Изменение роли женщины в обществе и семье диктует особое отношение к вопросам планирования беременности и контрацепции, которая рассматривается как одно из важнейших направлений сохранения здоровья и имеет большое значение для репродуктивной жизни женщин как с медицинской, так и с социальной точки зрения [1].
При подборе наиболее подходящего метода контрацепции в различные периоды жизни женщинами, мужчинами или парами должны учитываться многие факторы, включая в первую очередь безопасность, эффективность, доступность и приемлемость [2].
За последние годы выбор контрацептивов значительно увеличился. Тем не менее все доступные методы не могут быть универсальными для всех женщин. Особого внимания требуют женщины с заболеваниями или индивидуальными особенностями, у которых любая беременность, особенно незапланированная, подвержена повышенному риску. Безопасность контрацепции имеет решающее значение для этой группы пациенток.
Тот или иной метод контрацепции используется большинством (63,6%) женщин в возрасте 15–49 лет, состоящих в браке или живущих с партнером, почти во всех регионах мира [3]. Применение контрацепции помогает парам и отдельным лицам реализовать свое основное право свободно принимать решения и ответственно относиться к вопросу планирования семьи. Возрастающее использование противозачаточных средств, в свою очередь, приводит не только к уменьшению абортов, но и снижению таких показателей, как материнская и младенческая смертность, а также улучшению здоровья женского населения в целом.
В последнее десятилетие число женщин, применяющих гормональную контрацепцию, постепенно увеличивается. По данным «Выборочного обследования репродуктивного здоровья населения России 2011 года», 68% состоящих в партнерстве женщин и 74% сексуально активных женщин (независимо от брачного статуса) в возрасте от 15 до 44 лет применяют методы контрацепции – это средний уровень использования контрацепции для развитых стран. Схожие с российским показатели распространенности контрацепции характерны для таких стран, как Бельгия, Нидерланды, Испания, Германия. Можно сказать, что Россия по этому показателю не выделяется на фоне стран с близким уровнем рождаемости (не только развитых) [4].
Данные анкетирования (Прилепская В.Н. и др.) женщин репродуктивного возраста (16–45 лет) с целью оценки их контрацептивного поведения показали, что наибольшей популярностью среди способов контрацепции в настоящее время пользуются малоэффективные барьерные и естественные методы [5]. Так, ритмическим, или календарным, методом контрацепции пользовались 33,3% женщин, а 63,4% опрошенных использовали прерванный половой акт, барьерные методы (презерватив) применяли 95,5% респонденток. Спермициды использовали 6,1% женщин, а диафрагму – 0,8%. С помощью анализа частоты использования современных методов контрацепции выяснено, что различные КОК применяли 27,2% женщин и приблизительно столько же – ВМС (26,8%). Значительно меньшей популярностью пользовались трансдермальные гормональные контрацептивы (2,4%) и влагалищная гормональная рилизинг-система (4,5%).
Результаты исследования позволяют сделать вывод, что общее число пользователей контрацептивами является достаточным, однако в структуре используемых методов предпочтение отдается средствам с недостаточной эффективностью, несмотря на высокую информированность о современных гормональных и внутриматочных средствах. Это является важным для разработки контрацептивных стратегий, позволяющих влиять на частоту нежеланных беременностей в популяции современных российских женщин. Согласно данным статистики за 2015–2016 гг., 14,3% всех женщин фертильного возраста пользовались внутриматочными средствами контрацепции и 13,4% – гормональными [6, 7].
Современные гормональные контрацептивы представлены достаточно широко, включая прогестиновые оральные препараты, пролонгированные формы (имплантаты, инъекционные противозачаточные средства, внутриматочные системы), комбинированные гормональные контрацептивы.
В настоящее время более 100 млн женщин в мире в возрасте от 15 до 49 лет используют комбинированные оральные контрацептивы (КОК) [8]. КОК представляют собой таблетки, содержащие гормоны эстроген и прогестин, и являются высокоэффективным [9, 10] и удобным методом контрацепции, так как предлагают женскую репродуктивную самостоятельность и высокую надежность в предотвращении нежелательной беременности [11]. Кроме того, применение КОК влечет за собой ряд лечебных эффектов, которые широко используются: лечение предменструального синдрома, головных болей, акне [12–14], снижение риска развития рака яичников и эндометрия [15] и др.
Вопросы безопасности применения оральных контрацептивов занимали врачей и пациентов на протяжении десятилетий. Венозные тромботические риски, связанные с оральными контрацептивами, привлекают и по сей день особое внимание.
Существующие данные свидетельствуют о том, что первое поколение оральных контрацептивов было связано со значительным риском венозной тромбоэмболии (ВТЭ). Причиной считались эстрогеноподобные соединения в КОК.
В последние годы в контрацепции произошли глобальные изменения. К ним можно отнести уменьшение дозы эстрогенного компонента, входящего в состав КОК, появление новых прогестинов с уникальными свойствами, включая лечебные (диеногест, дроспиренон, номегэстрола ацетат), внедрение в клиническую практику препаратов, содержащих эстрадиол, – эстроген, идентичный эндогенному, разработку новых путей введения и режимов дозирования контрацептивных препаратов. Основной акцент при этом сделан на дополнительных лечебных эффектах контрацептивных гормонов и возможности применения индивидуального подхода при их назначении [16].
Уменьшение дозы эстрогена в КОК привело к снижению риска ВТЭ при приеме контрацептивных таблеток. Первые существенные изменения произошли в 1970-х гг., когда было предложено снижение дозы этинилэстрадиола (ЭЭ) с 50 мкг до 30–35 мкг [2]. Результатом явилось снижение венозных тромбозов на фоне приема КОК [17].
