Placental inflammatory changes associated with mRNA expression of transcription factor genes in term pregnancy
Objective: To investigate the mRNA expression levels of transcription factor genes (GATA3, RORC2, FOXP3) in the cervical canal, placenta, and fetal membranes in term gestation depending on the presence of histologic chorioamnionitis.Kaganova M.A., Spiridonova N.V.
Materials and methods: The expression of GATA3, RORC2, and FOXP3 mRNAs was detected in 42 pregnant women with a healthy pregnancy who underwent a planned cesarean section for fetal malpresentation and/or uterine scar at 37—41 weeks' gestation. The analysis was performed using RT-PCR and a morphological study of placentas.
Results: Histologic chorioamnionitis was detected in 28 (66.7%) patients without clinical manifestations. In the presence of funiculitis/funisitis (7.1%), there was a 10-fold increase in the expression of GATA3 mRNA in the placenta and a 4-fold decrease in the fetal membranes. Parietal chorioamnionitis (64.3%) was associated with a 2-fold increase in expression levels of RORC2 FOXP3 in the placenta. Leukocyte infiltration of the fetal membranes (26.1%) was associated with a 10-fold decrease in the expression of GATA3 mRNA in the cervical canal.
Conclusion: The present study showed that more than half of women with a healthy term pregnancy had histological chorioamnionitis. Analysis of the expression of transforming factors GATA3, RORC2, FOXP3 represents a promising direction in diagnosing placental and fetal membranes inflammatory process.
Keywords
In full-term pregnancies, chorioamnionitis may be categorized as clinical chorioamnionitis and histologic chorioamnionitis [1]. Histologic chorioamnionitis is defined as the presence of inflammatory changes in fetal membranes with infiltration by neutrophils, macrophages, and T-lymphocytes in the absence of clinical signs of the disease. Histologic chorioamnionitis is diagnosed in 20–34% of full-term pregnancies [2] without clinical manifestations of inflammation.
The diagnosis of clinical chorioamnionitis in pregnancy is commonly made based on the manifestation of local and systemic inflammation, including fever, purulent discharge, tachycardia, leukocytosis, increased C-reactive protein. Although the physiological mechanisms involved in the initiation of labor remain unclear, the predominance of the pro-inflammatory immune system has been shown to play an essential role in the initiation of labor, both in preterm and full-term pregnancy [3–6]. Local recruitment of macrophages and T lymphocytes into the decidual tissue and increased production of pro-inflammatory cytokines (tumor necrosis factor (TNF), interleukin (IL)-1, IL-6, IL-8) and prostaglandins are known to be involved in the processes of cervical maturation and early labor [1, 7–9].
Bacterial and viral lesions are considered the etiological factors of chorioamnionitis, and all the above mediators and immune system cells participate in the pathogenesis of chorioamnionitis. Recently, however, the view of the pathogenesis of chorioamnionitis has changed somewhat. Several authors argue that histologic chorioamnionitis is a reflection of the natural physiological process of childbirth, especially when there is no clinical evidence for infection [1, 3, 8, 10]. This concept is supported by the more frequent detection of histologic chorioamnionitis in the placentas of patients with vaginal delivery, compared to the placentas of patients undergoing a planned cesarean section [11]. According to other authors, histologic chorioamnionitis is an inflammatory process of infectious etiology, but at the time of delivery the process was localized and clinical manifestations of chorioamnionitis were absent [12].
It is not yet possible to determine what is primary: inflammatory histologic changes cause the onset of labor or are acquired during delivery. There is now accumulated evidence that histologic signs of inflammation in the placenta in labor also occur in the absence of any infectious agent, the so-called aseptic inflammation [1]. In fact, of 34% of histologic chorioamnionitis from all preterm births, only 4% have an established infectious factor [9].
In general, 96% of the cases of histologic chorioamnionitis were not accompanied by clinical manifestations of infection and had no adverse effects on maternal and newborn health. Any inflammatory process is accompanied by reactions of the immune system. They include migration of immunocompetent cells to the inflammatory focus, increased production of chemokines, pro-inflammatory cytokines, and changes in the profile of T-helpers in the inflammatory focus. The same is observed in physiological childbirth.
Evaluation of changes in the immunological environment, and in particular T-helpers, during the development of histologic chorioamnionitis in different tissues of the fetal-placental unit is an interesting promising direction. The leading role in the differentiation of T-helpers (Th1, Th2, Th17, Treg) with the subsequent production of their characteristic cytokines is played by transcription factors (TF). Their functions include cell differentiation with the expression of certain genes, stabilization of the patterns of expressed and available for induction of genes [12].
TF for Th2 is GATA3, for Th17 is RORC2, and for Treg is FOXP3. Few data in the current literature on the expression patterns of these TFs in healthy full-term healthy pregnancies and their interactions with each other. However, the balance of Treg/Th2 and Th17-types is an important factor in both healthy pregnancy and pregnancy complications [13–17].
Tregs control the immune response of Th1, Th2 and Th17-type effector immune response to their antigens and pathogens through FOXP3-dependent suppressor programs (forkhead box protein 3, FOXP3). FOXP3-producing cells have been identified in the decidual membrane, and their significant role in maintaining pregnancy and ensuring maternal-fetal tolerance has been showed [6, 15]. Infertility, miscarriage, and preeclampsia are often associated with deficits in Treg number and function, whereas normal pregnancy selectively stimulates the accumulation of maternal FOXP3 CD4(+) Treg cells with fetal specificity [6].
