Hyperandrogenism as a risk factor for isthmic-cervical insufficiency
Objective: To assess the association of isthmic-cervical insufficiency (ICI) with hyperandrogenism (HA). Materials and methods: A prospective cohort controlled study was performed (the study period was 2014–2019). The clinical base was Maternity Hospital One, Branch, L.A. Vorokhobov City Clinical Hospital Sixty-Seven (Moscow). The study involved 98 women, including 63 with ICI (a study group) and 35 without ICI (a control group). HA was evaluated by high-performance liquid chromatography according to the steroid profile and quantitative determination of urinary steroids. The data were statistically analyzed using the programs of Microsoft Excel 2007, Statistica 12.0. The association of HA with ICI was assessed. Results: Regardless of the presence of HA, ICI is associated with the factors of a compromised reproductive history (the number of pregnancies, р=0.00008; the term of premature birth (р=0.04); with the frequency of miscarriage (р<0.001); with the experience in using cerclage (р=0.49); with the pregestational weight exceeding the norm (OR=4.16; 95% CI, 1.32–13.31)). ICI is associated with HA (OR=3.48; 95% CI, 1.37–8.84). HA in the women with ICI is associated with an earlier age at menarche (р=0.01) and an older age during the current pregnancy (p=0.002). The gestational risk factors of HA-associated ICI are shown to be the absence of progesterone therapy in the preconception period/the first trimester of pregnancy (ОR=10.23; 95% CI. 1.12–93.35); the greater frequency of threatening spontaneous abortion (SA) (OR=5.38; 95% CI 1.83–15.79); that of imminent SA (p=0.01); the length of the cervix is 10 mm or less; ICI totals more than 6 scores; the significantly earlier gestational period in the diagnosis of ICI (19 weeks or less). Conclusion: The asymptomatic onset of ICI cannot implement modern prevention, limits the possibilities of medical management. Prescribing progesterones to woman with HA in the preconceptional period/the first trimester of pregnancy should be considered as a measure for the prevention of ICI. Authors' contributions: Levakov S.A. – development of the design of the clinical trial, supervision; Mokh A.V. – development of the design of the investigation, collection and analysis of the findings, writing the article. Conflicts of interest: The authors declare that there are no possible conflicts of interest. Funding: The investigation has not been sponsored. Ethical Approval: The investigation has been approved by the Ethics Committee, I.M. Sechenov First Moscow State Medical University (Sechenov University), Ministry of Health of Russia. Patient Consent for Publication: The patients have signed an informed consent form to the publication of their data. Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator. For citation: Levakov S.A., Mokh A.V. Hyperandrogenism as a risk factor for isthmic-cervical insufficiency. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (6): 69-79 (in Russian) https://dx.doi.org/10.18565/aig.2023.36Levakov S.A., Mokh A.V.
Keywords
The pathogenesis of isthmic-cervical insufficiency (ICI) has not been certainly studied [1], is heterogeneous; its idiopathic form is not excluded [3]. In the foreign guidelines, the risk factors of ICI primarily include habitual pregnancy loss in the second trimester [3, 4], a history of premature rupture of fetal membranes at less than 27 [4] or 32 [3, 5] weeks’ gestation; the length of the cervix is less than 25 mm at less than 27 weeks’ gestation; cervical trauma, congenital anomalies of the uterus or abnormalities of connective tissue development (for example, Ehlers-Danlos syndrome [1–5]; in utero diethylstilbestrol exposure in utero [1, 3, 4]. In Russia, the risk factors of ICI are clearly differentiated into 7 groups (functional, anatomical, the specific features of the current pregnancy, genetic and congenital, extragenital, the features of an obstetric and gynecological history, infectious and inflammatory diseases, and dysbiotic conditions), the primary condition of which is recognized to be hyperandrogenism (HA) of both ovarian (polycystic ovary syndrome) and adrenal (adrenogenital syndrome) genesis [2]. In the Russian ICI rating scale [6], HA during pregnancy along with the data of transvaginal echography (the location of the cervix uteri and the adjacent part of the fetus; the length of the cervix uteri, and opening of the internal orifice of the uterus) and a history (information about an earlier late spontaneous miscarriage) is evaluated as its independent sign (2 scores), while the sum of 5–6 scores or more already requires the correction of ICI.
HA is a pathological condition caused the excessive production of androgens by the ovaries and/or adrenal glands or by an increase in local tissue sensitivity to circulating androgens, the prevalence of which among women ranges from 10 to 20% [7]. The HA syndrome deserves the close attention of not only endocrinologists, but also obstetricians/gynecologists, reproductologists [8]. Information on the epidemiology of HA in women with ICI, on the methods and efficiency of its correction is not widely available. That on the role of androgens in the development of cardiovascular diseases and infertility is much more frequently reflected [9], on the features of the course of pregnancy and delivery [10], maternal metabolism, placental function, and fetal growth [11], vascular and placental changes that are significant in the pathogenesis of preeclampsia [12], hypertension and intestinal dysbiosis [13], reproductive losses [14, 15]. HA is generally recognized as a risk factor for reproductive losses [7, 8, 16], but controversial issues remain. The most frequently detected cause of HA is considered to be polycystic ovary syndrome (PCOS), which is along with obesity, diabetes mellitus, cardiovascular disorders, depression/anxiety disorders, etc. is predicted by the fertility disorder [17–19]. However, the Russian guidelines “Polycystic Ovary Syndrome” (2022) [20] notes that PCOS is indeed characterized by a 3–4-fold increase in the risk of pregnancy complications (gestational diabetes mellitus, hypertension, preeclampsia), especially in the “classical” phenotype of PCOS [21]. Nevertheless, an increase in the rate of miscarriage during natural conception in women with PCOS is denied, regardless of the presence of obesity, while the rate of miscarriage after ovulation induction is comparable with that in other forms of infertility [20]. The ambiguity of reproductive risks in PCOS is due to the presence of its four phenotypes proposed earlier by the National Institutes of Health and taken into account to this day in the “International Guidelines for the Diagnosis and Treatment of Polycystic Ovary Syndrome”, which are based on evidence-based medicine’ [22]. The phenotyping of PCOS is based on a set of three Rotterdam criteria: androgen excess (AE) + ovulatory dysfunction (OD) + polycystic ovarian morphology (POM). Androgen excess implies three of the four phenotypes of PCOS: Phenotype A = AE + OD + POM; Phenotype B = AE + OD; Phenotype C = AE + POM; and Phenotype D = OD + POM. The insufficient knowledge of the role of HA in the development of ICI determined the performance of this study.
