Взаимосвязь между тромботическими осложнениями и злокачественными новообразованиями показана во многих работах. Ее часто называют феноменом Труссо, или синдромом Труссо, но этот термин первоначально характеризовал состояние воспаления вен, которое приводило к мигрирующему поверхностному тромбофлебиту на фоне скрытой формы рака внутренних органов [1]. Синдром был описан Арманом Труссо в 1865 г. [2, 3]. Это взаимное влияние между тромбозом глубоких вен и раком формирует порочный круг патогенеза как роста и метастазирования новообразования, так и тромбоэмболических осложнений (рис. 1).
Риск развития венозной тромбоэмболии (ВТЭ) увеличивается у пациентов с онкологическими заболеваниями. В классическом исследовании P. Prandoni и соавт. [4] у 842 пациентов с ВТЭ риск рецидива тромбоза в течение 12 месяцев составил 20,7% среди пациентов с онкологическими заболеваниями и 6,8% – у пациентов без злокачественных новообразований. В то же время рак диагностируется впервые у 20% пациентов с предшествующим эпизодом ВТЭ. Кроме того, возникновение ВТЭ является фактором негативного прогноза для пациентов со злокачественными новообразованиями, поскольку тромботические осложнения являются маркером снижения выживаемости [5]. С другой стороны, у 10% пациентов с идиопатическими ВТЭ диагностирован рак в течение следующих 12 месяцев [6]. Таким образом, взаимосвязь между злокачественными новообразованиями и тромботическими осложнениями определенно прослеживается.
Факторы риска тромботических осложнений, ассоциированных со злокачественными новообразованиями
Факторы риска развития тромбоза у пациентов со злокачественными новообразованиями могут быть разделены на опухоль-зависимые, терапия-зависимые и пациент-зависимые (табл. 1).

Факторы риска, связанные непосредственно с новообразованием, иллюстрируют три механизма, описанные Рудольфом Вирховым (триада Вирхова): снижение кровотока из-за компрессии опухолевыми массами вены крупного калибра; повреждение сосуда в процессе инвазии или воздействия на эндотелий опухолевых факторов и, наконец, гиперкоагуляция, обусловленная опухоль-ассоциированными прокоагулянтами и проагрегантами, в частности, муцином или раковым прокоагулянтом (СР), выделенным из аденокарциномы, который непосредственно способен активировать X-фактор свертывания.
Таким образом, существуют некоторые биомаркеры, которые можно использовать для оценки риска того, что у пациента с тромботическим эпизодом, вероятно, может быть рак: растворимый тканевый фактор (ТФ), растворимый P-селектин, повышенный C-реактивный белок, высокий Д-димер после острой фазы тромбоза, количество тромбоцитов выше 350×109/л или количество лейкоцитов выше 11×109/л.
Скрининг новообразований у пациентов с ВТЭ
У значительного числа пациентов с тромбозом рак может быть диагностирован в течение 12 месяцев [6, 7]. Этот вывод был получен из систематического обзора литературы по данным 36 исследований. Более того, обширный скрининг на наличие онкологических заболеваний с использованием компьютерной томографии органов брюшной полости и таза увеличивал скорость обнаружения данной патологии с 49 до 70%. Конечной целью скрининга является раннее выявление рака, чтобы увеличить шансы на излечение и тем самым снизить смертность. Опубликованы три проспективных рандомизированных исследования, в которых сравнивается возможность обнаружения злокачественного процесса при применении стандартного и расширенного скринингов у пациентов с идиопатической ВТЭ (табл. 2) [8–10].