Th2 cells are defined by TF, GATA3 (GATA binding protein 3), which protects against helminth antigens and protein antigens and is also responsible for allergic reactions [10]. Studies [18] have shown that GATA3 is associated with the onset of labor by activating the cytokine production cascade (IL-1, IL-6, IL-8, cyclooxygenase (COX)-2, TNF, matrix metalloproteinase (MMP)-9). In contrast, GATA3 suppression has been demonstrated to occur in preterm rupture of membranes in preterm pregnancy [4, 19].
It is now recognized that Th17 cells constitute the third effector arm of Th cells, complementing the Th1 and Th2 clones. RORC2 is the main TF that can control Th17 cell differentiation; in addition, programming of human Th17 cells with RORC2 leads to partial resistance of these T cells to suppression by Treg.
The role of TF (GATA3, RORC2, and FOXP3) in the implementation of chronic inflammatory processes [12, 15, 16], fetal allograft rejection, preterm labor, and preeclampsia has been described in the literature [20].
The clinical significance and predictive value of histologic chorioamnionitis in the prognosis of septic complications are still unknown [11], as well as the role of TFs responsible for T-cell differentiation both in histologic chorioamnionitis and in its absence.
The present study aimed to investigate the mRNA expression levels of TF T-helper genes, including GATA3, RORC2, and FOXP3 in the cervical canal and fetoplacental cells in full-term pregnancy depending on the presence of histologic chorioamnionitis.
Materials and methods
The study was conducted in the maternity wards of N.I. Pirogov City Clinical Hospital No.1 of Samara. The study included 42 pregnant women with a low risk of obstetric complications at 37–41 weeks of gestation. All patients underwent elective cesarean section. Indications included fetal malpresentation and/or the presence of a post-cesarean uterine scar.
The exclusion criteria were: 1) high-risk pregnancy due to somatic comorbidities (diabetes mellitus, gestational diabetes), placentation features, and obstetric complications (preeclampsia, fetal growth restriction); 2) acute and exacerbation of chronic inflammatory diseases, including colpitis at the time of the study; 3) use of antibiotic therapy during pregnancy.
Cervical smear, placental tissue samples, and fetal membranes were examined by Reverse Transcription Polymerase Chain Reaction (RT-PCR) using molecular genetic methods at the laboratory of DNA-Technology LLC according to the manufacturer's instructions (DNA-Technology, Russia) using the Proba-NK reagent kit with additional phenol deproteinization. The expression level of GATA-3, RORC2, FOXP3 mRNAs was determined, as well as the expression of B2M mRNA, which belongs to the "housekeeping genes" and was used to normalize the obtained values. Cervical canal epithelium sampling was performed immediately before delivery using a cystoscope with a vaginal speculum, which was inserted into the cervical canal to a depth of 1.0–1.5 cm, and cervical epithelial cells were scraped with gentle rotary movements. The placenta and fetal membranes were sampled during cesarean section surgery within the operating field using aseptic techniques. Placenta sampling was performed in the middle of the distance from the place of umbilical cord attachment to the farthest point of the placental margin using a conchotome with a 9.4 mm working surface diameter. A standard placental tissue sample was cut off by capturing a section from the placental depth (with preliminary cutting off the amnion, chorionic plate, and decidual tissue from the maternal side). Fetal membranes were taken midway between the placenta and the internal os using a sterile conchotome with a 9.4 mm working surface diameter; a standard sample of fetal membranes was cut off. The obtained samples were placed in Eppendorf tubes with transport medium (Proba-NK produced by DNA-Technology LLC).
For pathomorphological study of placenta and fetal membranes, six specimens were taken, including umbilical cord in the fetal and maternal parts, fetal membranes of placenta (edge of rupture in the form of tape), twisted as rolls, second roll cut at the edge of placental disc, fragment of decidual plate with villi and fragment of chorionic plate with villi. The obtained samples were fixed in 10% neutral formalin on phosphate buffer, processed in Leica ST 4040 histology tissue guide machine (Germany) and were embedded in paraffin, then after preparation of sections according to the standard technique they were stained with hematoxylin and eosin and encapsulated with synthetic mounting medium Vitrogel. According to the classification of Amsterdam Placenta Workshop Group [21], all changes in the placenta were categorized into three groups: vascular disorders, inflammatory, and others. Histological preparations were used to assess the degree of maturity of the villous tree and its consistency with gestational age, the width of visceral space, the presence and nature of pathological changes. The severity of compensatory-adaptive (syncytial knots, syncytial-capillary membranes) and involutional dystrophic processes (deposition of perivortical fibrinoid, calcium salts), as well as the degree of villous edema were semi-quantitatively evaluated. Qualitative comparative assessment of the content of syncytiotrophoblast cells (fibroblasts, fibrocytes), cytotrophoblast cells, decidual cells, vascular component and signs of inflammation were also performed. Among inflammatory changes there were changes on the maternal side (acute chorioamnionitis) or on the fetal side (phlebitis, funisitis), lesions of placental villi and intervillous space – villitis and intervillositis), and decidual plate bordering the uterine cavity – deciduitis.
