Feasibility of using long noncoding RNAs for predicting malignant transformation of cervical epithelium
Objective: To investigate expression levels of long noncoding RNAs linc-ROR and MALAT1 in the normal cervical epithelium and cervical intraepithelial neoplasia and evaluate the change in their expression depending on the severity of cervical dysplasia. Materials and methods: The study included 89 reproductive age patients with histologically confirmed LSIL and HSIL and high-risk type of HPV. The comparison group included 40 patients with cytologically excluded cervical disease and test negative for HPV. The expression levels of long noncoding RNAs linc-ROR and MALAT1were analyzed by Real-Time PCR using primers specific for these genes. Results: The expression level of MALAT1 in cervical dysplasia was statistically significantly higher than in the control group, with 62.5% of patients having LSIL of 2.6±0.42 and 79.8% having HSIL of 4.9 ± 2.65 (p<0.001, Kruskal–Wallis test). No statistically significant differences between the groups were found in linc-ROR expression (LSIL 2.07±0.34, HSIL 1.91±0.86 in the study group, and 1.0±0.4 in the comparison group (p = 0.368) (Kruskal – Wallis test), which indicates no predictive value of linc-ROR for the diagnosis of precancerous lesions of the cervix. Conclusion: The search for early diagnostic markers of malignant transformation of the cervical epithelium is a priority research area aimed to reduce the incidence of cervical cancer. This study demonstrated an increase in the level of MALAT1 expression in LSIL in 62.5% and HSIL in 79.8% of patients compared with normal cervical epithelium, which indicates a high proliferative activity of cells, degradation of the gene p53 suppressing tumor growth, and blockage of apoptosis. Therefore, MALAT1 may become a promising biomarker for the progression of cervical dysplasia.Levakov S.A., Obukhova E.A., Sheshukova N.A., Bol'shakova O.V., Shakhparonov M.I., Antipova N.V.
Keywords
Over the past 20 years, significant progress has been made in diagnosing and treating cervical dysplasia. Despite the general decrease in the incidence of cervical cancer in developed countries after the introduction of effective screening and vaccination programs, there has been an increase in morbidity and mortality among women of reproductive age, especially in Latin America and Africa [1–5]. According to the World Health Organization statistics, the incidence rate of cervical cancer ranks second among female malignant tumors in the world, annually claiming more than 275,100 lives [1]. Many modern studies have reported a strong association between persistent oncogenic HPV infection in the cervical epithelium and a high risk of developing dysplasia and cervical cancer [1–5]. High-risk HPV types include two clinically significant types E6 and E7, which play an essential role in the pathogenesis of cervical cancer by neutralizing p53 and Rb tumor suppressor pathways leading to inhibition of cell differentiation and increased cell proliferation [6]. Even though the pathogenesis of cervical cancer is well understood, the molecular mechanisms of malignant transformation of cervical dysplasia have not been fully elucidated [6]. The development of new prognostic markers that determine the likelihood of cervical epithelium malignant transformation is still an urgent task of modern gynecology.
Long noncoding RNAs (lncRNAs) are non-coding transcripts longer than 200 nucleotides regulating physiological intracellular processes, intercellular interactions, and tumorigenesis [7]. lncRNAs have been reported to play critical roles in transcription, post-transcriptional processing, and translation, such as epigenetics, post-transcriptional regulation, chromatin modification, and regulation of the cell cycle [7, 8]. Besides, since ncRNAs can be packed into extracellular vesicles, including exosomes, they provide a mechanism for intercellular communication by transferring miRNAs and ncRNAs to recipient cells both locally and systemically [9]. LncRNAs play an essential role in the pathophysiology of human diseases, especially in the development and progression of tumors through the Wnt, Hedgehog, Notch, and PI3K/AKT/mTOR signaling pathways [6]. Dysregulation of several lncRNAs has been found in many types of cancers, including breast, ovarian, cervical, and prostate cancers, suggesting lncRNAs potential to act as a prognosis marker and treatment target of cancer.