The objective of this study was to assess the association of ICI with HA.
Materials and methods
A prospective cohort controlled study was performed in the period 2014 to 2019. Its clinical base was Maternity Hospital One, Branch, L.A. Vorokhobov City Clinical Hospital Sixty-Seven (Moscow). When planning the study stage, the sample size was justified using the formula:
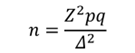
where n is the size of a sample; Z is the coefficient corresponding to the confidence interval; p is the proportion of respondents having the studied sign; q=1-p is the proportion of respondents who lack the studied sign; ∆ is the maximum sampling error.
Women were included into the study, by applying the random sampling principle according to a random number program.
The investigation involved 98 women with singleton pregnancy: 63 with ICI (a study group); 35 without ICI (a control group).
Outcomes studied. The final result of the investigation was to assess the association of ICI with HA.
Criteria for evaluating the outcome. The criteria for ICI corresponded to the Russian guidelines [2, 23]. HA was evaluated by high-performance liquid chromatography according to the steroid profile and to the quantitative determination of urinary steroids (µmole/24 hours): androsterone, etiocholanolone, dehydroepiandrosterone, 11-ketoandrosterone, 11-ketoethiocholanone, 11β-hydroxyandrosterone, 11β-hydroxyethiocholanolone, the sum of 17-ketosteroids (KS), van de Kalseide discriminant, pregnandiol, allo-pregnandiol, pregnanolone, delta5-pregnandiol, pregnantriol, 16-alpha-hydroxyetiocholanolone, 16-alpha-hydroxyandrosterone.
Potential confounding factors. The potential confounding factors included the features of menstrual, gynecological, and reproductive histories, general clinical factors (age, weight characteristics of a woman), and the specific features of the course of pregnancy.
The prospective follow-up time was considered to be an interval from the onset of pregnancy to the diagnosis of ICI (HA exposure) and to delivery (the outcome).
Statistical analysis
The data were statistically processed and analyzed using the programs of Microsoft Excel 2007, Statistica (Version 12.0; StatSoft; TIBCO Software). The quantitative data having a normal distribution were described using the arithmetic mean (M) and standard deviation (SD). Qualitative indicators were presented in absolute (n) and relative (%) values.
The significance of differences (p) was assessed in the presence of normality of the distribution of the signs and uniformity of variances according to the Student’s t-test. When interpreting the results of the statistical analysis, the critical value of the significance level (p) was taken to be less than 0.05. The significance of differences in the outcomes due to the studied factor was assessed by the chi-square (χ2) test, if there were less than 10 investigation participants minus 2 with the Yates correction. The relationship between of the outcome studied and the influencing factor was evaluated using the odds ratio (OR) with a 95% confidence interval (95% CI).
Results
The urinary steroid profile corresponded to HA in 32 (50.79%) women with ICI and in 8 (22.86%) women in the control group, with demonstrated the association of ICI with HA (OR=3.48, 95% CI 1.7–8.84).
Analyzing medical history data presented the predictors of ICI in women with HA. The menstrual history showed the differences characteristic of ICI, HA, and their concurrence (Table 1).
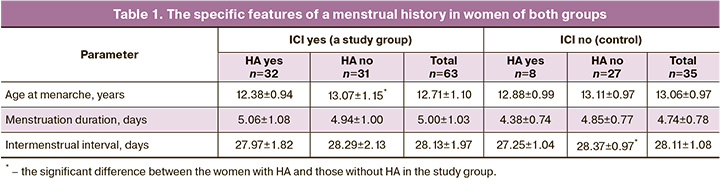
The women with HA in the presence of ICI showed an earlier age at menarche (12.38±0.94 years and 13.07±1.15 years; p=0.01); the control group exhibited a shorter intermenstrual interval (27.25±1.04 days and 28.37±0.97 days; р=0.01). The information obtained does not fit into the generally accepted ideas about the role of HA in later menarche and about the specific features of menstrual function. Impaired menstrual and ovulatory function (MOF) was detected in extremely small number of women, which was comparable in all the groups (Fig. 1).
Of interest were the specific features of a gynecological history in the perspective of predicting ICI and HA (Table 2).