All results were entered into MS Excel spreadsheets. Indicator cycles were compared using the comparative threshold cycle method (ΔΔCq). The mRNA expression levels of the studied genes were measured in relative units (10-3 RU), reflecting the representation of the transcript relative to the normalization factor calculated based on the expression level of reference gene mRNA (B2M).
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics v22 (USA) software. The normality of the distribution was tested by the Shapiro–Wilk test. Variables not meeting normality assumptions were reported as the median (Me) and interquartile range (Q1; Q3) and compared with a nonparametric Mann–Whitney test (2 groups) or Kruskal–Wallis test (3 groups). Differences were considered statistically significant at p<0.05; when comparing more than two groups for quantitative variables, Bonferroni correction was used, where the critical level of significance for three groups was p=0.017.
All women provided a written informed consent. The study was reviewed and approved by the Research Ethics Committee of the Samara SMU (Ref. No: 207 of May 20, 2020). Gestational age was calculated by ultrasound or from the number of weeks that have elapsed from the first day of the last normal menstrual period.
Results
The median age of participants was 28 years (28;34) and gestational age was 39.1 (39;39.4) weeks. There were 2.0 (1.0; 3.0) pregnancy per patient, and the number of nulliparous women was 14/42 (33.33%). The pregnant women had no severe somatic pathology or chronic or acute inflammatory diseases. All patients underwent cesarean section; the postoperative period was uneventful; there were no complications in the early neonatal period in the newborns as well.
All placentas were subjected to pathological examination. All patients had no clinical evidence of any obstetric pathology and infection (these were the exclusion criteria), but in some cases pathological examination of the placenta showed signs of an inflammatory process and chronic placental insufficiency. Thus, conformity of the placenta to gestational age was observed only in 13/42 cases (30.9%); placental hypoplasia was detected in 5/42 cases (11.9%); pathological immaturity of villi – in 29/42 cases (69%); dissociated villous development – in 2/42 (4.7%); impaired villous branching – in 8/42 (19.0%); stenosis of supporting villi – in 6/42 (14.2%); involutional dystrophic disorders – in 33/42 (78.5%); inadequate compensatory reactions – in 3/42 (7.1%); signs of postpartum and fetal membrane infection – in 28/42 (66.7%); phlebitis and funiculitis – in 3/42 (7.1%); placental chorioamnionitis – in 3/42 (7.1%); parietal chorioamnionitis – in 27/42 (64.3%) basal deciduitis in 20/42 (47.6%); leukocyte infiltration of fetal membranes in 11/42 (26.1%); lymphocyte infiltration in 15/42 (35.7%), and chronic placental insufficiency in 35/42 (83.3%).
Inflammatory changes in the placenta and fetal membranes are divided into acute (presented as leukocyte infiltration, caused, as a rule, by bacteria) and chronic changes (presented as lymphocyte infiltration, the causes of which may be viral infections, autoimmune processes). In the development of acute chorioamnionitis there are three stages: stage 1 – initial reaction, located in the subchorionic fibrin and internal choriodecidual membranes (subchorionitis, chorionic); stage 2 – lesion of connective tissue between chorion and amnion (chorioamnionitis); stage 3 – necrosis of amniotic epithelium (necrotic chorioamnionitis). According to the Amsterdam criteria, only stages 2 and 3 representing histologically developed chorioamnionitis should be used to evaluate the inflammatory response. In our study, we took stage 2 as histologic chorioamnionitis; there were no patients with signs of stage 3 in our study. On the fetal side, we also distinguished 3 stages in the inflammatory response: stage 1 – acute chorionic vasculitis/umbilical phlebitis/chorionic vasculitis): neutrophils were identified in the wall of any chorionic vessel or in the umbilical vein (small numbers of neutrophils are usually present in Wharton's jelly, but no aggregates or concentric bands of inflammatory infiltrates); stage 2 (umbilical arteritis) neutrophils are seen in one or both umbilical arteries and or in the umbilical vein; stage 3 (necrotic funisitis): presence of neutrophils, cellular debris and/or mineralization in concentric bands, ring, or halo of neutrophils around one or more umbilical vessels (Fig. 1) [22].
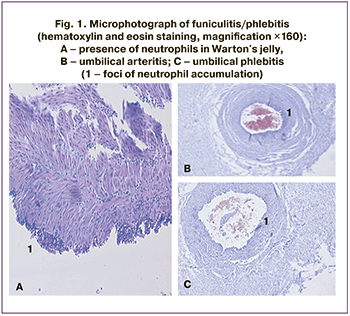
Analysis of associations between the expression of GATA3, RORC2, FOXP3 mRNA genes and the pathological characteristics of the placenta showed no correlation between the mass of the placenta and the features of TF mRNA expression (GATA3, RORC2, FOXP3).
