ могут отдавать предпочтение непрерывному режиму ЗГТ без менструальноподобных кровотечений по прошествии одного года аменореи или продолжать циклический режим до среднего возраста физиологической менопаузы [15]. В случае заинтересованности пациентки с ПНЯ в беременности, вероятность наступления которой в естественном цикле при этом заболевании составляет лишь 1–10%, рекомендуется циклический режим ЗГТ [15, 18]. В соответствии с рекомендациями Международного общества по менопаузе (IMS, 2020) [8] и Российского общества акушеров-гинекологов (РОАГ, 2021) [1] препаратами выбора являются трансдермальные формы эстрогенов, например, в виде пластыря или геля 17β-эстрадиола. Использование последних обеспечивает более физиологичное восполнение дефицита эстрогенов с созданием стабильной равновесной концентрации эстрадиола в сыворотке через постепенное высвобождение из подкожных жировых депо с минимальными рисками венозной тромбоэмболии и практически полным отсутствием влияния на гепатобилиарную систему [19–21]. Наличие первичного метаболизма в печени при назначении более привычных пероральных форм препаратов, большая часть которых подвергается пресистемной элиминации, требует их назначения в больших дозах по сравнению с трансдермальными формами [21]. Для достижения поставленных целей в лечении ПНЯ требуется, по последним рекомендациям IMS и РОАГ, назначение относительно более высоких доз эстрогенов, чем считалось ранее: в частности, 3–4 мг перорального эстрогена в сутки (ранее – до 2 мг) [1, 8]. Для эстрогенов с высоким профилем безопасности, вводимых трансдермально, эффективные терапевтические дозы препаратов также были пересмотрены: в настоящее время к высоким дозам трансдермальных эстрогенов относят назначение трансдермального пластыря 75–100 мг, 0,06% геля эстрадиола в дозе 3 мг, а 0,1% геля эстрадиола – 2 мг [1, 8].
Интересен тот факт, что рекомендации по дозировкам трансдермальных гелей перекликаются с ранее проведенным метаанализом Североамериканского общества по менопаузе, в котором сравнивали эффективность и безопасность применения различных трансдермальных гелей с эстрадиолом у женщин с естественной менопаузой, страдающих приливами. Результаты исследования показали, что показатели эффективности препарата 17β-эстрадиола в виде 0,1% геля («Дивигель») в дозе 0,5 мг аналогичны таковым для 0,06% геля в более высоких дозах – 0,75 и 1,5 мг. Более того, использование 0,1% геля 17β-эстрадиола приводило к уменьшению выраженности приливов (частоты и интенсивности) ко 2–5-й неделе, а 0,06% геля – лишь через 6–9 недель [22].
К прогестинам первой линии выбора при использовании высоких (off lable) доз эстрогенов при ПНЯ относят микронизированный прогестерон в дозе 200 мг в день или дидрогестерон 20 мг, назначаемые в течение последних 12 дней менструального цикла при циклическом режиме и ежедневно – при непрерывном. Для женщин, нуждающихся в надежной контрацепции, можно рассмотреть применение левоноргестрел-содержащей внутриматочной системы [1, 8].
Несмотря на то что КГК способны нивелировать вазомоторные симптомы, а также защищать от нежелательной беременности женщин с ПНЯ, их применение нецелесообразно. Указанное обусловлено тем, что КГК не снижают риски остеопороза, сердечно-сосудистых заболеваний и деменции. Прием КГК с 7-дневными безгормональными интервалами не обеспечивает женщине с ПНЯ ежедневного восполнения эстрогенов: суммарно до 3 месяцев в году пациентка, принимающая КГК, будет лишена эстрогенов [15]. Одно небольшое исследование показало, что ЗГТ с биоидентичными трансдермальными эстрогенами в низких дозах более эффективна для восстановления минеральной плотности костной ткани (МПК), чем синтетические эстрогены в более высоких дозах, входящие в состав КГК (этинилэстрадиол 30 мкг) [23].
В случае выявления ПНЯ необходимо провести деликатное консультирование об отсутствии в медицине надежных и доказанных методик и препаратов, способных восстановить нормальное функционирование яичников, тем самым увеличив вероятность естественного зачатия свыше 10% даже на фоне проводимой ЗГТ с эстрогенами и прогестинами [1, 8, 9]. Важно отметить, что циклическая ЗГТ не препятствует наступлению беременности и не относится к фармакологической группе «гормональные контрацептивные средства», так как даже в высоких дозах не блокирует спонтанные овуляции по механизму отрицательной обратной связи. При этом беременности, возникшие у женщин с ПНЯ в естественном цикле, в большинстве случаев протекают без осложнений и не нуждаются в поддержке эстрогенами. Рекомендуется незамедлительное направление пациенток с ПНЯ, страдающих бесплодием, к репродуктологу для проведения программы вспомогательных репродуктивных технологий (ВРТ) с донацией ооцитов (при невозможности получения собственных ооцитов) [1, 8 ,9].