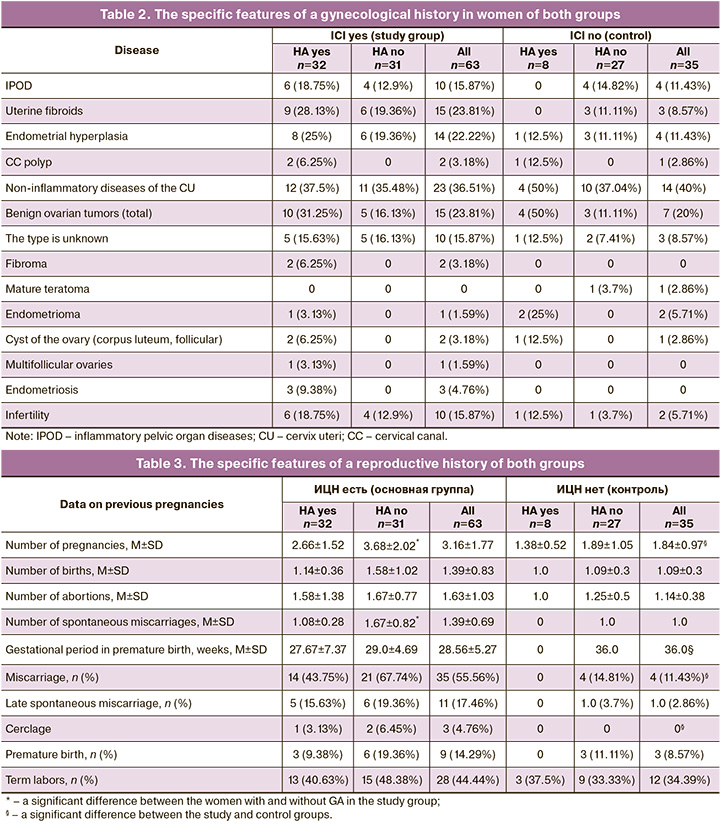
The women with ICI were distinguished from the control group by a statistically insignificant, but a higher incidence of uterine fibroids (23.81% and 8.57%; χ2=3.48; р=0.06), endometrial hyperplasia (22.22% and 11.43%; χ2=1.1; р=0.29), infertility (15.87% and 5.71%; χ2=1.32, р=0.25), endometriosis (4.76% and 0%; χ2=0.49; р=0.49). HA in the women with ICI was accompanied by benign proliferative female reproductive system diseases experienced in the history in 100% (n=32) with a higher incidence compared to the absence of HA (χ2=16.06; р<0.001).
Only women with HA (8 out of 40; χ2=10.1; р<0.002), regardless of the presence or absence of ICI, had a history of benign ovarian tumors known for their hormonal activity or hormone dependence. Data on multifollicular ovaries were available only in women with ICI and HA, but their incidence was extremely low (3.13%). Infertility was more frequently diagnosed in the study group women than in the control group (15.87% and 5.71%; χ2=1.32; р=0.25). The association of infertility with HA has not been established, although in women with ICI, its incidence in HA was higher than in its absence (18.75% and 12.9%; χ2=0.084, р=0.77).
The reproductive history in the women with ICI was also compromised (Table 3).
ICI was associated with a large number of pregnancies (3.16±1.77 and 1.84±0.97; р=0.00008), an earlier period of experienced preterm labor (28.56±5.27 and 36.0; р=0.04), with the rate of miscarriage (55.56% and 11.43%; χ2=18.99; р<0.001), with the need to use cerclage (4.76% and 0%; χ2=0.49; р=0.49). The dominant role of HA in reproductive dysfunction has not been established. The control group women with a history of HA had no miscarriage (spontaneous miscarriages, premature birth), did not previously use cerclage. The women with ICI in the presence of HA versus its absence had a history of a significantly lower number of pregnancies (2.66±1.52 and 3.68±2.02; р=0.03) and spontaneous miscarriages (1.08±0.28 and 1.67±0.82; р=0.02).
The current pregnancy occurred spontaneously in the natural menstrual cycle in all the women of the control group and in 88.89% of women with ICI. The women with ICI from the control group were distinguished by the use of assisted reproductive technologies (ART) (11.11% and 0%; χ2=2.68; р=0.1). The frequency of ART use in patients with HA was higher than in its absence (15.63% and 6.45%; χ2=0.57; р=0.45). The IVF protocols lacked information about the genesis (anovulatory, in particular), therefore, it was impossible to identify the association of ART with pregestational HA, ICI.
The mean age of women in the examined cohort was 31.38±5.23 years. The women with ICI were older than those in the control group, but statistically insignificant (р=0.1) (Table 4).
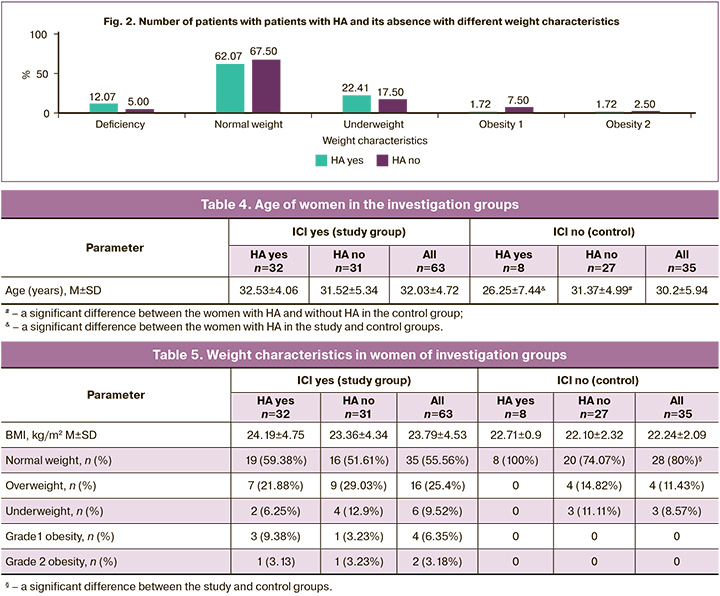
Age was an important differentiating criterion for women with HA in the study and control groups: a significantly older age was established in the presence of ICI (32.53±4.06 and 26.25±7.44 years; р=0.002). Comparing the age of women with and without HA revealed significant intergroup differences for the women of the control group, rather than those of the study group. In the control group, the age of women with HA was considerably younger than in those without HA (26.25±7.44 and 31.37±4.99 years; р=0.03).
Assessment of pregestational weight in the cohort showed a wide range of body mass index (BMI) (from 16.44 to 39.9 kg/m2), averaging 23.23±3.89 kg/m2. There were no significant BMI differences in women with ICI and in those of the control group, in the presence or absence of HA (Table 5).