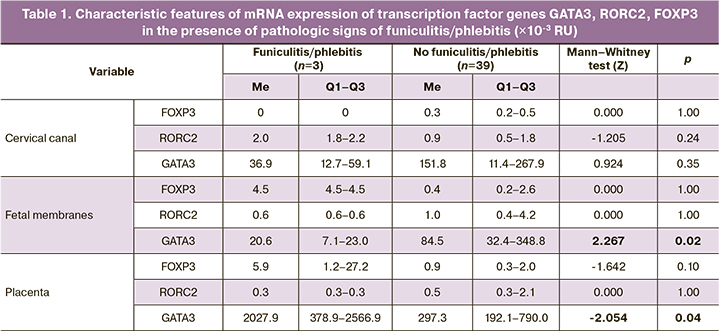
Among the histological signs of funiculitis / phlebitis, there were significant differences between the expressions of GATA3 mRNA genes (Table 1). In funiculitis/phlebitis, there was increased expression in the placenta (2027.9×10-3 RU (378.9×10-3; 2566.9×10-3) and 297.3×10-3 RU (192.1×10-3; 790.0×10-3) (p=0.04)) and decreased in fetal membranes (20.6×10-3 RU (7.1×10-3;23.0×10-3) and 84.5×10-3 RU (32.4×10-3;348.8×10-3) (p=0.02)).
In the presence of parietal chorioamnionitis, there was a concordant increase in RORC2 and FOXP3 in the placenta: 0.7×10-3 RU (0.5×10-3; 3.0×10-3) and 1.4×10-3 RU (0.9×10-3; 2.9×10-3), compared to placentas without evidence of chorioamnionitis: 0.3×10-3 (0.1×10-3; 1.4×10-3) RU and 0.6×10-3 (0.3×10-3; 1.2×10-3) RU (p=0.04 and p=0.02), respectively (Table 2). GATA3 gene mRNA expression was independent of the presence of parietal chorioamnionitis and remained high in all loci.
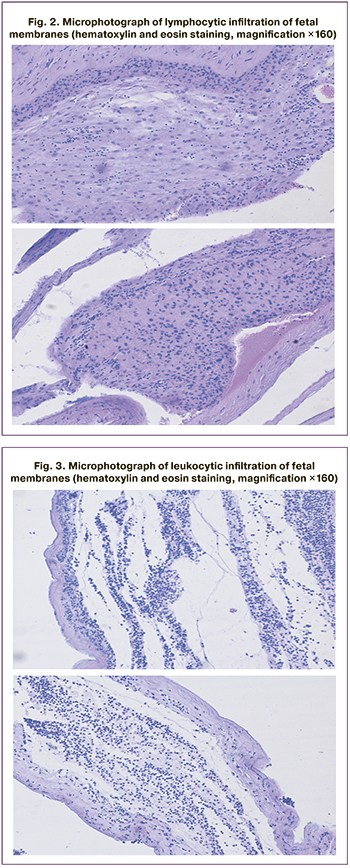
The morphological patterns of lymphocytic infiltration of the fetal membranes (Fig. 2) and leukocytic infiltration are presented below (Fig. 3).
The patients were divided into 3 subgroups classified by predominance of leukocytic or lymphocytic infiltration, in which the expression of transcription factor genes mRNA was compared (Table 3).
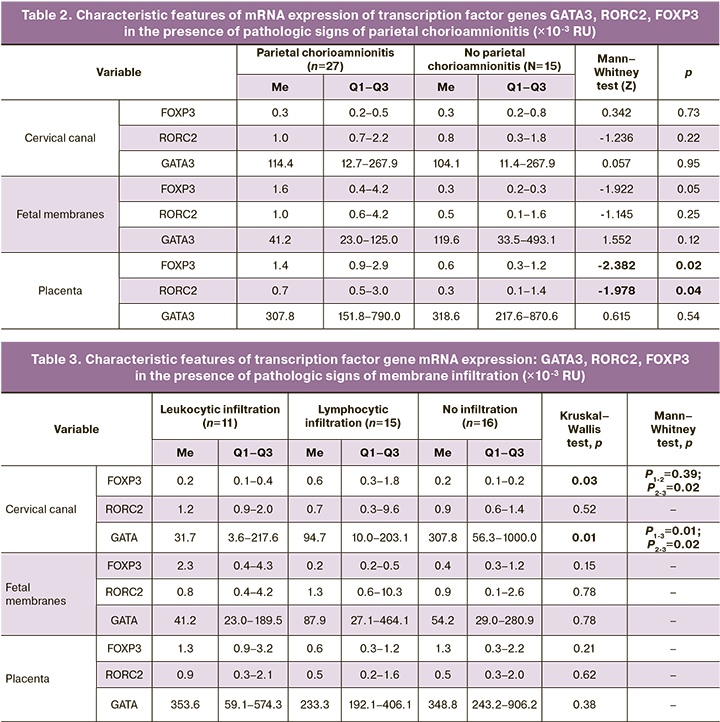
In the cervical canal, there was a decreased expression of GATA3 in both leukocytic and lymphocytic infiltration of the fetal membranes (Table 3). However, given the performance comparison in the three groups, significant differences were observed only for the presence of leukocytic infiltration, 31.7×10-3 RU (3.6×10-3;217.6×10-3) compared to fetal membranes without evidence of infiltration, 307.8×10-3 RU (56.3×10-3;1000.0×10-3; p=0.01). For lymphocytic infiltration, these differences were on the margin of statistical significance (p=0.02). RORC2, FOXP3 mRNA expression at all loci as well as GATA3 mRNA expression in the fetal membranes and in the placenta did not change significantly in the presence of histologic signs of fetal membrane infiltration.