К основным стратегическим ошибкам в диагностике и менеджменте ПНЯ, которые допускаются в реальной клинической практике, можно отнести позднее распознавание заболевания, назначение КГК в качестве первой линии терапии с целью «нормализации цикла», применение низких доз эстрогенов в составе ЗГТ короткими или прерывистыми курсами, а также уменьшение дозы эстрогенов в процессе лечения задолго до достижения возраста физиологической менопаузы, отсрочку в назначении ЗГТ вследствие избыточного обследования (как у женщин в пери- и постменопаузе для назначения менопаузальной гормональной терапии). Серьезным заблуждением клиницистов является надежда на восстановление активности яичников с помощью различных вмешательств с недоказанными эффектами, что обусловливает несвоевременное направление к репродуктологу женщин с ПНЯ, заинтересованных в беременности с собственными ооцитами.
Синдром Ашермана
При выявлении у женщины с отсутствием менструаций нормальных значений ФСГ и ЛГ и при наличии указаний на недавно перенесенные внутриматочные хирургические вмешательства, чаще всего послеабортные или послеродовые выскабливания полости матки, следует исключить внутриматочные синехии или синдром Ашермана (рисунок). Вторичная аменорея как следствие болезни Ашермана составляет около 7% [14]. Подозрение на внутриматочные синехии как причину аменореи может быть подтверждено пробой с эстрогеном и прогестероном, которая будет отрицательной, гистеросальпингографией или гистеросонографией, однако золотым диагностическим стандартом считается гистероскопия, при которой, в случае подтверждения синехий, выполняется резекция спаек (адгезиолизис) [10]. Рецидивы внутриматочных синехий после хирургического удаления спаек регистрируются у каждой третьей женщины с легкой и умеренной стадией заболевания, у двух женщин из трех – с тяжелой [10]. Существуют различные стратегии предотвращения повторного образования спаек, включающие введение в матку внутриматочной спирали, стента или катетера, использование гиалуроновой кислоты, послеоперационное гормональное лечение эстрогенами в сочетании или без прогестинов [10, 24]. В настоящее время неизвестны оптимальные режимы применения эстрогенов с указанием определенных доз и форм, длительности приема для профилактики рецидивов синехий, преимущества которых были бы доказаны в рандомизированных клинических исследованиях. Одно из немногих исследований, сравнивающее эффективность использования 2 и 6 мг пероральных эстрогенов после адгезиолизиса у 121 женщины с синдромом Ашермана, показало, что обе дозы значительно уменьшили образование синехий в полости матки, но отличий между группами выявлено не было [25]. В соответствии с протоколом РОАГ (2021) [1] после иссечения внутриматочных синехий рекомендованы послеоперационное применение пероральных форм эстрадиола валерата 4 мг/сут или трансдермальные формы эстрадиола в форме геля 2 мг/сут либо 50–100 мкг/сут в виде пластыря в течение 4 недель в комбинации с прогестагенами в дозе 200 мг/сут или дидрогестероном 20 мг/сут на срок не менее 10 дней с 16-го дня цикла.
Синдром поликистозных яичников
При нормальных концентрациях сывороточных ФСГ, ЛГ и Е2 у женщин с клиническими проявлениями гиперандрогении (акне, гирсутизм, алопеция) потребуется дифференциальная диагностика между тремя заболеваниями: СПКЯ, неклассической (с поздним началом) формой ВГКН и андроген-продуцирующими опухолями яичников или надпочечников (рисунок). Если на СПКЯ приходится до 30% всех вторичных аменорей, то доля нВГКН и андроген-продуцирующих опухолей в совокупности не превышает 1% [14].
Около 90% пациентов с ВГКН имеют дефицит 21-гидроксилазы, распространенность неклассической формы дефицита 21-гидроксилазы составляет 0,1–0,2% в мировой популяции. Для исключения у женщин с олиго/аменореей нВДКН необходимо измерить уровень 17-гидроксипрогестерона (предшественника кортизола, находящегося непосредственно над ферментативным блоком) в утренние часы в раннюю фолликулярную фазу при сохраненном менструальном цикле или в любой день на фоне аменореи. При концентрации гормона менее 6 нмоль/л (или менее 2 нг/мл) нВГКН практически не встречается. В случае значений 17-гидроксипрогестерона более 30 нмоль/л (или 10 нг/мл), диагноз нВГКН считается подтвержденным. При сывороточных концентрациях 17-гидроксипрогестерона в пределах так называемой «серой зоны» в диапазоне 6–30 нмоль/л (или 2–10 нг/мл), выявленных минимум при двукратном определении, необходимо проведение пробы с адренокортикотропным гормоном, которая отсутствует в РФ. Поэтому для подтверждения диагноза рекомендуется исследование на наличие мутаций в гене CYP-21. Лечение нВГКН находится в компетенции эндокринолога, подготовка к беременности также осуществляется при его участии [26].