Despite the intergroup comparability of BMI, the corresponding normal weight was detected in a significantly smaller number of women with ICI compared to the control group (55.56% and 80%; χ2=4.84; р=0.03) (Table 5). A relationship was found ICI and the pregestational weight characteristics that go beyond the normal values (below or above those (44.44% and 20%; OR=3.2, 95% CI 1.22–8.41). The main contribution to this relationship was made by the weight above the norm (overweight, grades 1 and 2 obesity) (34.92% and 11.43%; χ2=5.22; р=0.02; OR=4.16, 95% CI 1.32–13.31), rather than underweight (9.23% and 8.57%; χ2=0.04; р=0.84; OR=1.12, 95% CI 0.26–4.8).
In women of the entire cohort under study (regardless the presence or absence of ICI), weight characteristics (underweight, normal weight, overweight, obesity) were not a significant differentiating criterion for HA or its absence (χ2=1.74; p=0.78) (Fig. 2).
Pregnancy in women of both groups occurred with complications, but their features and frequency differed significantly (Table 6).
The first trimester of pregnancy was complicated by threatening spontaneous miscarriage in both groups with a comparable frequency, but exceeding in the ICI group (49.21% and 17.14%; χ2=8.53; р=0.004; OR=4.68; 95% CI 1.71–12.83). The frequency of threatening spontaneous miscarriage was higher in women with HA than in those without HA, was statistically significant in the presence of ICI (68.75% and 29.03%; χ2=8.41; р=0.004; OR=5.38; 95% CI, 1.83–15.79); that was absent in the control group (37.5% and 11.11%; χ2=1.45; р=0.23). Imminent spontaneous miscarriage complicated pregnancy only in 6.35% of women with ICI and only in the presence of HA (χ2=6.77; р=0.01).
In the presence of HA, the number of women receiving progesterone in the preconception period/the first trimester of pregnancy with ICI was much smaller than in the control group (40.63% and 87.5%; р=0.049). The association of the absence of progesterone therapy in the preconception period/the first trimester of pregnancy with ICI was established for women with HA (OR=10.23; 95% CI 1.12–93.35).
Analysis of second-trimester complications did not reveal an expected increase in the incidence of iron deficiency anemia and gestational diabetes mellitus.
Prenatal amniorrhea complicated pregnancy in both the study and control groups (15.87% and 8.57%; χ2=0.51; р=0.48). The highest rate of prenatal amniorrhea was observed in the study group women with ha, but it was comparable with that in women without HA (21.88% and 9.68%; χ2=0.96; р=0.33).
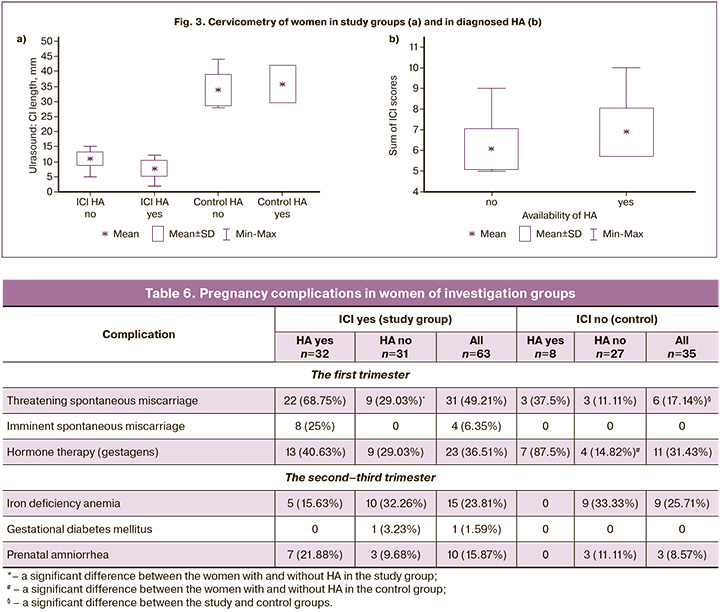
Ultrasound cervicometry indicated that the length of the cervix uteri in the study group women did not reach 20 mm (Fig. 3); it varied from 2 to 12 mm in women with HA from 5 to 15 mm in women without HA (7.84±2.58 and 10.97±2.25 mm; р=0.000004).
All the women of the study group had indications for suturing; however, in the presence of HA, the total number of ICI assessment scores was significantly higher than in its absence (6.88±1.16 and 6.07±0.99 mm; р=0.004).
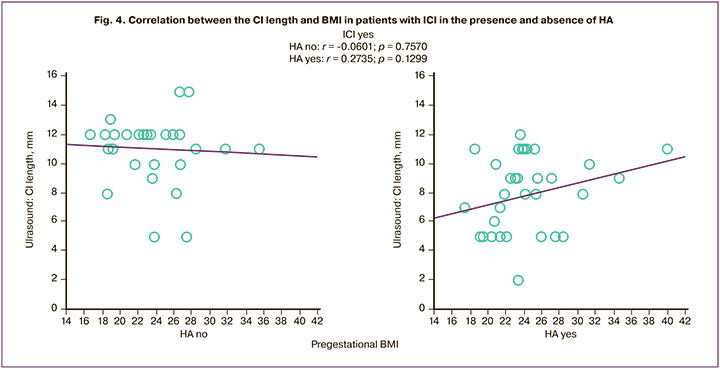
The trend of a multidirectional correlation between the ultrasonic length of the cervix uteri and BMI was established to be positive in the presence of HA (r=0.27; p=0.13) and negative in its absence (r=-0.06; p=0.76) (Fig. 4).