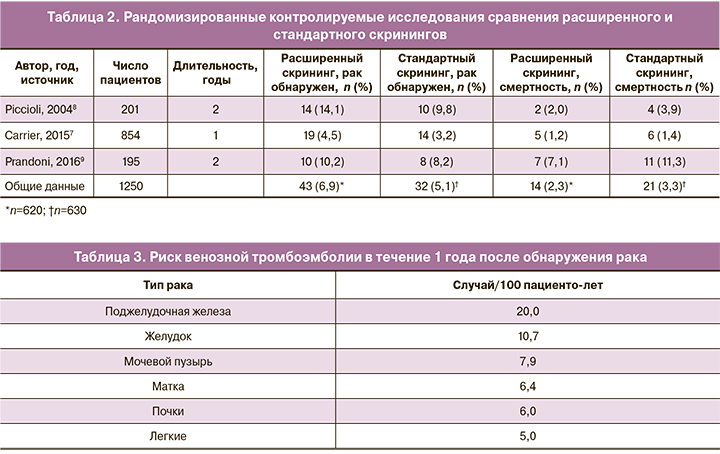
Увеличение частоты выявления случаев рака при проведении обширного скрининга статистически незначимое, однако смертность ниже в группе расширенного скрининга: относительный риск (ОР) составляет 0,67 (95% доверительный интервал (ДИ) 0,33–1,33). На основании чего мы не можем рекомендовать проводить расширенный скрининг на наличие онкологической патологии у каждого пациента с идиопатической ВТЭ, а только с учетом дополнительных факторов риска, связанных с полом и возрастом пациентов [8, 11].
Прогнозирование риска тромбоза у онкологических больных
Пациенты с онкологическими заболеваниями могут иметь повышенный риск кровотечения из-за тромбоцитопении, связанной с химиотерапией, или из-за локализации опухоли вблизи сосудов, что ограничивает возможности проведения рутинной тромбопрофилактики с помощью антикоагулянтов. При анализе 235 149 случаев рака H. Chew и соавт. [5] обнаружили, что в течение первого года после постановки диагноза наибольший риск тромбоэмболии наблюдался у пациентов с метастатическим раком поджелудочной железы (табл. 3).
Было разработано несколько моделей оценки риска развития ВТЭ для лучшего прогнозирования того, пациентам с какими онкологическими заболеваниями профилактика ВТЭ будет особенно полезна. Наиболее известной и достоверной является шкала Khorana по оценке риска ВТЭ, предназначенная для амбулаторных пациентов, получающих химиотерапию (табл. 4) [12].
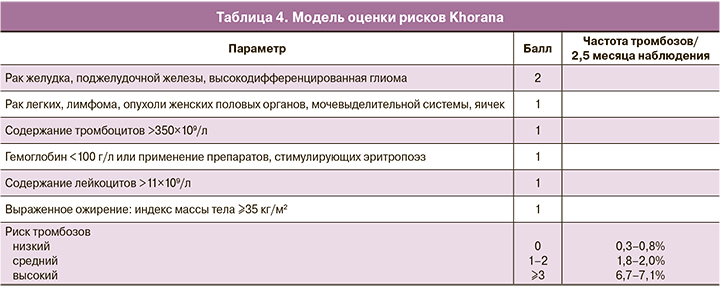
Спустя два года Ay и соавт. [13] использовали данные Венского исследования рака и тромбоза (CATS) для проверки шкалы оценки риска Khorana, а затем для проверки диагностической значимости двух биомаркеров: растворимого P-селектина и D-димера. Риск развития ВТЭ в течение 6 месяцев наблюдения по шкале оценки риска Khorana составил 0, 1, 2 и 3 у 1,5%, 3,8%, 9,6% и 17,7% соответственно. При добавлении двух биомаркеров разброс значений увеличился: низкий, средний и высокий риск (0, 3 и ≥5 по шкале CATS) был обнаружен у 1,0%, 10,3% и 35,0% соответственно. Впоследствии Verso и соавт. [14] разработали шкалу «Профилактика тромбоэмболии во время химиотерапии» (Protecht), используя данные рандомизированного исследования. Применение химиотерапии с использованием цисплатина, карбоплатина или гемцитабина добавило еще один пункт к шкале оценки Khorana. У пациентов с высоким риском (≥3) по шкале оценки Khorana либо по шкале Protecht лечение низкомолекулярным гепарином (НМГ) (надропарином) уменьшало риск ВТЭ, хотя и статистически значимо только по шкале оценки Protecht (ОР 0,27; 95% ДИ 0,09–0,78).