К угрожающим здоровью и жизни гиперандрогенным состояниям относят андроген-продуцирующие опухоли яичников или надпочечников, которые выявляются по недавно возникшему (обычно менее одного года) прогрессирующему гирсутизму и симптомам вирилизации, включающим андрогенную алопецию, акне, клиторомегалию, увеличение мышечной массы и снижение тембра голоса, при повышении сывороточной концентрации тестостерона более 150 нг/дл (5,2 нмоль/л), при опухолях надпочечников – дегидроэпиандростерона сульфата (ДГЭА-с) более 700 мкг/дл (18,9 мкмоль/л) [27, 28].
Для уточнения локализации опухоли необходимо проведение трансвагинального ультразвукового исследования (УЗИ). В случае отсутствия УЗ-признаков опухоли яичника проводят компьютерную томографию надпочечников. Лечение опухолей хирургическое [27, 28].
После исключения иных причин гиперандрогении у женщин с олиго- и аменореей следует направить диагностический поиск в сторону подтверждения СПКЯ – одного из самых распространенных (10–13% женской популяции) генетически-обусловленных эндокринных заболеваний, «патогенетическим субстратом» которого, наряду с гиперандрогенией, являются инсулинорезистентность (ИР) и «компенсаторная гиперинсулинемия» даже у женщин с нормальным весом [1, 2, 5, 6]. Инсулин действует на клетки теки, усиливая продукцию андрогенов, преждевременно лютеинизирует клетки гранулезы и ингибирует выработку в печени глобулина, связывающего половые стероиды, еще более увеличивая концентрацию биодоступного тестостерона. Кроме того, инсулин функционирует как гонадотропин через свой родственный рецептор, что модулирует стероидогенез в яичниках, а тека-клетки при СПКЯ гиперчувствительны к стимулирующему действию инсулина как к триггеру секреции андрогенов [29]. СПКЯ относится к нормогонадотропным аменореям и не сопровождается дефицитом эстрогенов [5].
Несмотря на кажущуюся простоту диагностики СПКЯ на основании присутствия минимум двух из трех критериев (клиническая и/или биохимическая гиперандрогения, олиго- или ановуляция, поликистозная морфология яичников по УЗИ) [1, 5, 6], почти 50% опрошенных женщин вынуждены были посетить более трех гинекологов до постановки диагноза, а у трети пациенток отсрочка диагностики СПКЯ составляла два и более года [30].
Трудности в объективной оценке клинических проявлений гиперандрогении (гирсутизма по шкале Ферримана–Голлвея) обусловлены применением женщинами косметических процедур. В соответствии с клиническими протоколами [5], для исследования андрогенов рекомендуется жидкостная или газовая хроматография с масс-спектрометрией, которые ограниченно применяются в рутинной практике, что также приводит к гиподиагностике СПКЯ. Биохимическая гиперандрогения подтверждается повышением концентраций свободного тестостерона, расчетными показателями индекса свободных андрогенов и биодоступного тестостерона, определяемыми не ранее 8–12 недель после отмены КГК [5, 6]. В ряде случаев можно определять уровень андростендиона и ДГЭА-с, хотя клиническое значение данных тестов при СПКЯ окончательно не установлено [5].
УЗИ, необходимое для выявления «поликистозной трансформации яичников» (второго диагностического критерия СПКЯ), не следует использовать для диагностики данной эндокринопатии в первые 8 лет после менархе. В этой связи рекомендуется пересмотр диагноза у молодых женщин по прошествии 8 лет после начала менструаций, у которых в подростковом периоде обнаруживались все критерии СПКЯ. Если ранее «поликистозная морфология яичников» устанавливалась при обнаружении 12 и более фолликулов диаметром 2–9 мм, то при использовании высокочастотных УЗ-датчиков (>8 МГц) необходимо визуализировать 20 и более антральных фолликулов на одном яичнике и/или увеличение объема любого яичника ≥10 см3 (без желтых тел, кист, доминантных фолликулов) [1, 5, 6].