Recent studies have shown that lncRNAs may play a vital role in developing cervical cancer [9]. Linc-ROR (Long Intergenic Non-Protein Coding RNA, Regulator of Reprogramming) as a new intergenic non-protein coding RNA, has been considered to be a pivotal regulatory factor that affects the occurrence and development of human tumors, including breast cancer, colorectal cancer, pancreatic cancer, hepatocellular carcinoma and others [10]. Linc-ROR is a novel and essential carcinogenic 2.6 kb lncRNA located in chromosome 18, initially identified as a highly expressed transcript of pluripotent and embryonic stem cells [7, 8]. Linc-ROR regulates the reprogramming of pluripotent stem cells [1]. This RNA suppresses the p53 signal pathway after DNA damage [7]. This RNA can also play an extracellular role in modulating the response to hypoxia in tumor cells [11]. In recent years, many studies investigating linc-ROR and tumorigenesis have shown that overregulation of linc-ROR positively correlates with clinical and pathological characteristics and tumor progression [11]. The growth and metastasis of tumors are stimulated by linc-ROR through the activation of epithelial-mesenchymal transformation [8]. Additionally, studies have found that the expression level of linc ROR was substantially upregulated in samples of papillary thyroid carcinomas and samples of metastatic PTC and PTC cell lines [11]. Since linc-ROR can regulate cell proliferation, apoptosis, migration, and invasion, it can thus be used as a potential biomarker for tumors and has potential clinical significance as a therapeutic target.
Another promising biomarker for the progression of cervical dysplasia is ncRNA MALAT1 (Metastasis associated lung adenocarcinoma transcript 1), a multifunctional RNA that forms molecular scaffolds for ribonucleoprotein complexes that regulate cell proliferation and migration [12, 13]. MALAT1 may act as a transcriptional regulator for numerous genes, including some genes involved in cancer metastasis and cell migration, and it is involved in cell cycle regulation [12]. The increased expression of MALAT1was associated with breast, ovarian, cervical, and endometrial cancers [14]. In 2015, a group of Chinese researchers led by L. Yang revealed increased expression of MALAT1 in cervical cancer compared to the normal epithelium, while MALAT1 expression depended on tumor size, FIGO stage, vascular invasion, and lymph node metastases. Knockdown of MALAT1 decreases cell proliferation, increases apoptosis, inhibits migration and invasion [12]. Thus, linc-ROR and MALAT1 may play a potential role in the pathogenesis of cervical cancer.
This study aimed to investigate expression levels of long noncoding RNAs linc-ROR and MALAT1 in the normal cervical epithelium and cervical intraepithelial neoplasia and evaluate the change in their expression depending on the severity of cervical dysplasia.
Materials and methods
The study included 89 reproductive age patients with histologically confirmed LSIL and HSIL and high-risk types of HPV. Group I comprised 43 patients with LSIL + high-risk HPV. Group II included 46 patients with HSIL + high-risk HPV. The comparison group included 40 patients with cytologically excluded cervical disease and tested negative for HPV. All participants provided signed informed consent to take part in the study.
Study inclusion criteria:
Groups I and II
1. Women of reproductive age (ages 18 to 40);
2. A signed informed consent to participate in the study;
3. Radio-wave excision of the cervix for LSIL and HSIL and histopathology reporting LSIL (CINI) and HSIL (CIN II-III);
4. Regular menstrual cycle;
5. The presence of high-risk HPV types including 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68 types.
Comparison group
1. Women of reproductive age (ages 18 to 40);
2. A signed informed consent to participate in the study;
3. Absence of HPV infection;
4. Regular menstrual cycle;
4. Cytological diagnosis of NILM.
Exclusion criteria from the study:
1. Acute pelvic inflammatory diseases;
2. Pregnancy, puerperium, and lactation;
3. Oncological and autoimmune diseases;
4. Decompensated extragenital diseases;
5. Hormonal therapy;
6. Refusal of the patient to participate in the study;
7. Violation of the study protocol.
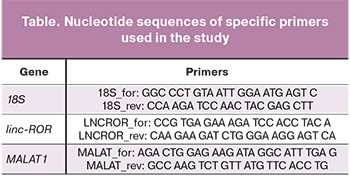 The expression levels of linc-ROR and MALAT1 were analyzed by Real-Time PCR using primers specific for these genes. Samples for molecular genetic study were obtained during a gynecological examination from the transformation zone of the cervical epithelium using an endocervical brush of type D2. The cytobrush was broken off and placed in a 1.5 ml Eppendorf micro-centrifuge tube with 1 ml of Trizol reagent to inhibit RNases and isolate total mRNA. Within 2 hours after taking the biological material, the samples were homogenized and frozen at a temperature of -80°C. Total mRNA was isolated according to the Invitrogen protocol. The MMLV reverse transcription kit (Evrogen, Russia) was used to synthesize the first cDNA strands with specific primers complementary to the highly conserved regions of the linc-ROR and MALAT1 gene loci (table).