Discussion
Despite numerous studies on the role of inflammatory biomarkers in labor activity, its mechanism has not yet been definitively determined. The concordant increase in pro-inflammatory markers in all tissues of the fetal-placental unit in labor indicates their harmonious work in the initiation of labor activity [4, 20, 23]. Proinflammatory cytokines play an important pathogenic role in the activation of any inflammatory process, including chorioamnionitis. Histologic chorioamnionitis, in fact, is a heterogeneous condition with different histological characteristics related to gestational age at the time of delivery, the presence of spontaneous labor, and the involvement of bacterial and viral agents. In most cases, there is no association between histological diagnosis and clinical manifestations of chorioamnionitis [1, 8, 11, 24].
According to the literature [25], histologic chorioamnionitis in preterm pregnancy is more often limited to the decidual membrane and the chorionic plate. Histological signs of maternal-related fetal membrane inflammation are found in 20-34% of preterm pregnancies [7, 24]. In preterm birth, the process is more frequently associated with funisitis, which is characterized by an increased risk of neonatal sepsis and other long-term complications, such as cerebral palsy [1, 24, 25]. However, in several studies, changes characteristic of histologic chorioamnionitis have been associated with maternal malnutrition and exposure to endocrine-disrupting chemicals (compounds that may be present in pesticides, plastic food containers, etc.) [7, 25]. In our study, histological signs of placental and fetal membrane infection were observed in 28/42 (66.7%) cases, phlebitis and funiculitis in 3/42 (7.1%), placental chorioamnionitis in 3/42 (7.1%), parietal chorioamnionitis in 27/42 (64.3%), basal deciduitis in 20/42 (47.6%), leukocytic infiltration of fetal membranes in 11/42 (26.1%), lymphocytic infiltration in 15/42 (35.7%) without clinical signs of inflammation. Therefore, 2/3 of patients with a term pregnancy shortly before delivery have any histological signs of placental and fetal membrane inflammation, but they play a low predictive role for the mother and fetus in terms of the risk of clinical infection in term pregnancy, as confirmed by the results of other authors [8, 25]. The detection of funiculitis/funiculitis is considered the most unfavorable for the fetus [24]. There were 3/42 (7.1%) such cases in our study; however, no pathological changes in the neonates were subsequently observed. The literature also suggests that one in three patients usually shows signs of funiculitis/phlebitis [3]. The presence of inflammatory changes in the fetal membranes without clinical chorioamnionitis reaches 20–34% [3, 8, 25], but in our study inflammatory changes were found in the fetal membranes in 27/42 (64.3%) cases. Perhaps the absence of clinical manifestations of chorioamnionitis and its complications for the mother and fetus indicates that the process was identified at an early preclinical stage, or the formation of an inflammatory process is inherent in the act of delivery [3].
In this aspect, of interest is the behavior of immune cells and their TFs as pathogenic and predictive markers of the inflammatory process in term pregnancy and histological signs of chorioamnionitis. GATA3 is a TF responsible for Th2 differentiation, whose role is to produce anti-inflammatory cytokines (IL-4, IL-10), form allergic responses, anthelmintic protection, cell proliferation, and regeneration. The progression and maintenance of pregnancy is based on the switch in the immune response from Th1 to Th2 [13], ie, from pro-inflammatory to anti-inflammatory cascade, and back with the onset of labor. According to the literature, the presence of inflammatory changes in the reproductive tract correlates with a decrease in GATA3 [13, 17, 26, 27]. O.V. Burmenskaya et al. [17], O.V. Budilovskaya et al. [26] found that in the presence of local vaginal inflammatory components, there was a significant decrease in the level of GATA3 expression. Katkova N.Yu. also reported a decrease in the level of GATA3 expression in the cervical canal in preterm prelabor rupture of membranes [26]. In our study, leukocytic infiltration of the fetal membranes revealed a 10-fold decrease in GATA3 mRNA expression in the cervical canal compared to unchanged membranes. One may assume that these pregnant women had a subclinical inflammatory process in the vagina accompanied by a decrease in GATA3 expression by cervical canal cells, while the leukocytic infiltration was a consequence of inadequate protection of fetal membranes from ascending infection from the lower genital tract. Infiltration of the fetal membranes with leukocytes and lymphocytes is one of the histological signs of chorioamnionitis. Acute chorioamnionitis caused by bacteria is supported by leukocytic infiltration, while chronic chorioamnionitis of viral etiology is associated with lymphocytic infiltration [27, 28].
In contrast, an increase in GATA3 expression with activation of the cytokine production cascade (IL-1, IL-6, IL-8, COX-2, TNF, MMP-9) has been shown in labor [18]. We observed a significant increase in GATA3 expression in the placenta and a decrease in the fetal membranes in the presence of phlebitis/funiculitis. The increased expression of GATA3 mRNA in the placenta in phlebitis/funiculitis, in our opinion, is associated with the response of placental immunocompetent cells, including dendritic cells and macrophages (producers of GATA3) to the inflammatory process. It can be assumed that the cause of phlebitis/funiculitis in this case was intracellular agents or protein antigens initiating an increase in GATA3 expression. An alternative hypothesis is that phlebitis without infectious factors may be due to allergic and autoimmune reactions. This hypothesis is supported by the work of Tsuda S. at al. (2018) [16], Xu Y., Romero R. (2018) [3], Medjidova M.K., Burmenskaya O.V. (2012) [29].