Несколько усложняет диагностику СПКЯ существование четырех фенотипов заболевания, формирующихся в зависимости от различных комбинаций трех диагностических критериев [5, 6]. Сомнения в правильности постановки диагноза часто возникают при «овуляторном» и «неандрогенном» клинических вариантах. Не исключено, что СПКЯ ускользает из поля зрения клинициста при «овуляторном» фенотипе, поскольку женщины с регулярным менструальным циклом реже испытывают проблемы с зачатием и чаще обращаются к дерматологу/косметологу для борьбы с клиническими проявлениями избытка андрогенов. А у половины пациентов с фенотипом СПКЯ без гиперандрогении (олигоаменорея и поликистозная морфология яичников) может быть диагностирована ФГА [31]. Хотя оценка уровня сывороточного антимюллерова гормона (АМГ), соотношения ЛГ/ФСГ может показаться полезной для верификации СПКЯ, для диагностических целей указанные тесты в настоящее время не применяются [1, 5, 6].
Принимая во внимание повышенные кардиометаболические риски, ассоциированные с СПКЯ, а также 2–7-кратное увеличение риска гиперплазии/рака эндометрия в перименопаузе на фоне продолжительной ановуляции в репродуктивном периоде необходимо [5], чтобы диагноз СПКЯ был установлен в максимально короткие сроки. Дополнительные исследования, такие как липидограмма, определение уровня глюкозы, гликированного гемоглобина, проведение перорального глюкозотолерантного теста, а также регулярный мониторинг артериального давления, изменений веса, окружности талии позволяют своевременно выявить метаболические нарушения при СПКЯ [5, 6].
Лечебные цели СПКЯ определяются фенотипом заболевания, репродуктивными планами и предпочтениями пациентки [1, 5, 6]. Можно выделить несколько задач при лечении пациенток с СПКЯ: устранение клинических проявлений гиперандрогении, коррекция инсулинорезистентности и/или ожирения, предотвращение аномальных (ановуляторных) маточных кровотечений, индукция овуляции при ановуляторном бесплодии, нивелирование депрессивно-тревожных расстройств и сексуальной дисфункции, в долгосрочной перспективе – профилактика гиперплазии и рака эндометрия, а также сахарного диабета, ишемической болезни сердца.
Учитывая, что у 50–75% женщин с СПКЯ, в том числе с нормальным индексом массы тела, присутствует инсулинорезистентность (ИР) [5], патогенетическое лечение СПКЯ рекомендовано начинать с модификации образа жизни, направленной на снижение веса через ограничение суточной калорийности питания и увеличение физической активности [32]. Показано, что восстановление овуляторных циклов у женщин с СПКЯ регистрируется уже при снижении массы тела на 5–10% [33]. При отсутствии эффекта от проводимых мероприятий пациентке предлагается фармакологическая коррекция ИР [5, 6]. В качестве наиболее исследованного препарата для этих целей используют инсулиносенситайзер метформин – бигуанид, улучшающий толерантность тканей к глюкозе, снижающий синтез глюкозы в печени, ограничивающий кишечную абсорбцию глюкозы, а также обладающий анорексигенным эффектом [5, 6, 34]. Несмотря на рекомендованное пошаговое (на 500 мг 1–2 раза в неделю) увеличение суточной дозы метформина до терапевтической дозы 2000 мг, а также использование пролонгированных форм препарата, частота нежелательных реакций со стороны желудочно-кишечного тракта, таких как диарея, тошнота, метеоризм и других, остается крайне высокой и нередко служит основанием для отказа от дальнейшего лечения [5]. Юридическим ограничением для назначения метформина пациенткам, планирующим беременность, является наличие противопоказаний к применению препарата во время гестации [6]. Кроме того, необходимо принимать во внимание тот факт, что в соответствии с инструкцией по применению препарата метформин нарушает всасывание витамина B12 в кишечнике на 30% и снижает сывороточную концентрацию витамина B12 у 5–10% пациентов, что в редких случаях приводит к развитию мегалобластной анемии [35]. В международном руководстве по менеджменту пациентов с СПКЯ в качестве альтернативной патогенетической терапии предлагается применение инозитола как инсулиносенситайзера, который, по данным многочисленных исследований, приводит к восстановлению овуляторных циклов у пациенток с СПКЯ [5]. Инозитол является структурным компонентом клеточных мембран, а также преимущественно накапливается в тканях с высокой активностью метаболических процессов, в том числе в органах репродуктивной системы (в сперме, ооцитах, тканях эмбриона). По химической структуре инозитол представляет собой шестиатомный спирт циклогексана и