The expression levels of linc-ROR and MALAT1 were analyzed by Real-Time PCR using primers specific for these genes. Samples for molecular genetic study were obtained during a gynecological examination from the transformation zone of the cervical epithelium using an endocervical brush of type D2. The cytobrush was broken off and placed in a 1.5 ml Eppendorf micro-centrifuge tube with 1 ml of Trizol reagent to inhibit RNases and isolate total mRNA. Within 2 hours after taking the biological material, the samples were homogenized and frozen at a temperature of -80°C. Total mRNA was isolated according to the Invitrogen protocol. The MMLV reverse transcription kit (Evrogen, Russia) was used to synthesize the first cDNA strands with specific primers complementary to the highly conserved regions of the linc-ROR and MALAT1 gene loci (table).
The expression level of the linc-ROR and MALAT1 genes was determined by real-time reverse transcription-polymerase chain reaction (qRT-PCR) using a LightCycler 96 Real-Time PCR System (Roche). PCR was carried out under the following conditions: preliminary incubation 150 s at 95ºC; 3-step amplification: 20 sec at 95ºC, 20 sec at 60ºC, 20 sec at 72 ºC 45 cycles; temperature detection - melting of reaction products. The cDNA samples were normalized using ribosomal 18S RNA as internal standard. The linc-ROR and MALAT1 genes relative expressions were calculated using the 2-ΔΔCT method. The absence of PCR by-products was determined by the melting curve. For each pair of primers in all samples, each sample's same PCR melting peaks were observed in triplicate. The obtained cycle thresholds (Ct) for each sample did not exceed 35.
Statistical analysis
Statistical analysis was performed using Microsoft Excel and StatTech v. 1.2.0 (Stattech LLC, Russia). Descriptive statistics included means and standard deviations M (SD). The median (Me) was used as a measure of the central tendency of all quantitative variables, and the lower Q1 (0.25) and upper Q3 (0.75) quartiles were used as an interval estimate. Categorical variables were summarized using frequency counts and percentages (%). The distribution of continuous variables was tested for normality using the Shapiro–Wilk test (with the number of subjects being less than 50) or the Kolmogorov–Smirnov test (with the number of subjects being more than 50). The statistical significance of between-group differences for continuous variables was tested with Mann–Whitney U test, and differences between the groups were considered statistically significant at p ≤ 0.05.
Results
There were no statistically significant differences between the groups regarding age, anthropometric data, menstrual function, and chronic diseases. The most common HPV types were 16 – 50/89 (56.18%), 18 – 12/89 (13.48%), 31 – 8/89 (8.9%), 52 – 7/89 (7.8%). HSV-2 was detected in 11/43 (25.58%) patients with LSIL, 10/46 (21.73%) patients with HSIL, and 2/40 (5%) in the comparison group. Bacterial infections (Chlamydia trachomatis, Neisseria gonorrhoeae, Micoplasma genitalium, Trichomonas vaginalis) were detected in 35/89 (39.32%) patients with cervical dysplasia. The number of sexual partners in patients with HPV infection was higher than in the comparison group: group I – 3.1 (1.9), group II – 4.6 (1.8), and comparison group – 1.2 (0.7). The largest number of sexual partners was observed in patients with severe cervical dysplasia. On average, patients with HSIL had more than four sexual partners. According to colposcopy findings, changes in the cervical epithelium were statistically more frequent in the groups with cervical dysplasia and HPV infection compared with the control group (p<0.05). The most pronounced colposcopic changes were observed in severe cervical dysplasia. They included dense acetowhite epithelium in patients with HSIL was detected in 29/46 (63%) women; coarse mosaic and punctate patterns were observed in 13/46 (28.2%) and 9/46 (19.5%) patients, respectively.
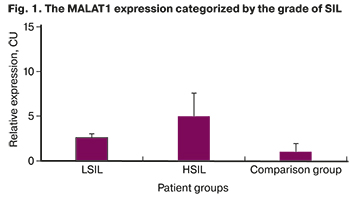 The results of a molecular genetic study demonstrated a higher expression of MALAT1 in cervical dysplasia compared to the control group: 2.6 (0.42) in 27/43 (62.8%) patients with LSIL and 4.97 (2.65) in 36/46 (78.26%) patients with HSIL (p<0.001, Mann–Whitney U-test, Fig. 1.)
The results of a molecular genetic study demonstrated a higher expression of MALAT1 in cervical dysplasia compared to the control group: 2.6 (0.42) in 27/43 (62.8%) patients with LSIL and 4.97 (2.65) in 36/46 (78.26%) patients with HSIL (p<0.001, Mann–Whitney U-test, Fig. 1.)