When signs of parietal chorioamnionitis were detected in our study, we observed a concordant increase in RORC2 and FOXP3 in the placenta, which is consistent with the literature [30, 31]. RORC2 is a key TF that coordinates Th17 cells, which are significant participants in the inflammatory process and several autoimmune diseases (rheumatoid arthritis, multiple sclerosis) [29, 30, 32].
TF FOXP3, according to various sources, is one of the factors responsible for limiting the inflammatory process [12, 29, 30]. In our study, too, TF FOXP3 was elevated in parietal chorioamnionitis. Recent studies have shown that FOXP3-expressing human Treg T cells can also secrete IL-17, a cytokine produced predominantly by Th17 [10, 14], and some cells show concomitant expression of RORC and FOXP3 [3], which we actually observed in our study.
Without clinical signs of inflammation in the mother and child, histological signs of chorioamnionitis were observed in 66.7% of the cases. In the presence of signs of funiculitis/funisitis, there was a 10-fold increase in the expression of GATA3 mRNA in the placenta and a 4-fold decrease in the fetal membranes. In parietal chorioamnionitis, the expression level of RORC2, FOXP3 increased 2-fold in the placenta. With leukocytic infiltration of the fetal membranes, there was a 10-fold decrease in the expression of GATA3 mRNA in the cervical canal.
Conclusion
The present study showed that more than half of women with a healthy term pregnancy had histologic chorioamnionitis. Analysis of the expression of transforming factors GATA3, RORC2, FOXP3 represents a promising direction in the diagnosis of the inflammatory process of the placenta and fetal membranes.
References
1. Conti N., Torricelli M., Voltolini C., Vannuccini S., Clifton V.L., Bloise E. et al. Term histologic chorioamnionitis: a heterogeneous condition. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015; 188: 34-8. https://dx.doi.org/10.1016/ j.ejogrb.2015.02.034.
2. Lee S.M., Lee K.A., Kim S.M., Park C.W., Yoon B.H. The risk of intra-amniotic infection, inflammation and histologic chorioamnionitis in term pregnant 420 women with intact membranes and labor. Placenta. 2011; 32(7): 516-21. https://dx.doi.org/10.1016/j.placenta.2011.03.012.
3. Xu Y., Romero R., Miller D., Silva P., Panaitescu B., Theis K.R. et al. Innate lymphoid cells at the human maternal-fetal interface in spontaneous preterm labor. Am. J. Reprod. Immunol. 2018; 79(6): e12820. https://dx.doi.org/10.1111/aji.12820.
4. Каткова Н.Ю., Бодрикова О.И., Сергеева А.В., Безрукова И.М., Покусаева К.Б. Состояние локального иммунного статуса при различных вариантах преждевременных родов. Вестник Российского государственного медицинского университета. 2017; 3: 57-61. [Katkova N.Yu., Bodrikova O.I., Sergeeva A.V., Bezrukova I.M., Pokusaeva K.B. The local immune profile of the woman and different scenarios of preterm delivery. Vestnik Rossiiskogo gosudarstvennogo meditsinskogo universiteta/ Bulletin of Russian State Medical University. 2017; 3: 57-61. (in Russian)].
5. Krop J., Heidt S., Claas F.H.J., Eikmans M. Regulatory T cells in pregnancy: it is not all about FoxP3. Front. Immunol. 2020; 11: 1182. https://dx.doi.org/10.3389/fimmu.2020.01182.
6. La Rocca C., Carbone F., Longobardi S., Matarese G. The immunology of pregnancy: regulatory T cells control maternal immune tolerance toward the fetus. Immunol. Lett. 2014.; 162(1, Pt A): 41-8. https://dx.doi.org/10.1016/ j.imlet.2014.06.013.
7. Roberts K.A., Riley S.C., Reynolds R.M., Barr S., Evans M., Statham A. et al. Placental structure and inflammation in pregnancies associated with obesity. Placenta. 2011; 32(3): 247-54. https://dx.doi.org/10.1016/ j.placenta.2010.12.023.
8. Ремнева О.В., Колядо О.В., Песоцкая А.В., Стародубцев Е.Г., Гуменюк И.С. Патоморфологические особенности последов у пациенток с различными клиническими фенотипами спонтанных преждевременных родов. Акушерство и гинекология. 2021; 8: 111-8. [Remneva O.B., Kolyado O.V., Pesotskaya A.V., Starodubtsev E.G., Gumenyuk I.S. Pathomorphological features of placenta in patients with different clinical phenotypes of spontaneous preterm births. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 8: 111-8. (in Russian)]. https://dx.doi.org/10.18565/ aig.2021.8.111-118.
9. Lee Y.H., Shynlova O., Lye S.J. Stretch-induced human myometrial cytokines enhance immune cell recruitment via endothelial activation. Cell. Mol. Immunol. 2015; 12(2): 231-42. https://dx.doi.org/10.1038/ cmi.2014.39.