встречается в виде 9 стереоизомеров, к биологически значимым из которых относятся мио-инозитол и Д-хиро-инозитол. Функционально инозитол играет роль вторичного мессенджера, передающего внутриклеточные сигналы для обеспечения биологических эффектов ФСГ, ЛГ, инсулина и ряда других гормонов и факторов роста [5, 36]. Несмотря на то что оба стереоизомера участвуют, подобно инсулину, в метаболизме глюкозы, действие их принципиально различается. Если мио-инозитол стимулирует процесс транслокации транспортера глюкозы к цитоплазматической мембране, тем самым увеличивая поглощение глюкозы клетками, Д-хиро-инозитол реализует анаболические эффекты инсулина, активизируя синтез гликогена в печени [36, 37]. Указанное, вероятно, объясняет максимально высокую концентрацию мио-инозитола в тканях и клетках с высокой метаболической активностью, в том числе в яичниках и семенной жидкости [37]. Кроме того, ключевую роль в развитии ооцитов играет именно мио-инозитол, концентрация которого в фолликулярной жидкости в 100 раз превышает таковую для Д-хиро-стереоизомера. Последнему принадлежит функция инсулиноопосредованного синтеза тестостерона в яичнике, поэтому повышение уровня Д-хиро-инозитола может усугублять негативное влияние андрогенов на фолликулогенез. Мио-инозитол, напротив, поддерживает нормальный рост фолликулов в яичниках, обеспечивая передачу сигналов ФСГ, а также снижает соотношение ЛГ/ФСГ, увеличивает уровень глобулина, связывающего половые гормоны, что приводит к снижению тестостерона в сыворотке. Учитывая вышеизложенное, оптимальное соотношение мио-инозитола и Д-хиро-инозитола в препаратах не должно быть меньше 40:1, что соответствует физиологическому соотношению стереоизомеров в плазме крови. По данным исследований, инозитол у женщин с СПКЯ применяют в течение 3–12 месяцев как в комплексном лечении заболевания, так и для подготовки к беременности в естественном цикле или в программе вспомогательных репродуктивных технологий (ВРТ) [36, 37]. Преимуществом использования «естественного» инсулиносенситайзера инозитола является его безопасность, в том числе при беременности. Последнее явилось основанием для изучения новой терапевтической стратегии для профилактики гестационного сахарного диабета с помощью мио-инозитола у женщин с СПКЯ [38], относящихся к группе высокого риска по его развитию [5, 6].
Помимо монотерапии метформином или инозитолом, возможны комбинации инозитола с метформином для пациенток с СПКЯ, которые страдают непереносимостью высоких доз метформина из-за нежелательных явлений препарата [39].
Для женщин, планирующих беременность, оптимальными могут считаться комплексы, содержащие инозитол с фолиевой кислотой (или метаболитом фолиевой кислоты – L-метилфолатом кальция) в дозах, обеспечивающих ежедневное поступление в организм 4000 мг мио-инозитола и 400 мкг фолиевой кислоты/метафолина [40].
В исследовании, проведенном с участием 140 российских женщин с диагнозом СПКЯ, было продемонстрировано восстановление овуляторной функции у 78,6% женщин с СПКЯ на фоне 6-месячного применения негормонального нутриентного комплекса «мио-инозитол и фолиевая кислота» («Фертина»), а также существенное сокращение висцерального жира – основного триггера ИР. Авторами были подтверждены ранее существующие сведения о положительном влиянии комплекса «мио-инозитол и фолиевая кислота» на показатели углеводного и липидного обменов, уровень андрогенов [41].
Для женщин с СПКЯ, не заинтересованных в беременности, основной терапевтической «мишенью» является гиперандрогения, которая устраняется назначением КГК, антиандрогенов и препаратов, снижающих ИР (метформин, мио-инозитол), а также косметическими методами лечения. В настоящее время отсутствуют рекомендации по конкретным типам или дозам КГК, но, вероятно, имеет смысл избегать назначения некоторых КГК, в состав которых входят гестагены с андрогенной активностью метаболитов. Препараты на основе 35 мг этинилэстрадиола и ципротерона ацетата не следует рассматривать в качестве первой линии лечения при СПКЯ из-за относительно частых эпизодов тромбозов [5, 6].
Данные по применению антиандрогенов при лечении симптомов СПКЯ весьма ограничены, но считается, что их назначение возможно после 6–12 месяцев безрезультатного приема КГК, когда появляется объектовая возможность оценки влияния терапии на выраженность гирсутизма. Наиболее часто используется спиронолактон в дозе 50–100 мг 2 раза в день, ципротерона ацетат 10–100 мг в циклическом или непрерывном режиме, гораздо реже – флутамид или финастерид [5, 6].