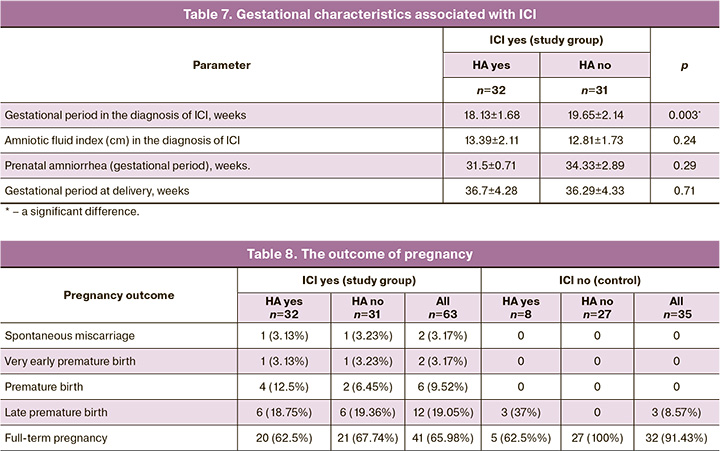
Asymptomicity did not allow one to accurately determine the gestational period of the onset of ICI. HA differed significantly earlier in pregnancy in the diagnosis of ICI as compared to the absence of HA (18.13±1.68 and 19.65±2.14 weeks; р=0. 003) (Table 7).
Analyzing the term of the outcome of pregnancy (Table 8) revealed that spontaneous miscarriage occurred in women with HA (n=1) and without HA (n=1) at 21 and 20 weeks’ gestation, respectively.
The term of delivery in the women of the study group was comparable in the presence of HA and in its absence (36.7±4.27 and 36.29±4.33 weeks; р=0.71), demonstrating the efficiency of cerclage (Fig. 5).
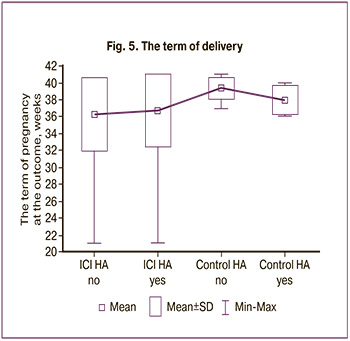
The demonstration of the risks of miscarriage associated with HA was late premature birth in 37% of women with HA in the control group; therefore, the mean delivery time was significantly lower than in its absence (38.0±1.69 and 39.41±1.22 weeks; р=0.01).
Discussion
The different genesis of HA-induced extragestational and gestational disorders in a woman’s body is reflected by investigations from all over the world [8, 16, 24–36]. The main causes of androgen excess are PCOS, congenital adrenal hyperplasia, adrenal tumors, and racial characteristics.
The additional gestational causes of HA are luteoma and theca lutein ovary cysts, placental aromatase deficiency, and congenital fetal adrenal hyperplasia [16]. Our findings confirm the complexity of diagnosing HA outside and during pregnancy and understanding its pathogenesis. The absence of data on the level of blood hormones and ultrasound examination protocols on the endocrine glands before pregnancy in the absolute number of women with HA in the examined cohort, spontaneous conception in 87.5%, the absence of obesity in 67.5% and the presence of underweight in 5%, normal MOF in 92.5% complicate the differential diagnosis of HA, suggesting its gestational outcome.
The pathogenesis of miscarriage is determined by the well-known role in the remodeling of the cervix uteri in PCOS, especially that concurrent with insulin resistance [15], premature birth [10, 29]. The data supporting and justifying the role of HA in the genesis of ICI are extremely few and are focused either only on PCOS [37], or on obesity [38, 19]. The Canadian Society of Obstetricians/Gynecologists in its Clinical Protocol “Cervical Insufficiency and Cervical Cerclage No. 373” (2019) considers PCOS as an independent risk factor, especially in South Asians or black women [3]. It is also emphasized that 85% of the dry weight of the cervix uteri is represented by collagen and that in the women with a history of ICI, the concentration of hydroxyproline in the cervix uteri is significantly lower than in those without ICI. Despite the lack of knowledge about the reasons for this difference, it is considered as a key factor in the mechanism of ICI. According to the findings, HA detected during pregnancy increases the likelihood of ICI by more than 3 times (OR=3.48; 95% CI, 1.37–8.84). Given the asymptomatic nature of ICI, the prevention of miscarriage requires the timely diagnosis of HA before pregnancy or in its early periods.
A separate attention in assessing the risks of ICI is given to obesity, the role of which is ambiguous. There is evidence of a positive correlation between the level of cervicovaginal cytokines (tumor necrosis factor-α (TNF-α) and interleukins (IL6) and (L10) and BMI in a woman (r=0.303; p=0.005; r=0.285; p=0.008; r=0.247; p=0.023, respectively), especially in the presence of premature birth in the history [39, 40].
Other researchers [41] have shown that an increase in BMI by 1.0 kg/m2 corresponds to an increase in the length of the cervix uteri (CI) by 0.25 mm, i.e. obesity is characterized by its large length.
We have not found the similar data on the assessment of the CI length, on the risks of developing ICI in obesity associated with HA. The described relationship between obesity and the risk of over-pregnancy [42–45] makes a disagreement in the evaluation of the efficiency of surgical ICI correction. According to the observational cohort study by Kandeel M.S. et al. (2014) [37], the premature birth (in primigravidas, with a single fetus) is characterized by a women’s underweight, whereas by overweight or obesity for over-pregnancy. The systematic review by Prodromidou A., Frountzas M. (2016) [38], has failed to establish a relationship between obesity and the term of pregnancy at delivery, a significant impact on the efficiency of cervical cerclage. The findings were consonant with those of the studies by Venkatesh K.K. et al. (2017) [41]: HA is characterized by a trend of positive correlation between the ultrasonic CI length anf BMI and a trend of negative one for the absence of HA.
To study the trigger mechanisms of ICI, the cofactors determining the efficiency of its therapy makes up a platform of the present-day investigations [37, 40, 46]. The impact of HA on female health and reproductive function is significant [47, 48]; its concomitant effect with other factors (age, weight characteristics, heredity, acquired diseases and disorders, etc.), remain the subject of an in-demand study. The fundamental stage of preventing premature birth is the prediction and timely diagnosis of ICI that is extremely difficult, by taking into account its asymptomatic course embedded in its definition.
Conclusion
Consolidating the findings, it should be stated that ICI associated with HA is extremely difficult to predict in the pregravid period and in the early periods of pregnancy. The asymptomatic onset of ICI does not allow one to implement the current opportunities of its prevention, limits the possibility of medical management.