10. Kwak-Kim J., Bao S., Lee S.K., Kim J.W., Gilman-Sachs A. Immunological modes of pregnancy loss: inflammation, immune effectors, and stress. Am. J. Reprod. Immunol. 2014; 72(2): 129-40. https://dx.doi.org/10.1111/aji.12234.
11. Низяева Н.В., Карапетян А.О., Гапаева М.Д., Синицына В.А., Баев О.Р. Структурные особенности плодных оболочек при преждевременных родах. Акушерство и гинекология. 2019; 8: 63-9. [Nizyaeva N.V., Karapetyan A.O., Gapaeva M.D., Sinitsyna V.A., Baev O.R. Structural features of fetal membranes in preterm labor. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; 8: 63-9 (in Russian)]. https://dx.doi.org/10.18565/ aig.2019.8.63-69.
12. Хаитов P.M., Ярилин А.А., Пинегин Б.В. Иммунология: атлас. М.: ГЭОТАР- Медиа; 2011. 624с. [Khaitov R.M., Yarilin A.A., Pinegin B.V. Immunology: Atlas. M.: GEOTAR-Media; 2011. 624 p. (in Russian)].
13. Areia A., Vale-Pereira S., Alves V., Rodrigues-Santos P., Moura P., Mota-Pinto A. Membrane progesterone receptors in human regulatory T cells: a reality in pregnancy. BJOG. 2015; 122(11): 1544-50. https://dx.doi.org/10.1111/ 1471-0528.13294.
14. Wilczynski J.R. Immunological analogy between allograft rejection, recurrent abortion and pre-eclampsia - the same basic mechanism? Hum. Immunol. 2006; 67(7): 492-511. https://dx.doi.org/10.1016/j.humimm.2006.04.007.
15. Bar E., Whitney PG., MoorK., Reis e Sousa C., LeibundGut-Landmann S. IL-17 regulates systemic fungal immunity by controlling the functional competence of NK cells. Immunity. 2014; 40(1): 117-27. https://dx.doi.org/10.1016/ j.immuni.2013.12.002.
16. Tsuda S., Zhang X., Hamana H., Shima T., Ushijima A., Tsuda K. et al. Clonally expanded decidual effector regulatory T cells increase in late gestation of normal pregnancy, but not in preeclampsia, in humans. Front. Immunol. 2018; 9: 1934. https://dx.doi.org/10.3389/fimmu.2018.01934.
17. Bourmenskaya O., Bayramova G., Nepsha O., Rebrikov D., Trofimov D., Muravieva V., Sukhikh G. Vaginal smear TNF-alpha, IL18, TLR4, and GATA3 mRNA levels correlate with local inflammation. Int. J. Biomed. 2014; 4(4): 204-8.
18. Chen J., Shi Y., Huang J., Luo J., Zhang W. Neuromedin B receptor mediates neuromedin B-induced COX-2 and IL-6 expression in human primary myometrial cells. J. Investig. Med. 2020; 68(6): 1171-8. https://dx.doi.org/10.1136/jim-2020-001412.
19. Zhang C., Wang W., Liu C., Lu J., Sun K. Role of NF-kB/GATA3 in the inhibition of lysyl oxidase by IL-1b in human amnion fibroblasts. Immunol. Cell Biol. 2017; 95(10): 943-52. https://dx.doi.org/10.1038/icb.2017.73.
20. Крукиер И.И., Левкович М.А., Авруцкая В.В., Чурюкина Э.В., Никашина А.А., Чикина Л.Г. Роль продукции цитокинов, органических кислот в сыворотке крови и амниотической жидкости в развитии спонтанных преждевременных родов. Акушерство и гинекология. 2021; 9: 60-5. [Krukier I.I., Levkovich M.A., Avrutskaya V.V, Churyukina E.V., Nikashina A.A., Chikina L.G. The role of cytokines and organic acids production in the blood serum and amniotic fluid in spontaneous preterm labor. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 9: 60-5. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.9.60-65.
21. Щеголев А.И. Современная морфологическая классификация повреждений плаценты. Акушерство и гинекология. 2016; 4: 16-23. [Shchegolev A.I. Current morphological classification of damages to the placenta. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2016; 4: 16-23. (in Russian)]. https://dx.doi.org/10.18565/aig.2016.4.16-23.
22. Щеголев А.И., Дубова Е.А., Павлов К.А. Морфология плаценты. Пособие для врачей. М.: Форза; 2010. 46с. [Shchegolev A.I., Dubova E.A., Pavlov K.A. Morphology of the placenta: a manual for doctors. M.: Forza; 2010. 46 p. (in Russian)].
23. Lima J., Martins C., Nunes G., Sousa M.J., Branco J.C., Borrego L.M. Regulatory T cells show dynamic behavior during late pregnancy, delivery, and the postpartum period. Reprod. Sci. 2017; 24(7): 1025-32. https://dx.doi.org/10.1177/1933719116676395.
24. Romero R., Pacora P., Kusanovic J., Jung E., Panaitescu B., Maymon E. et al. Clinical chorioamnionitis at term X: microbiology, clinical signs, placental pathology, and neonatal bacteremia - implications for clinical care. J. Perinat. Med. 2021; 49(3): 275-98. https://dx.doi.org/10.1515/ jpm-2020-0297.