В качестве профилактики гиперплазии/рака эндометрия и предотвращения ановуляторных маточных кровотечений при длительности аменореи более 90 дней также рекомендуется прием КГК или гестагенов [5].
Кратковременный прием КГК с ожиданием повышения вероятности наступления беременности на отмене («ребаунд-эффект») показал свою неэффективность. В рамках рандомизированного клинического исследования было показано, что 16-недельный прием КГК у женщин с ожирением не увеличивал частоту зачатия по сравнению с пациентками с СПКЯ, не использующими КГК. При этом у женщин, снизивших вес на 6,5% без фармакологической поддержки путем модификации образа жизни или с помощью лекарственных препаратов, показатели частоты овуляции и живорождения превышали таковые у тех, кто предварительно не снижал вес перед зачатием (частота овуляции 62 и 45%, частота живорождения 25 и 10,2% соответственно) [42].
Для женщин с СПКЯ-ассоциированным ановуляторным бесплодием индукция овуляции проводится кломифеном, летрозолом (off lable), гонадотропинами, второй линией лечения является лапароскопический дриллинг яичников и гонадотропины, а третьей – программы ВРТ [5, 6].
Диагностические и терапевтические неудачи при оказании медицинской помощи женщинам с СПКЯ связаны с многоликостью заболевания, назначением КГК, гестагенов с целью восстановления фертильности, а также ЗГТ биоидентичными эстрогенами, рассмотрение программ ВРТ или лапароскопического дриллинга яичников в качестве первой линии лечения СПКЯ-ассоциированного бесплодия.
Функциональная гипоталамическая аменорея
Снижение сывороточных уровней ФСГ, ЛГ и Е2 у женщин со вторичной олиго- и аменореей (рисунок), обозначающееся как гипогонадотропный гипогонадизм, после исключения других причин может свидетельствовать о развитии у пациентки ФГА – аменореи, обусловленной энергетическим дефицитом вследствие ограничения пищи и/или избыточной физической нагрузки и стрессом, но не связанной с органическими поражениями ГГЯ-оси [1, 7]. Частота ФГА в структуре вторичных аменорей составляет 20–35% [7, 14]. Без преувеличения ФГА можно отнести к наиболее сложно идентифицируемым нозологиям, особенно при нормальных значениях ФСГ и ЛГ, когда пациентки годами не получают необходимую терапию и считаются либо здоровыми, либо проходят «лечение» КГК или гестагенами. Интересен тот факт, что патогенетические механизмы формирования гипоэстрогении при ФГА кардинально отличаются от таковых при ПНЯ, при которой «разрыв» ГГЯ-оси происходит на уровне яичников вследствие истощения овуляторного резерва или резистентности тканей гонад к ФСГ. При аменорее, ассоциированной с недостаточным питанием или активным спортом, из-за резкого падения уровня лептина на фоне сокращения массы подкожножировой клетчатки нарушается работа гипоталамических центров регуляции репродуктивной системы, ответственных за продукцию кисспептина, который, в свою очередь, через стимуляцию синтеза ГнРГ обеспечивает физиологическую «импульсную» секрецию гонадотропных гормонов. Аномальная выработка ГнРГ, характерная для ФГA, приводит к изменению пульсативного выброса гонадотропинов, отсутствию пика ЛГ, ановуляции, аменорее, гипоэстрогении и, конечно, к ановуляторному бесплодию [1, 7]. Практически всегда при ФГА наблюдается более выраженное снижение уровня ЛГ по сравнению с нормальным или низким уровнем ФСГ, что напоминает эндокринологию препубертата [7]. Напротив, смещение соотношения сывороточных концентраций ФСГ/ЛГ в сторону ЛГ позволяет в ряде случаев заподозрить СПКЯ, а не ФГА. В случае если патогенез ФГА связан со стрессом, высвобождение гонадотропных гормонов нарушается вследствие ингибирующего эффекта кортикотропин-рилизинг-гормона и кортизола на гипоталамо-гипофизарные уровни ГГЯ-оси. Ключевым звеном патогенеза ФГА, детерминирующим в последующем выбор патогенетической терапии заболевания, является снижение синтеза в печени инсулиноподобного фактора роста 1 (ИФР-1), несмотря на усиление выработки соматотропного гормона по механизму обратной связи [43].