Эти три шкалы оценки риска и четвертая шкала CONKO были проверены в исследовании с участием 876 больных раком [15–17], однако высокой достоверности результатов продемонстрировано не было. Лучшее разделение пациентов с низким и высоким риском (оценка ≥3) было обнаружено при применении Венской шкалы оценки CATS и шкалы Protecht. Таким образом, на настоящий момент ни одна из этих моделей оценки не может быть безусловно рекомендована для повседневного использования в клинической практике.
Профилактика ВТЭ у пациентов с онкологическими заболеваниями, проходящими стационарное лечение
Международное руководство рекомендует проводить профилактику ВТЭ у госпитализированных пациентов с онкологическими заболеваниями, если их состояние связано с иммобилизацией [18]. Применяются препараты НМГ, нефракционированный гепарин или фондапаринукс (класс 1B). То же самое руководство не рекомендует проводить рутинную профилактику пациентам во время химиотерапии (класс 1B). Однако у пациентов с местнораспространенным, или метастатическим раком поджелудочной железы (класс 1B), или раком легкого (класс 2B) профилактика тромбозов показана. Пациенты с раком головного мозга могут восприниматься как группа высокого риска по кровотечениям, однако этот риск низкий, и профилактика, например после оперативного вмешательства, им не противопоказана.
Оперативные вмешательства. Пациенты с онкологическими заболеваниями, требующими хирургического вмешательства, имеют выраженный риск развития ВТЭ по причине комбинированного риска из-за наличия рака, хирургического вмешательства и иммобилизации. Из нескольких исследований видно, что доза нефракционированного гепарина, применяемого в общей хирургии, то есть 5000 ЕД дважды в день, является неоптимальной у пациентов с онкологической патологией [19]. Эффективным является применение нефракционированного гепарина по 5000 ЕД три раза в день или НМГ один раз в день (класс 1А) [18]. Первая доза должна быть назначена за 2–12 ч до начала операции (класс 1А), курс не менее 7–10 дней. Механические методы профилактики сами по себе недостаточно эффективны, но если антикоагулянтная терапия противопоказана из-за риска кровотечения, то применение механических методов обязательно (класс 2С) [18].
Для пациентов с большим объемом хирургического вмешательства при абдоминальной или тазовой локализации рака риск тромбоза повышен в течение нескольких недель. Результаты проспективного когортного исследования с участием более чем 2000 пациентов показали двухфазную кривую динамики эпизодов тромбоза после такой операции, большинство из которых пришлись на первые 5 дней, уменьшаясь к 16–20 дню, а затем был продемонстрирован еще один пик [20]. Это объясняется тем, что в большинстве случаев профилактика была прекращена как раз через 2–3 недели после операции. Три рандомизированных клинических исследования (ENOXACAN II, FAME и CANBESURE), в которых применяли три различных препарата НМГ в течение одного месяца, по сравнению со стандартной продолжительностью послеоперационной терапии продемонстрировали, что риск ВТЭ снижался без значительного увеличения риска кровотечения (рис. 2) [21–23].
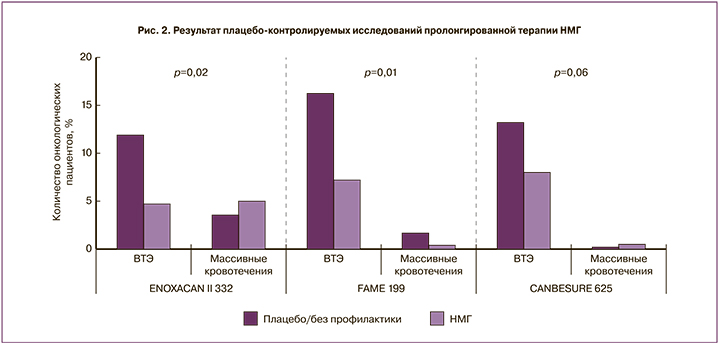
В настоящее время несколько руководств по клинической практике рекомендуют пролонгированную профилактику до 4 недель после обширного хирургического вмешательства на органах брюшной полости или таза у пациентов с высоким риском развития ВТЭ, то есть с онкологией [24–26].