HA is a well-known risk factor of different gestational complications, but the absence of a unique phenotype of HA explains its hypodiagnosis. ICI is associated with HA. The latter, regardless of the presence or absence of ICI, is related to the weight characteristics of women who have previously experienced benign ovary tumors known by their potential of hormonal activity and/or dependence.
The predictor of HA-associated ICI is shown to be an earlier age at menarche (12.38±0.94 years) and an older age of a pregnant woman (32.53±4.06 years). The gestational factors for developing ICI concurrent with HA are the absence of progesterone therapy in the preconceptional period/the first trimester of pregnancy. Prescribing progesterones to women in the preconceptional period/the first trimester of pregnancy should be considered as a measure to prevent ICI.
The findings are important to form practical recommendations for the prevention, diagnosis, and timely treatment of ICI in women with HA.
References
- ACOG Practice Bulletin No.142: Cerclage for the management of cervical insufficiency. American College of Obstetricians and Gynecologists. Obstet. Gynecol. 2014; 123(2, Pt 1): 372-9. https://dx.doi.org/10.1097/01.AOG.0000443276.68274.cc.
- Информационное письмо МАРС по клиническим рекомендациям «Истмико-цервикальная недостаточность» [Текст]: Направлены письмом Минздрава России от 28 декабря 2018 года №15-4/10/2-7991. М.: Редакция журнала StatusPraesens; 2019. 52c. [MARS Informational letter on clinical recommendations " Isthmic-cervical insufficiency»: Sent by letter of the Ministry of health of the Russian Federation dated 28 December 2018 no 15-4/10/2-7991. Moscow: Editorial office of StatusPraesens; 2019. 52p.(in Russian)].
- Brown R., Gagnon R., Delisle M.F. No. 373-сervical insufficiency and cervical cerclage. J. Obstet. Gynaecol. Can. 2019; 41(2): 233-47.https://dx.doi.org/10.1016/j.jogc.2018.08.009.
- South Australian Perinatal Practice Guidelines. Cervical Insufficiency and Cerclage. 2017. 13 р.
- Diagnosis and Management of Cervical Insufficiency GLM0055. Maternity Guidelines Group Christchurch Women’s Hospital. New Zealand. 2017. 8 р.
- Шалина Р.И. Коррекция истмико-цервикальной недостаточности. Акушерство и гинекология: новости, мнения, обучение. 2015; 1(7): 40-3. [Shalina R.I. Correction of isthmic-cervical insufficiency. Obstetrics and Gynecology: News, Opinions, Training. 2015; 1(7): 40-3. (in Russian)].
- Луценко Л.А. Надпочечниковые гиперандрогении: мультидисциплинарный подход к решению проблемы. Международный эндокринологический журнал. 2016; 8: 29-34. [Lutsenko L.A. Adrenal hyperandrogenism: a multidisciplinary approach to solving the problem. International Journal of Endocrinology. 2016; (8): 29-34. (in Russian)].
- Доброхотова Ю.Э., Рагимова З.Э., Ильина И.Ю., Ибрагимова Д.М. Гиперандрогения и репродуктивное здоровье женщины. М.: ГЭОТАР-Медиа; 2015. 140c. [Dobrokhotova Yu.E., Ragimova Z.E., Ilyina I.Yu., Ibragimova D.M. Hyperandrogenism and reproductive health of women. Moscow: GEOTAR-Media; 2015. 140p. (in Russian)].
- Mossa F., Latham K.E., Ireland J.J., Veiga-Lopez A. Undernutrition and hyperandrogenism during pregnancy: Role in programming of cardiovascular disease and infertility. Mol. Reprod. Dev. 2019; 86(9): 1255-64.https://dx.doi.org/10.1002/mrd.23239.
- Makieva S., Saunders P.T.K., Norman J.E. Androgens in pregnancy: roles in parturition. Hum. Reprod. Update. 2014; 20: 542-59.
- Fornes R., Maliqueo M., Hu M., Hadi L., Jimenez-Andrade J.M., Ebefors K.et al. The effect of androgen excess on maternal metabolism, placental function and fetal growth in obese dams. Sci. Rep. 2017; 7(1): 8066.https://dx.doi.org/10.1038/s41598-017-08559-w.
- Kumar S., Gordon G., Abbott D., Mishra J. Androgens in maternal vascular and placental function: implications for preeclampsia pathogenesis. Reproduction. 2018; 156(5): R155-67.
- Sherman S.B., Sarsour N., Salehi M., Schroering A., Mell B., Joe B., Hill J.W. Prenatal androgen exposure causes hypertension and gut microbiota dysbiosis. Gut Microbes. 2018; 9(5): 400-21. https://dx.doi.org/10.1080/19490976.2018.1441664.
- Semeniuk L.M., Likhachov V.K., Yuzvenko T.Y., Dobrovolska L.М., Makarov O.G. Risk markers of reproductive loss in women with hyperandrogenism. Wiad. Lek. 2018; 71(8): 1550-3.
- Hu M., Zhang Y., Guo X., Jia W., Liu G., Zhang J. et al. Hyperandrogenism and insulin resistance induce gravid uterine defects in association with mitochondrial dysfunction and aberrant reactive oxygen species production. Am. J. Physiol. Endocrinol. Metab. 2019; 316(5): E794-E809. https://dx.doi.org/10.1152/ajpendo.00359.2018.
- Hakim Ch., Padmanabhan V., Vyas A.K. Gestational hyperandrogenism in developmental pro-gramming. Endocrinology. 2017; 158(2): 199-212.https://dx.doi.org/10.1210/en.2016-1801.
- Синдром поликистозных яичников в репродуктивном возрасте (современные подходы к диагностике и лечению). Клинические рекомендации (Протокол лечения). М.; 2015. 22с. [Polycystic ovary syndrome in reproductive age (modern approaches to diagnosis and treatment). Clinical guidelines (Treatment Protocol). Moscow; 2015. 22p. (in Russian)].