Считаясь «диагнозом исключения», ФГА верифицируется при наличии в анамнезе указаний на изменения в образе жизни женщины со вторичной аменореей (снижение веса по причине диет и/или интенсивных занятий спортом, перенесенный стресс), но только после полного клинико-лабораторного обследования, включающего, помимо измерения уровня гормонов в сыворотке и УЗИ органов малого таза, магнитно-резонансную томографию головного мозга. Отсутствие кровотечения отмены при проведении пробы с прогестероном выявляет снижение эстрогенной насыщенности, при этом «маточный фактор» позволяет исключить тест с эстрогенами и прогестероном, сопровождающийся менструальноподобным кровотечением [1, 7]. После исключения иных причин вторичной аменореи и подтверждения ФГА в качестве окончательного клинического диагноза необходимо оценить МПК с помощью двухэнергетической рентгеновской абсорбциометрии для подбора оптимальных доз эстрогенов в составе ЗГТ [1, 7]. Последнее обусловлено тем, что гипоэстрогения при ФГА приводит к потере МПК, а также вагинальной сухости, сексуальной дисфункции, но, в отличие от ПНЯ, не сопровождается вазомоторными симптомами (приливами, ночной потливостью) [7]. Наглядным клиническим примером ФГА являются профессиональные спортсменки, которым требуется поддержание низкой массы тела за счет интенсивных физических нагрузок и ограничения питания (фигуристки, гимнастки, бегуньи и другие). На фоне вторичной (иногда первичной) аменореи и гипоэстрогении у девушек-спортсменок могут формироваться остеопороз и склонность к малотравматичным переломам (при падении с высоты собственного тела). В англоязычной медицинской литературе появился термин «триада спортсменок» («женская атлетическая триада»), под которым понимают наличие аменореи, остеопороза как последствий основного «компонента» триады – энергетического дефицита [7, 44]. Однако, по данным опросов, только 17% врачей-гинекологов справляются с идентификацией всех трех компонентов «триады спортсменок» у женщин с ФГА, что дополнительно подтверждает сложность диагностики этого заболевания [45].
Первой линией лечения ФГЯ являются коррекция психологических нарушений, в том числе связанных с РПП, изменение питания в сторону увеличения суточной калорийности и/или снижение избыточных физических нагрузок. Задача акушера-гинеколога – не только установить диагноз ФГА и своевременно направить пациентку к психологу/психотерапевту и/или диетологу/эндокринологу, но и нивелировать негативные последствия гипоэстрогении в молодом женском организме и оказать помощь в преодолении ановуляторного бесплодия [1, 7].
Несмотря на теоретическую эффективность бисфосфонатов и деносумаба при ФГА, пока имеется недостаточное количество исследований, изучающих безопасность использования антирезорбтивных препаратов среди молодых женщин. Существуют гипотетические опасения, связанные с потенциальными тератогенными эффектами бисфосфонатов, которые, предположительно, надолго и прочно связываются с костной тканью с последующим медленным обратным высвобождением в системный кровоток, что проявляется длительным обнаружением бисфосфонатов в низких концентрациях в плазме крови [7]. Проспективные исследования не смогли продемонстрировать преимуществ приема КГК по восстановлению МПК у девушек, страдающих РПП по типу нервной анорексии [7, 46]. В этой связи единственно эффективным и безопасным способом восполнения дефицита эстрогенов, приводящего к потере МПК и остеопорозу, считается использование биоидентичных эстрогенов. Однако динамика восстановления МПК на фоне ЗГТ пероральными эстрогенами и в группе плацебо статистически значимо не отличалась [47]. Объяснением полученных результатов считают подавление пероральными эстрогенами в процессе первичного прохождения через печень синтеза ИФР-1, который является важнейшим костно-трофическим фактором, регулирующим метаболизм в костной ткани [1, 7].
В соответствии с международными рекомендациями и клиническим протоколом РОАГ [1, 7] биоидентичные трансдермальные эстрогены являются препаратами первой линии выбора для патогенетической терапии женщин с ФГА: в качестве эстрогенного компонента предпочтительнее назначать эстрадиол в форме геля 1–2 мг/сут или эстрадиол 50–100 мкг/сут в виде пластыря в сочетании с микронизированным прогестероном в дозе 200 мг/сут или дидрогестероном 10 мг/сут на срок не менее 10–12 дней с 16-го дня цикла для профилактики гиперпластических процессов эндометрия.
У женщин, заинтересованных в беременности, эксперты не рекомендуют начинать индукцию овуляции без ликвидации дефицита энергии и при значениях индекса массы тела менее 18 кг/м2, равно и восстановление МПК не может обеспечиваться только приемом эстрогенов при сохраняющемся дефиците микронутриентов и питательных веществ. Для овариальной стимуляции следует использовать гонадотропины, отдавая предпочтение ЛГ-содержащим препаратам [1, 7]. В качестве экспериментального лечения при достаточном уровне эстрадиола для индукции овуляции можно использовать кломифен при достаточном уровне E2 [1, 3, 7]. В целом при лечении ФГА важно придерживаться стратегии междисциплинарного взаимодействия, поскольку без устранения энергетического дефицита, психологической поддержки шансы на успешное излечение и наступление беременности даже в программах ВРТ крайне низкие.