НМГ и выживаемость при онкологических заболеваниях
По данным метаанализа, среди пациентов, изначально получавших терапию для профилактики ВТЭ с использованием НМГ, уровень смертности был ниже, чем среди получавших нефракционированный гепарин [27]. Повышение выживаемости было значительным у пациентов с онкологическими заболеваниями. Кроме того, в исследовании, посвященном сравнению далтепарина с варфарином, в частности у пациентов с онкологическими заболеваниями, вновь была продемонстрирована более низкая смертность в первом случае [28].
Также для подтверждения влияния на улучшение выживаемости у пациентов с онкологическими заболеваниями без анамнеза ВТЭ были проведены плацебо-контролируемые исследования с применением надропарина или тинзапарина [29]. Были включены пациенты с немелкоклеточным раком легкого, гормонально-независимым раком предстательной железы и с местнораспространенным раком поджелудочной железы, которым была проведена терапия в течение 6 недель. В исследование с тинзапарином были включены только пациенты с немелкоклеточным раком легкого, и их лечили в течение 12 недель. Ни одно из исследований, однако, не показало никакого повышения выживаемости. В связи с чем на настоящий момент единственным доказанным показанием применения НМГ у онкологических больных является профилактика или лечение ВТЭ.
Лечение ВТЭ при онкологических заболеваниях
НМГ. В течение более 10 лет золотым стандартом для лечения рак-ассоциированных тромботических осложнений была терапия НМГ. Эти выводы были сделаны на основании исследования CLOT, в котором было показано значительное снижение риска рецидива ВТЭ при применении далтепарина по сравнению с варфарином (ОР 0,48) у 672 пациентов [30]. Причем не было найдено различий в частоте кровотечений, в том числе и массивных. Более того, в исследовании CANTHANOX (применялся эноксапарин), опубликованном годом ранее, было продемонстрировано статистически значимое снижение рецидивов ВТЭ и массивных кровотечений по сравнению с варфарином в группе из 146 пациентов [31]. Пациенты в обоих исследованиях получали терапевтические дозы НМГ на протяжении всего исследования (3–6 месяцев), хотя с 25% сокращением дозы после первого месяца в исследовании CLOT. Десять лет спустя в исследовании CATCH оценивался эффект тинзапарина по сравнению с варфарином в течение 6 месяцев у 900 пациентов [32]. Было отмечено, что применение тинзапарина связано с уменьшением риска рецидива ВТЭ (ОР 0,65; 95% ДИ 0,41–1,03), при отсутствии различий частоты тяжелых кровотечений, но уменьшения частоты легких кровотечений (ОР 0,58; 95% ДИ 0,40–0,84).
В метаанализе 5 исследований, сравнивающих НМГ с антагонистами витамина К у пациентов с тромбозом и онкологическими заболеваниями, было выявлено почти 50% снижение риска рецидива ВТЭ на фоне применения НМГ (ОР 0,53; 95% ДИ 0,36–0,76) без какого-либо влияния на риски развития тяжелых или легких кровотечений или смертности от всех причин [33].
Таким образом, в целом продемонстрировано стабильно подтверждаемое преимущество при применении НМГ по сравнению с варфарином, и это оправдывает рекомендацию по его использованию в течение по крайней мере первых 3 месяцев (класс 2B или класс 1B) после эпизода ВТЭ [18, 34]. Применение варфарина у онкологических больных может быть затруднено из-за взаимодействий с многими лекарственными препаратами или рвоты. Тем не менее в долгосрочной перспективе пациенты более склонны к использованию перорального препарата, а не ежедневных подкожных инъекций. Более того, НМГ намного дороже, чем варфарин, и в некоторых странах недоступен для поддерживающей антикоагулянтной терапии в течение нескольких месяцев.