- Johnson T., Kaplan L., Ouyang P., Rizza R. National Institutes of Health Evidence-Based Methodology Workshop on Polycystic Ovary syndrome. National Institutes of Health Bethesda: National Institutes of Health; 2012: 1-14.
- Cheng J., Wu H., Liu H., Li H., Zhu H., Zhou Y. et al. Exposure of hyperandrogen during pregnancy causes depression- and anxiety-like behaviors, and reduced hippocampal neurogenesis in rat offspring. Front. Neurosci. 2019; 13: 436. https://dx.doi.org/10.3389/fnins.2019.00436.
- Адамян Л.В., Андреева Е.Н., Абсатарова Ю.С., Григорян О.Р., Дедов И.И.,Мельниченко Г.А., Сутурина Л.В., Филиппов О.С., Шереметьева Е.В., Чернуха Г.Е., Ярмолинская М.И. Клинические рекомендации«Синдром поликистозных яичников». Проблемы эндокринологии. 2022; 68(2): 112-27. [Adamyan L.V., Andreeva E.N., Absatarova Yu.S., Grigoryan O.R., Dedov I.I., Melnichenko G.A., Suturina L.V., Filippov O.S., Sheremetyeva E.V., Chernukha G.E., Yarmolinskaya M.I. Clinical guidelines «Polycystic Ovary syndrome». Problems of Endocrinology. 2022; 68(2):112-27.(in Russian)].
- Zawadski J.K., Dunaif A. Diagnostic criteria for Polycystic Ovary syndrome: towards a rational approach. In: Dunaif A., Givens J.R., Haseltine F.P., Merriam G.R., eds. Polycystic Ovary syndrome. Boston: Blackwell Scientific Publications; 1992: 377-84.
- International evidence-based guideline for the assessment and management of Polycystic Ovary Syndrome (PCOS). 2018. Available at:https://www.monash.edu/__data/assets/pdf_file/0004/1412644/PCOS_Evidence-Based-Guidelines_20181009.pdf Accessed 10 June 2020.
- Айламазян Э.К., Кулаков В.И., Радзинский В.Е., Савельева Г.М., ред. Акушерство. Национальное руководство. М.: ГЭОТАР-Медиа; 2014. 1200 с. [Ailamazyan A.K., Kulakov V.I., Radzinsky V.E., Saveleva G.M., ed. Obstetrics: National guidance. Moscow: GEOTAR-Media; 2014. 1200p.(in Russian)].
- Леваков С.А., Габитова Н.А. Система гемостаза у беременных с гиперандрогенией. Лечение и профилактика. 2015; 2: 20-3. [Levakov S.A., Gabitova N.A. Hemostasis system in pregnant women with hyperandrogenism. Treatment and Prevention. 2015; (2): 20-3. (in Russian)].
- Мельниченко Г.А., Трошина Е.А., Молашенко Н.В., Сазонова А.И., Ужегова Ж.А. Клинические рекомендации Российской ассоциации эндокринологов по диагностике и лечебно-профилактическим мероприятиям при врожденной дисфункции коры надпочечников у пациентов во взрослом возрасте. Consilium Medicum. 2016; 4: 8-19. [Melnichenko G.A., Troshina E.A., Molashenko N.V., Sazonova A.I., Uzhegova Z.A. Russian Association of Endocrinologists clinical practice guidelinesfor diagnosis, treatment and preventive measures in congenital adrenal hyperplasia due to 21-hydroxylase deficiency patients in adulthood. Consilium Medicum. 2016; (4): 8-19. (in Russian)].
- Меликова А.В. Гиперандрогения: мифы и реальность. Эффективная фармакотерапия. 2018; 3: 40-5. [Melikova A.V. Hyperandrogenism: mythsandreality. Effective Pharmacotherapy. 2018; (3): 40-5. (in Russian)].
- Malinowski A.K., Sen J., Sermer M. Hyperreactio luteinalis: maternal and fetal effects. J. Obstet. Gynaecol. Can. 2015; 37(8): 715-23.https://dx.doi.org/10.1016/S1701-2163(15)30176-6.
- Yang C., Wang H., Zou Y., Cui W., Yang J., Li T., Li C., Jiang J. Hyperreactio luteinalis after delivery: a case report and literature review. Int. J. Clin. Exp. Med. 2015; 8(4): 6346-8.
- Palomba S., de Wilde M.A., Falbo A., Koster M.P., La Sala G.B., Fauser B.C. Pregnancy complications in women with polycystic ovary syndrome. Hum. Reprod. Update. 2015; 21(5): 575-92. https://dx.doi.org/10.1093/humupd/dmv029.
- Stangler Herodež Š., Fijavž L., Zagradišnik B., Kokalj Vokač N. Detection of mutations in the CYP21A2 gene: genotype-phenotype correlation in Slovenian couples with conceiving problems. Balkan J. Med. Genet. 2016 ;18(2): 25-32.
- Edell H., Shearkhani O., Rahmani M.R., Kung R.C. Incidentally found hyperreactioluteinalis in pregnancy. Radiol. Case Rep. 2018; 13(6): 1220-3. https://dx.doi.org/10.1016/j.radcr.2018.08.022.
- Wei D.M., Zhang Z.Z., Wang Z., Li P., Wang J.F., Liu Y.J. et al. Effect of hyperandrogenism on obstetric complications of singleton pregnancy from in vitro fertilization in women with polycystic ovary syndrome. Zhonghua Fu Chan Ke Za Zhi. 2018; 53(1): 18-22. (in Chinese). https://dx.doi.org/10.3760/cma.j.issn.0529-567X.2018.01.005.
- Gomes L.G., Bachega T.A.S.S., Mendonca B.B. Classic congenital adrenal hyperplasia and its impact on reproduction. Fertil. Steril. 2019; 111(1): 7-12. https://dx.doi.org/10.1016/j.fertnstert.2018.11.037.