В реальной клинической практике нередко встречаются некоторые искажения в понимании этиологии, патогенеза ФГА как «неорганической» эндокринопатии и, как следствие, ошибки в диагностике и лечебном подходе. Последние включают в себя попытки лечения заболевания без участия специалиста по психиатрии и диетолога (эндокринолога), назначение КГК с целью нормализации ритма менструаций, создающих «иллюзию благополучия», индукцию овуляции без устранения энергетического дефицита, использование слишком низких доз эстрогенов в составе ЗГТ, а также применение пероральных форм эстрогенов. Кроме того, ограничение длительности ЗГТ при ФГА из-за опасений блокирования овуляции беспочвенно, поскольку трансдермальные формы эстрогенов создают физиологические концентрации эстрадиола в сыворотке, недостаточные для подавления ФСГ и ЛГ.
Необходимо отметить, что существование одной эндокринопатии не исключает наличие другой, поэтому даже при обнаружении, например, повышенной концентрации пролактина в сыворотке не следует останавливать процесс диагностического поиска, а исключить остальные нозологии, в частности ПНЯ или СПКЯ, которые могут «скрываться» под симптомом «аменорея».
Вне зависимости от формы аменореи (гипер-, гипо- или нормогонадотропная), репродуктивных планов женщин в основополагающих международных клинических протоколах указывается необходимость назначения женщинам с СПКЯ, ПНЯ и ФГА витамина D [5, 7, 8]. Классические «костные эффекты» витамина D, обеспечивающие регуляцию фосфорно-кальциевого гомеостаза, являются обоснованием для его использования с целью профилактики и лечения остеопороза при ФГА и ПНЯ [7–9]. Неклассическое «внекостное» гормоноподобное действие витамина D опосредуется рецепторами витамина D, которые экспрессируется в различных органах и системах, в том числе в тканях органов репродукции. Несмотря на продолжающиеся исследования, обнаруженные к настоящему моменту биологические эффекты витамина D, связанные с эндокринологией репродукции [48], расширили показания для его применения у субфертильных женщин, страдающих ановуляторным бесплодием, и у пациенток с нарушением функционирования ГГЯ-оси на любом ее уровне. Так, в исследованиях установлена способность витамина D стимулировать увеличение продукции прогестерона на 13%, E2 – на 9% и эстрона – на 21% [49]. Более того, витамин D регулирует экспрессию и секрецию ХГЧ в синцитиотрофобластах человека и увеличивает выработку стероидных гормонов плацентой, а также регулирует экспрессию гена HOXA10 в клетках эндометрия человека, необходимого для успешной имплантации эмбриона как при естественном зачатии, так и в программах ВРТ [47, 48]. Недостаток витамина D ассоциируется с повышенным риском ожирения и ИР, что определяет его ключевую роль в патогенетической терапии СПКЯ [48]. При этом исследование по применению витамина D в различных суточных дозах в виде водного раствора или жевательных таблеток («Ультра-Д») с участием 247 пациентов репродуктивного возраста с дефицитом витамина D из различных регионов Российской Федерации продемонстрировало преимущества в скорости восстановления уровня витамина D и достигаемом сывороточном уровне при использовании жевательных таблеток в дозе 4000 МЕ в течение 8 недель. Последнее объясняется оптимальными условиями всасывания жевательных таблеток в богато васкуляризированной слизистой ротовой полости, высокой биодоступностью благодаря отсутствию первого прохождения через печень, а также точным дозированием при приеме таблетированных форм по сравнению с водным раствором [50]. В этой связи назначение витамина D при фармакологической коррекции заболеваний, сопровождающихся олиго- или аменореей, повышает эффективность патогенетической терапии, в том числе у пациенток, страдающих бесплодием.
Заключение
Таким образом, диагностический путь от симптома «олиго- или аменорея» к конкретной нозологической форме или синдрому требует тщательного и последовательного анализа и синтеза жалоб, анамнеза, данных объективного осмотра и, конечно же, результатов дополнительных методов исследования. Врач, консультирующий пациенток с олиго- и аменореей, порой должен обладать знаниями, выходящими за границы компетенций по специальности «акушерство и гинекология», а четкое пошаговое исключение заболеваний в соответствии с предложенным алгоритмом позволит в кратчайшие сроки установить причину аменореи и разработать план терапевтических мероприятий, опираясь на российские и международные клинические протоколы.