Прямые пероральные антикоагулянты. Опыт применения пероральных антикоагулянтов, которые непосредственно ингибируют Х-фактор свертывания или тромбин (II фактор), в большей степени касается пациентов без онкологических заболеваний, однако и в онкологии эти препараты также применялись. В метаанализе, включающем исследования сравнения дабигатрана, ривароксабана, апиксабана и эдоксабана с антагонистами витамина K для лечения ВТЭ, ОР рецидива ВТЭ у пациентов с онкологическими заболеваниями был значительно ниже при применении прямых антикоагулянтов (ОР 0,57; 95% ДИ 0,36–0,91) [35]. Однако количество пациентов с онкологическими заболеваниями в этих исследованиях было всего около 5% от всей популяции исследования, и, вероятно, в целом их прогноз был изначально лучше, поскольку смертность в этой группе была намного ниже, чем в исследованиях, включавших только онкологических пациентов, в частности CLOT.
Значительно важнее сравнить прямые антикоагулянты с НМГ. Эдоксабан в исследовании Hokusai VTE Cancer [36] и ривароксабан в экспериментальном исследовании SELECT-D34 показали тенденции к лучшей эффективности, чем НМГ (табл. 5).

Интересно, что в обоих исследованиях наблюдался повышенный риск кровотечения при приеме прямых антикоагулянтов в основном за счет желудочно-кишечного кровотечения у пациентов с новообразованиями желудочно-кишечного тракта. Есть несколько объяснений этого феномена, но, скорее всего, причина в том, что прямые антикоагулянты выводятся из кровотока через кишечник посредством Р-гликопротеина и обнаруживаются в высоких концентрациях в стуле. Если в слизистой оболочке кишечника на фоне онкологического процесса образуются аномальные сосуды, то даже небольшое кровотечение может усилиться при взаимодействии с активным антикоагулянтом [37].
Небольшое исследование (287 пациентов), сравнивающее апиксабан с НМГ – ADAM VTE Trial, на настоящей момент опубликовано только в виде аннотации [38]. В нем не наблюдалось тяжелых кровотечений при применении апиксабана в отличие от дальтепарина – 3 случая, в то время как колоректальный рак был одним из наиболее распространенных злокачественных новообразований в рамках исследования. Рецидивы ВТЭ возникали значительно реже при применении апиксабана (ОР 0,26; 95% ДИ 0,09–0,80). Пока неясно, будут ли подтверждены эти благоприятные результаты с апиксабаном в более крупном исследовании CARAVAGGIO (ClinicalTrials.gov NCT03045406).
Кава-фильтр нижней полой вены. В качестве рутинного метода имплантация кава-фильтра нижней полой вены у пациентов с рак-ассоциированной ВТЭ не рекомендована. Крупные исследования на данный момент отсутствуют, но общая рекомендация заключается в том, чтобы имплантировать фильтр только в случае острого тромбоза в проксимальной части ноги и/или тромбоэмболии легочной артерии при наличии противопоказаний к антикоагулянтной терапии вследствие активного кровотечения или риска кровотечения в критической области [18]. У пациентов с рецидивирующим или прогрессирующим тромбозом, несмотря на то, что они уже получают терапевтическую дозу антикоагулянта, предпочтительнее повышение дозы НМГ.
Тромболитическая терапия. Существует несколько ретроспективных когортных исследований применения тромболитической терапии у пациентов с тромбозом и онкологическими заболеваниями. Kim и соавт. [39] сравнивали эффективность катетер-опосредованного тромболизиса у 61 пациента со злокачественными новообразованиями и 117 – без рака. Лизис сгустка был достигнут в обеих группах в равной степени, и риск развития массивного кровотечения не был статистически увеличен у онкологических пациентов (4,9% против 3,4%). В другом исследовании, проведенном Maleux и соавт. [40], включавшем 35 пациентов с активной формой рака и 33 – без, у всех пациентов был центральный тромбоз в области грудной клетки. Катетер-опосредованный лизис сгустка наблюдался в 88,6 и 93,8% соответственно, и частота тяжелых кровотечений у пациентов со злокачественными новообразованиями была гораздо меньше (2 против 7). Тем не менее тромболизис не следует использовать, если у пациента обнаружены метастазы в головном мозге.