The expression of MALAT1 was increased by 62.8% and 78.26% in patients with LSIL and HSIL, respectively, and with the progression of cervical dysplasia, which indicates an increase in proliferative activity and apoptosis blockade.
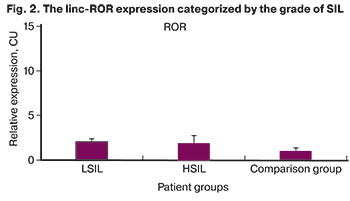
There were no statistically significant differences between the groups in the expressions of linc-ROR with 2.07 (0.34) in patients with LSIL and 1.91 (0.86) with HSIL compared with 1 (0.4) in the comparison group (p=0.368) (Mann–Whitney U-test), which demonstrates the absence of invasive growth in the studied samples (Fig. 2).
 Discussion
Discussion
The present study analyzed the expression of linc-ROR and MALAT1 in the normal cervical epithelium and the presence of squamous intraepithelial lesions. The findings showed that changes in the expression of linc-ROR and MALAT1 were associated with the severity of cervical dysplasia.
Increased expression of MALAT1 was detected in 62.8% of patients with LSIL and 78.26% with HSIL. The level of increase depended on the grade of cervical dysplasia, which reflects impaired cell proliferation and apoptosis in the cervical epithelium, more pronounced along with dysplasia progression (HSIL). Thus, the level of MALAT1 expression is an essential predictor of neoplastic transformation of the cervical epithelium.
The role of MALAT1 in the pathogenesis of cervical cancer is currently being actively studied [12-14]. Studies have been carried out to confirm the relationship between the presence of HPV infection and an increase in the MALAT1 expression [8]. A high level of MALAT1 in patients with cervical cancer was associated with poor survival [8, 9].
It was reported that the suppression of MALAT1 by shRNA in cervical cancer cells led to a decrease in tumor cell invasion and metastasis in vitro and in vivo [12]. Proteomic analysis of cell lines with knockdown MALAT1 revealed activation of epithelial markers E-cadergin and ZO1 (Zonula Occludens-1 Protein) and suppression of mesenchymal markers β-catenin and vimentin [12]. The Snail transcription factor, which modulates the epithelial-mesenchymal transition (EMT) mechanism, was also suppressed at the transcript and protein levels due to the suppression of MALAT1 [12]. These results suggest that an increase in MALAT1 expression promotes invasion and metastasis of cervical cancer through the induction of the epithelial-mesenchymal transition and might also be a promising therapeutic and preventive target in cervical cancer.
We found that the expression of linc-ROR is not significantly associated with cervical dysplasia, which is confirmed by the absence of stromal invasion in the studied samples. At present, the signaling pathways and molecular mechanisms underlying linc-ROR involvement are not fully understood. Many studies strongly indicate its carcinogenic role [7–11]. It has been shown that both tumor progression and metastasis are caused by linc-ROR by activating the epithelial-mesenchymal transition in various cancers [11, 15]. Activation of linc-ROR promotes invasive growth and metastasis of hepatocellular carcinoma [15]. A high expression of linc-ROR was associated with resistance to chemotherapy in pancreatic and breast cancer and resistance of colorectal cancer cells to radiation therapy [11]. Linc-ROR can function as a competing endogenous RNA (cerRNA), influencing the post-transcriptional mechanisms of tumor progression, stimulating cell migration and invasion, neoangiogenesis, metastasis, and the formation of tumor stem cells [16]. The role of linc-ROR in the pathogenesis of cervical cancer remains unexplored, which requires further research.
Conclusion
Cervical cancer remains one of the leading causes of death from female reproductive cancers. This study demonstrated an increase in MALAT1 expression in LSIL in 62.5% and HSIL in 79.8% of patients compared with normal cervical epithelium, which indicates a high proliferative activity of cells mediated by neutralizing p53 tumor suppressor pathway and blocking apoptosis. Therefore, MALAT1 may become a promising biomarker for cervical dysplasia progression. The development of new diagnostic and prognostic markers of the transition from dysplasia to invasive cancer will allow more effective cervical cancer prevention.
References
- Пак Р.В. Эпидемиологические особенности рака шейки матки в мире. Вестник КазНМУ. 2019; 1: 675-7. [Pak R.V. Epidemiological features of cervical cancer in the world. Bulletin of KazNMU. 2019; 1: 675-7. (in Russian)].