- Kocova M., Anastasovska V., Bitovska I. The impact of CYP21A2 (P30L/I172N) genotype on female fertility in one family. Eur. J. Med. Res. 2019; 24: 21. https://dx.doi.org/10.1186/s40001-019-0379-4.
- Thurston L., Abbara A., Dhillo W.S. Investigation and management of subfertility. J. Clin. Pathol. 2019; 72(9): 579-87. https://dx.doi.org/10.1136/jclinpath-2018-205579.
- Schwandner A., Zámečník M., Kaščák P. Hyperreactio luteinalis - two accidental findings during cesarean section. Ceska Gynekol. 2019; 84(6): 439-42.
- Wang Y., Gu X., Tao L., Zhao Y. Co-morbidity of cervical incompetence with polycystic ovarian syndrome (PCOS) negatively impacts prognosis: A retrospective analysis of 178 patients. BMC Pregnancy Childbirth. 2016; 16(1): 308.
- Kandeel M.S., Sanad Z.F., Sayyed T.M., Elyazid Elmenawy S.A. The effect of body mass index on cervical characteristics and on the length of gestation in low-risk pregnancies. Menoufia Med. J. 2014; 27 :518-23.
- Prodromidou A., Frountzas M., Perrea D., Vlachos G.D., Pergialiotis V. The impact of obesity on cervical cerclage efficacy: A systematic review of the literature. J. Neonatal. Perinatal. Med. 2016; 9(1): 59-65. https://dx.doi.org/10.3233/NPM-16915058.
- Zaher Y.A., Al-Kholy A.F. Cervicovaginal inflammatory cytokines, obesity and inter-pregnancy interval negatively affect pregnancy duration in pregnant women at high-risk for recurrent spontaneous preterm birth. Adv. Reprod. Sci. 2019; 7: 125-37. https://dx.doi.org/10.4236/arsci.2019.74014.
- Venkatesh K.K., Cantonwine D.E., Zera C., Arjona M., Smith N.A., Robinson J.N., McElrath T.F. Is there an association between body mass index and cervical length? Implications for obesity and cervical length management in pregnancy. Am. J. Perinatol. 2017; 34(6): 568-75. https://dx.doi.org/10.1055/s-0036-1594242.
- Иловайская И.А. Влияние ожирения у женщин на фертильность и вынашивание беременности. РМЖ. 2016; 1: 32-7. [Ilovaiskaya I.A. Influence of obesity in women on fertility and pregnancy. Russian Medical Journal. 2016; (1): 32-7. (in Russian)].
- Радынова С.Б., Иванова Е.А. Осложнение беременности и родов у женщин с ожирением. Современные проблемы науки и образования. 2018; 5: 45. [Radynova S.B., Ivanova E.A. Complication of pregnancy and childbirth in women with obesity. Modern problems of science and education. 2018; (5): 45. (in Russian)].
- Радзинский В.Е., Фукс А.М., ред. Акушерство. Учебник. М.: ГОЭТАР-Медиа; 2016. 1040с. [RadzinskyV.E., FuksA.M., eds. Obstetrics. Textbook. Moscow: GOETAR-Media; 2016. 1040p. (in Russian)].
- Жабченко И.А., Коваленко Т.Н., Сюдмак О.Р. Пути минимизации акушерских рисков у беременных с ожирением. Охрана материнства и детства. 2016; 2: 69-75. [Zhabchenko I.A., Kovalenko T.N., Sudmak O.R.The ways of obstetrical risks minimization in pregnant women with obesity. Protection of Motherhood and Childhood. 2016; (2): 69-75.(in Russian)].
- Доброхотова Ю.Э., Боровкова Е.И., Залесская С.А., Нагайцева Е.А.,Раба Д.П. Диагностика и тактика ведения пациенток с истмико-цервикальной недостаточностью. Гинекология. 2018; 2: 41-5.[Dobrokhotova Yu.E., Borovkova E.I., Zalesskaya S.A., Nagaitseva E.A.,Raba D.P. Diagnosis and management of patients with isthmic-cervical insufficiency. Gynecology. 2018; (2): 41-5. (in Russian)].
- Белова И.С., Хащенко Е.П., Уварова Е.В. Современные аспекты роли инсулинорезистентности, системного воспаления и оксидативного стресса в патогенезе гиперандрогении и нарушений фолликулогенеза у пациенток с синдромом поликистозных яичников. Акушерство и гинекология. 2021; 5: 55-63. [Belova I.S., Khashchenko E.P., Uvarova E.V. Current aspects of the role of insulin resistance, systemic inflammation, and oxidative stress in the pathogenesis of hyperandrogenism and abnormal folliculogenesis in patients with polycystic ovary syndrome. Obstetrics and Gynecology. 2021; (5): 55-63. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.5.55-63.
- Чернуха Г.Е., Найдукова А.А., Каприна Е.К., Донников А.Е. Молекулярно-генетические предикторы формирования синдрома поликистозных яичников и его андрогенных фенотипов. Акушерство и гинекология. 2021; 4: 120-7. [Chernukha G.E., Naidukova A.A., Kaprina E.K., Donnikov A.E. Molecular genetic predictors of polycystic ovary syndrome and its androgenic phenotypes. Obstetrics and Gynecology. 2021; (4): 120-7. (in Russian)].https://dx.doi.org/10.18565/aig.2021.4.120-127.
Received 08.02.2023
Accepted 22.06.2023
About the Authors
Sergey A. Levakov, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology of the N.V. Sklifosovsky Institute of Clinical Medicine, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University), levakoff@yandex.ru , 119991, Russia, Moscow, Trubetskaya str., 8-2.Artur V. Mokh, Head of the Maternity Department, M.P. Konchalovsky City Clinical Hospital, Perinatal Center, mohar2205@mail.ru,
124489, Russia, Moscow, Zelenograd, Kashtanovaya alleya, 2-1.