25. Курносенко И.В., Долгушина В.Ф., Пастернак А.Е. Воспалительные изменения в последе у женщин в преждевременными и своевременными родами. Современные проблемы науки и образования. 2016; 3: 172. [Kurnosenko I.V., Dolgushina V.F., Pasternak A.E. Inflammatory changes in the placenta in women with preterm and timely delivery. Sovremennye problemy nauki i obrazovaniya/Modern Problems of Science and Education. 2016; 3: 172. (in Russian)].
26. Будиловская О.В., Шипицына Е.В., Переверзева Н.А., Воробьева Н.Е., Спасибова Е.В., Григорьев А.Н., Савичева А.М. Сравнение методов оценки воспалительной реакции нижних отделов женского репродуктивного тракта. Журнал акушерства и женских болезней. 2018; 67(5): 13-20. [Budilovskaya O.V., Shipitsina E.V., Pereverzneva N.A., Vorobyova N.E., Spasibova E.V., Grigoryev A.N. et al. Comparison of methods for assessing the inflammatory response of the lower parts of the female reproductive tract. Zhurnal akusherstva i zhenskikh boleznei/Journal of Obstetrics and Women's Diseases. 2018; 67(5): 13-20. (in Russian)]. https://dx.doi.org/10.17816/ JOWD67513-20.
27. Гомболевская Н.А., Бурменская О.В., Демура Т.А., Марченко Л.А., Коган Е.А., Трофимов Д.Ю., Сухих Г.Т. Оценка экспрессии мРНК генов цитокинов в эндометрии при хроническом эндометрите. Акушерство и гинекология. 2013; 11: 35-40. [Gombolevskaya N.A., Burmenskaya O.V., Demura T.A., Marchenko L.A., Kogan E.A., Trofimov D.Yu. et al. Estimation of the mRNA expression of cytokine genes in the endometrium in chronic endometritis. Akusherstvo i Ginekologiya/Obstetrics and gynecology. 2013; 11: 35-40. (in Russian)].
28. Низяева Н.В. Гистологические критерии воспалительных изменений плодных оболочек плаценты и пуповины. Международный журнал при-кладных и фундаментальных исследований. 2018; 3: 180-8. [Nizyaeva N.V. Histological criteria of inflammatory diseases of placenta membranes and umbilical cord. Mezhdunarodnyi zhurnal prikladnykh i fundamental'nykh issledovanii/International journal of applied and fundamental research. 2018; 3: 180-8 (in Russian)].
29. Меджидова М.К., Бурменская О.В., Донников А.Е., Непша О.С., Трофимов Д.Ю., Касабулатов Н.М., Тютюнник В.Л., Сухих Г.Т. Оценка локальной воспалительной реакции во влагалище по профилю экспрессии мРНК генов цитокинов у беременных накануне родов. Акушерство и гинекология. 2012; 3: 26-31. [Medzhidova M.K., Burmenskaya O.V., Donnikov A.E., Nepsha O.S., Trofimov D.Yu., Kasabulatov N.M. et al. Evaluation of local vaginal inflammatory response from the profile of cytokine gene mRNA expression in pregnant women before birth. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2012; 3: 26-31. (in Russian)].
30. Ganjalikhani Hakemi M., Ghaedi K., Homayouni V., Andalib A., Hosseini M., Rezaei A. Positive and negative regulation of Th17 cell differentiation: evaluating the impact of RORC2. Cell J. 2014 Autumn; 16(3): 343-52.
31. Eghbal-Fard S., Yousefi M., Heydarlou H., Ahmadi M., Taghavi S., Movasaghpour A. et al. The imbalance of Th17/Treg axis involved in the pathogenesis of preeclampsia. J. Cell. Physiol. 2019; 234(4): 5106-16. https://dx.doi.org/10.1002/jcp.27315.
32. Figueiredo A.S., Schumacher A. The T helper type 17/regulatory T cell paradigm in pregnancy. Immunology. 2016; 148(1): 13-21. https://dx.doi.org/10.1111/ imm.12595.
Received 09.12.2021
Accepted 24.02.2022
About the Authors
Maria A. Kaganova, Ph.D., Associate Professor at the Department of Obstetrics and Gynecology, Institute for Postgraduate Education, Samara State Medical University, Ministry of Health of Russia, +78462071968, mkaganova@yandex.ru, https://orcid.org/0000-0001-5879-418x, 443100, Russia, Samara, Polevaya str. 80.Natalia V. Spiridonova, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology, Institute for Postgraduate Education, Samara State Medical University, Ministry of Health of Russia, +78462071968, nvspiridonova@mail.ru, eLibrary SPIN: 3079-3658; ORCID: 0000-0003-3390-8034; Web of Science S-6918-2016;
Scopus 56089251400, 443100, Russia, Samara, Polevaya str. 80.
Authors' contributions: Spiridonova N.V., Kaganova M.A. - conception and design of the study; Kaganova M.A. - data collection and analysis, statistical analysis, manuscript drafting; Spiridonova N.V - manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Samara SMU, Ministry of Health of Russia (Ref. No: 207 of May 20, 2020).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Kaganova M.A., Spiridonova N.V. Placental inflammatory changes associated with mRNA expression of transcription factor genes in term pregnancy.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 3: 49-58 (in Russian)
https://dx.doi.org/10.18565/aig.2022.3.49-58