- Шахтахтинская Ф.Ч., Намазова-Баранова Л.С., Таточенко В.К., Новикова Д.А., Ткаченко Н.Е. Вирус папилломы человека. Профилактика ВПЧ-ассоциированных заболеваний. Педиатрическая фармакология. 2015; 12(1): 74-8. [Shakhtakhtinskaya F.Ch., Namazova-Baranova L.S., Tatochenko V.K., Novikova D.A., Tkachenko N.E. Human Papilloma Virus. Prevention of HPV-Associated Diseases. Pediatricheskaya farmakologiya/Pediatric pharmacology. 2015; 1(12): 74-8. (in Russian)].
- Arbyn M., Gultekin M., Morice P., Nieminen P. et al. The European response to the WHO call to eliminate cervical cancer as a public health problem. Int J Cancer. 2021; 148: 277-84. https://doi.org/10.1002/ijc.33189.
- Ашрафян Л.А., Киселев В.И., Кузнецов И.Н., Серова О.Ф., Узденова З.Х., Герфанова Е.В. Рак шейки матки: проблемы профилактики и скрининга в Российской Федерации. Доктор.ру. 2019; 11(166): 50-4. [Ashrafyan L.A., Kiselev V.I., Kuznetsov I.N., Serova O.F., Uzdenova Z.Kh., Gerfanova E.V. Cervical Cancer: Issues with Prevention and Screening in the Russian Federation. Doctor.Ru. 2019; 11(166): 50-4. (in Russian)]. https://doi.org/10.31550/1727-2378-2019-166-11-50-54.
- Bedell S.L., Goldstein L.S., Goldstein A.R., Goldstein A.T. Cervical Cancer Screening: Past, Present, and Future. Sex Med Rev. 2020; 8: 28-37. https://doi.org/10.1016/j.sxmr.2019.09.005.
- Рябая О.О., Прокофьева А.А. Взаимодействие аутофагии и эпителиально-мезенхимального перехода в развитии опухолевой прогрессии. Успехи молекулярной онкологии. 2020; 7(2): 8-19. [Ryabaya O.O., Prokofieva A.A. The interplay of autophagy and epithelial-to-mesenchymal transition in cancer progression. Advances in Molecular Oncology. 2020; 7(2): 8-19. https://doi.org/10.17650/2313-805X-2020-7-2-8-19.
- Pan Y., Zhang K., Li C., Chen J. et al. The Emerging Roles of Long Noncoding RNA ROR (lincRNA-ROR) and its Possible Mechanisms in Human Cancers. Cellular Physiology and Biochemistry. 2016; 40(1-2): 219-29. https://doi.org/10.1159/000452539.
- Xu X.Y., Zhang J., Qi Y.-H., Kong M., Liu S.-A., Hu J.-J. Linc-ROR promotes endometrial cell proliferation by activating the PI3K-Akt pathway. Eur Rev Med Pharmacol Sci. 2018; 22(8): 2218-25. https://doi.org/10.26355/eurrev_201804_14807.
- Бейлерли О.А., Гареев И.Ф., Павлов В.Н., Shiguang Z., Xin C., Кудряшов В.В. Экзосомальные длинные некодирующие РНК как биомаркеры и терапевтические мишени при раке. Креативная хирургия и онкология. 2019; 9(4): 297-304. [Beylerli O.A., Gareev I.F., Pavlov V.N., Shiguang Z., Xin C., Kudriashov V.V. Exosomal Long NonCoding Rnas as Cancer Biomarkers and Therapeutic Targets. Creative surgery and oncology. 2019; 9(4): 297-304. (in Russian)]. https://doi.org/10.24060/2076-3093-2019-9-4-297-304.
- Архангельская П.А., Бахидзе Е.В., Берлев И.В., Самсонов Р.Б., Иванов М.К., Малек А.В. Микро-РНК, ВПЧ-инфекция и цервикальный канцерогенез: молекулярные аспекты и перспективы клинического использования. Сибирский онкологический журнал. 2016; 15(4): 88-97. [Arkhangelskaya P.A., Bakhidze E.V., Berlev I.V., Samsonov R.B., Ivanov M.K., Malek A.V. MicroRNA, HPV and Cervical Carcinogenesis: Molecular Aspects and Prospects of Clinical Application. Siberian journal of oncology. 2016; 15(4): 88-97. (in Russian)]. https://doi.org/10.21294/1814-4861-2016-15-4-88-97.