Длительная вторичная профилактика
Лечение НМГ обычно продолжается в соответствии с рекомендациями в течение как минимум 3 месяцев и до 6 месяцев, в зависимости от того, как пациент переносит инъекции. Исследования, проводимые с НМГ в течение 6 месяцев, трудновыполнимы. В частности, исследование LONGHEVA было прекращено досрочно из-за крайне медленного набора пациентов в него (ClinicalTrials.gov NCT01164046).
В исследовании HOKUSAI VTE Cancer, которое длилось 360 дней, приняли участие 168 пациентов в группе эноксабана и 174 пациента – в группе далтепарина. Рецидивы ВТЭ происходили с одинаковой частотой в обеих группах до 90 дня исследования, но после этого самочувствие пациентов, принимавших эноксабан, становилось все лучше. Риск тяжелых кровотечений был выше в группе эноксабана с самого начала и увеличивался с течением времени.
Таким образом, на настоящий момент сложно отдать предпочтение НМГ или пероральным антикоагулянтам. Очевидно, что пероральное применение один раз в день предпочтительнее для пациентов и, кроме того, значительно менее дорогостоящее, чем НМГ.
Ведение пациентов с рецидивами ВТЭ
Случаи рецидива ВТЭ на фоне антикоагулянтной терапии у онкологических больных выявляются у 7–27% пациентов, что значительно выше, чем у пациентов без злокачественных новообразований (рис. 3а) [4, 41, 42]. Риск кровотечения также выше у онкологических больных, но относительно ниже, чем риск рецидива ВТЭ (рис. 3б).
В международном исследовании [43] 212 пациентов со злокачественными новообразованиями и рецидивами ВТЭ наблюдались в течение 3 месяцев. Из них у 73% был метастатический рак, и доминирующим типом рака среди всех пациентов была аденокарцинома (59%). Большинство (70%) пациентов на момент рецидива ВТЭ уже получали НМГ. Единого протокола назначения антикоагулянтов не было, увеличение интенсивности антикоагулянтной терапии после случая ВТЭ не уменьшало частоту рецидивов по сравнению с теми, кто продолжал прием препаратов по той же схеме. Но пациенты с терапией НМГ показали лучшие результаты, чем те, кто принимал антагонисты витамина К. Однако по результатам двух небольших ретроспективных когортных исследований оказалось, что при увеличении дозы НМГ на 20–25% риск рецидива уменьшается [44, 45].
Таким образом, существует 3 варианта ведения таких пациентов – переход от антагонистов витамина К на НМГ, увеличение дозы НМГ примерно на 25% или имплантация кава-фильтра в нижнюю полую вену [18].
Способствует ли тромбоз развитию злокачественного процесса?
Риск выявления рака после эпизода ВТЭ примерно в 3–4 раза выше, чем у сопоставимой популяции без ВТЭ [46]. В целом разумно полагать, что онкологический процесс присутствовал уже в то время, когда был обнаружен тромбоз, и способствовал его развитию.
Тем не менее существует еще и повышенный риск нахождения новообразования через 4–6 лет после случая ВТЭ, и неясно, как долго может существовать рак и вызывать тромбоз. Более убедительную эпидемиологическую информацию можно получить в результате исследований пациентов с гипокоагуляцией, а именно на фоне терапии антикоагулянтами.
В раннем исследовании Zacharski и соавт. обнаружили повышенный уровень выживаемости у пациентов с мелкоклеточным раком легкого на фоне терапии варфарином [47, 48]. В исследовании с участием 897 пациентов с первым эпизодом ВТЭ, которым проводилась терапия варфарином в течение 6 недель или 6 месяцев, частота рецидива рака была выше на 50% среди пациентов, получивших короткий курс терапии антикоагулянтами [46]. Разница в частоте возникновения рецидивов была значимой после 6 лет при любом виде онкологии, в том числе для урогенитального рака.
В исследовании Tagalakis и соавт. [49] была продолжена эта идея и проведено сравнение 19 412 случаев среди пациентов, у которых развился урогенитальный рак, и группой контроля – 160 470 человек. У больных, которым проводилась терапия варфарином в течение многих лет, был выявлен более низкий риск развития этого вида рака, по сравнению с теми, кто вообще не получал варфарин (ОР 0,80; 95% ДИ 0,65–0,99). Аналогичным образом в итальянском исследовании среди пациентов в возрасте 65 лет у 3231 пациентов, которым проводилась терапия антагонистами витамина К, риск заболеваемости был ниже, чем у 72 777 пациентов контрольной группы (ОР 0,88; 95% ДИ 0,80–0,98) [50]. В отношении рака предстательной железы снижение риска было еще более весомым (ОР 0,69; 95% ДИ 0,50–0,97). Таким образом, антагонисты витамина К, по-видимому, оказывают защитное действие в отношении развития урогенитального рака или по крайней мере рака предстательной железы.
Для понимания механизма онкологической защиты было проведено проспективное когортное исследование G.J. Miller и соавт., наблюдавших за 3052 мужчинами среднего возраста без онкологических заболеваний. Ежегодно проводили лабораторный контроль уровней фрагментов протромбина 1+2 (F1+2) и фибринопептида А (FPA), которые являются маркерами активации свертывания крови [51, 52]. При обнаружении значений в верхнем квартиле 2 года подряд исследуемый относился к группе имеющих «персистирующую активацию свертывания крови». Таким образом, были выявлены 111 пациентов, тогда как на основании статистических ожиданий их должно было быть гораздо меньше – 43 человека. У пациентов с устойчивым повышением F1+2 и/или FPA была зарегистрирована более высокая смертность от всех причин (1,71% против 0,97%, p=0,015), более высокая смертность от рака (1,13% против 0,51%, p=0,01) и более высокая смертность от рака желудочно-кишечного тракта (0,63% против 0,19%, p=0,004).
Другим потенциальным механизмом защитного действия антагонистов витамина K является снижение активации: посредством тканевого фактора VIIа, что приводит к снижению рецепторной экспрессии урокиназы и уменьшению инвазии опухоли [53, 54]; путем ингибирования тромбина, приводящего к меньшему индуцированному тромбином высвобождению матричной металлопротеиназы-2 и, следовательно, к уменьшению деградации белков внеклеточного матрикса [55].
Заключение
Повышенный риск развития тромбоза у пациентов с онкологическими заболеваниями хорошо известен. Некоторым группам пациентов высокого риска проводится профилактика амбулаторно во время химиотерапии или после обширной операции в течение длительного периода.
В случае рак-ассоциированных ВТЭ доказано, что предпочтительно проводить терапию НМГ, а не антагонистом витамина К. Новые данные о прямых антикоагулянтах свидетельствуют, что они могут быть эквивалентно хорошей альтернативой с более доступным способом приема препарата и более низкой стоимостью.
Однако есть группы пациентов, которым не подходит терапия прямыми оральными антикоагулянтами, в частности, пациенты с раком желудочно-кишечного тракта, с тяжелой почечной недостаточностью или совместным приемом препаратов, которые являются сильными ингибиторами или энхансерами P-гликопротеина или CYP 3A4, что может обусловливать опасно высокие или низкие концентрации прямых антикоагулянтов.
Связь между раком и тромбозом является двунаправленной. Накопленные данные показывают, что воздействие антагонистов витамина К снижает риск урогенитального рака (рак предстательной железы) и, возможно, также желудочно-кишечного рака, скорее всего, путем снижения активации свертывания крови. Вполне возможно, что аналогичный эффект может быть достигнут при приеме прямых пероральных антикоагулянтов, но этот механизм требует дальнейшего изучения.