- Chen W., Yang J., Fang H., Li L., Sun J. Relevance Function of Linc-ROR in the Pathogenesis of Cancer. Front Cell Dev Biol. 2020; 8: 696. https://doi.org/10.3389/fcell.2020.00696.
- Yang L., Bai H.-S., Deng Y., Fan L. High MALAT1 expression predicts a poor prognosis of cervical cancer and promotes cancer cell growth and invasion. Eur Rev Med Pharmacol Sci. 2015; 19(17): 3187-93.
- Shen F., Zheng H., Zhou L., Li W., Xu X. Overexpression of MALAT1 contributes to cervical cancer progression by acting as a sponge of miR-429. J Cell Physiol. 2019; 234(7): 11219-26. https://doi.org/10.1002/jcp.27772.
- Бейлерли О.А., Гареев И.Ф. Роль длинных некодирующих РНК в биологии опухолей. Бюллетень сибирской медицины. 2020; 19(1): 125-33. [Beylerli O.A., Gareev I.F. The role of long, non-coding RNA in the biology of tumors. Bulletin of Siberian Medicine. 2020; 19(1): 125-33. (in Russian)]. https://doi.org: 10.20538/1682-0363-2020-1-125-133.
- Li C., Lu L., Feng B., Zhang K., Han S., Hou D.-R., Chen L.-B., Chu X., Wang R. The lincRNA-ROR/miR-145 axis promotes invasion and metastasis in hepatocellular carcinoma via induction of epithelial-mesenchymal transition by targeting ZEB2. Scientific Reports. 2017; 7(1): 4637. https://doi.org/10.1038/s41598-017-04113-w.
- Zhou Q., Guo J., Huang W., Yu X., Xu C., Long X. Linc-ROR promotes the progression of breast cancer and decreases the sensitivity to rapamycin through miR-194-3p targeting MECP2. Mol Oncol. 2020; 14(9): 2231-50. https://doi.org/10.1002/1878-0261.12700.
Received 08.06.2021
Accepted 07.09.2021
About the Authors
Sergey A. Levakov, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology, N.V. Sklifosovsky ICM, I.M. Sechenov First MSMU,Ministry of Health of Russia (Sechenov University), levakoff@yandex.ru, 119991, Russia, Moscow, Trubetskaya str., 8-2.
Elizaveta A. Obukhova, Teaching Assistant at the Department of Obstetrics and Gynecology, N.V. Sklifosovsky ICM, I.M. Sechenov First MSMU,
Ministry of Health of Russia (Sechenov University), liza_obukhova@mail.ru, 119991, Russia, Moscow, Trubetskaya str., 8-2.
Natalia A. Sheshukova, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology, N.V. Sklifosovsky ICM, I.M. Sechenov First MSMU,
Ministry of Health of Russia (Sechenov University), dr.sheshukova@mail.ru, 119991, Russia, Moscow, Trubetskaya str., 8-2.
Olesya V. Bolshakova, Ph.D., Associate Professor at the Department of Obstetrics and Gynecology of the Academy of Postgraduate Education, FNCC FMBA of Russia, 125371, Russia, Moscow, Volokolamsk highway, 91.
Mikhail I. Shakhparonov, Dr. Sci. (Chemistry), Head of the Laboratory of Membrane and Bioenergetic Systems, Institute of Bioorganic Chemistry, shakhparonov@gmail.com, 117997, Russia, Moscow, Miklukho-Maklaya str., 16/10.
Nadezhda V. Antipova, Ph.D. (Bio), Senior Researcher at the Laboratory of Membrane and Bioenergetic Systems, Institute of Bioorganic Chemistry; Associate Professor at the Department of Pharmaceutical and Toxicological Chemistry, RUDN University, Nadine.antipova@gmail.com, 117997, Russia, Moscow, Miklukho-Maklaya str., 16/10.
Authors’ contributions: Levakov S.A. – research leader; Obukhova E.A. – patient selection, material collection;
Sheshukova N.A. – manuscript editing; Bolshakova O.V. – maintaining patient database, statistical analysis; Shakhparonov M.I. – concept and design of the study; Antipova N.V. – PCR study, manuscript preparation.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Levakov S.A., Obukhova E.A., Sheshukova N.A., Bol'shakova O.V., Shakhparonov M.I., Antipova N.V. Feasibility of using long noncoding RNAs for predicting malignant transformation of cervical epithelium.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 9: 120-126 (in Russian)
https://dx.doi.org/10.18565/aig.2021.9.120-126