Интерес вызывают исследования, посвященные действию гормональных контрацептивов на сердечно-сосудистую систему в целом и на артериальное давление в частности, из-за присутствия рецепторов эстрогена и прогестерона во всех составляющих слоях кровеносных сосудов [18]. Более ранние исследования показали, что использование противозачаточных таблеток потенциально увеличивало систолическое артериальное давление (САД) в группах женщин, которые обычно имели более высокий уровень АД [19]. Недавние исследования также подчеркивают, что комбинированные гормональные контрацептивы, содержащие ЭЭ, всегда изменяют артериальное давление (АД) даже при низких дозах. ЭЭ, благодаря своей биологической активности по сравнению с эстрадиолом, усиливает выработку печеночного ангиотензиногена, что, в свою очередь, вызывает повышение артериального давления системой ренин-ангиотензин-альдостерон (РААС) [20].
Несмотря на то что КОК не вызывают клинических последствий у здоровых женщин, их следует избегать женщинам с высоким АД [21]. Кроме того, необходимо учитывать и существующие факторы риска.
Значимыми факторами риска сердечно-сосудистых заболеваний при приеме КОК являются курение (>15 сигарет в день), возраст (>35 лет), ожирение, гипертония, некоторые наследственные тромбофилии, отягощенный анамнез (ВТЭ или другие факторы риска тромбоза, например, длительная иммобилизация) [22].
Использование КОК может увеличивать риски некоторых угрожающих жизни состояний, включая ВТЭ и/или ишемический инсульт, особенно среди женщин с наследственной тромбофилией (тромбогенными мутациями), такими как фактор V Лейдена, мутация гена протромбина, недостаток белка С, белка S, антитромбина [23–27]. Всемирная организация здравоохранения (ВОЗ) классифицирует «известную тромбогенную мутацию» как «состояние, представляющее неприемлемый риск для здоровья, если используется комбинированная гормональная контрацепция» [28]. И наоборот, в пояснении к этой рекомендации ВОЗ указывает рутинный скрининг на тромбофилию до начала применения КОК как нецелесообразный в свете низкой распространенности тромбогенных мутаций и высокой стоимости скрининга [29].
Прогестины, входящие в состав современных КОК, такие как левоноргестрел, этоногестрел и дроспиренон, имеют хорошо описанный профиль побочных эффектов в целом. В 2000-х гг. стали появляться свидетельства того, что дроспиренонсодержащие КОК могут вызывать более высокий риск ВТЭ, чем КОК более раннего поколения. Некоторые систематические обзоры показали повышенный риск ВТЭ [19, 30], но другие подчеркивают скромный абсолютный риск: «Независимо от того, повышается ли тромботический риск дроспиренона по сравнению с левоноргестрелом в 1,5 или 3 раза, абсолютный риск все еще остается низким» [11].
Отмечено, что риск тромботических осложнений при приеме современных низкодозированных оральных контрацептивов (несмотря на некоторые различия, связанные с составом препарата) в 1,5–2 раза ниже, чем у курящих женщин, и значительно уступает частоте тромбозов при беременности и в послеродовом периоде. При этом потенциальная польза от применения КОК превышает все возможные риски у здоровых женщин моложе 40 лет [11, 31–34].
На сегодняшний день разработаны препараты, содержащие 20 мкг ЭЭ, благодаря чему было достигнуто еще большее снижение частоты побочных эффектов, связанных с эстрогенным компонентом [8, 9].
Среди неврологических расстройств, представляющих проблему при подборе контрацепции, особое внимание было уделено мигрени, которая распространена у 13% женщин репродуктивного возраста [35]. Была установлена последовательная связь между мигренью и различными сердечно-сосудистыми заболеваниями, в частности ишемическим инсультом [28, 29, 35]. Было отмечено, что при приеме КОК могут ухудшаться симптомы мигрени и увеличивается риск инсульта из-за протромботического эффекта эстрогена [36–38]. На основании этих клинических данных ВОЗ ограничила использование гормональной контрацепции у пациентов с мигренью [22].
Особое внимание уделяется при подборе контрацепции у пациенток с раком молочной железы. Такие женщины нуждаются в особом руководстве по выбору метода контрацепции. Рак молочной железы является наиболее распространенной злокачественной опухолью у женщин репродуктивного возраста [39, 40]. Несмотря на то что лечение может повлиять на фертильность женщины, контрацепция является ключевым аспектом во время и после лечения данного заболевания. Беременность во время процесса лечения настоятельно не рекомендуется, так как потенциально может увеличить риск врожденных пороков развития плода [41, 42]. Но выбор метода контрацепции должен быть ограничен обратимыми негормональными методами [31, 40, 43], так как современные данные подтверждают неприемлемость гормональной контрацепции для женщин с раком молочной железы [32].
Медьсодержащие внутриматочные системы (ВМС) и барьерные методы считаются безопасными вариантами первой линии (независимо от их эффективности) у данной когорты женщин в любом случае [22].
Среди эндокринных расстройств сахарный диабет становится все более распространенным во всем мире. Беременные женщины с плохо контролируемым сахарным диабетом являются группой повышенного риска неблагоприятного течения и исходов беременности (самопроизвольный аборт, гипертония, преэклампсия, ухудшение существующей ретинопатии или нефропатии), а также развития осложнений у плода/новорожденного [33]. Поэтому контрацепция для молодых женщин с сахарным диабетом имеет большое значение, так как дает возможность отложить зачатие до оптимального гликемического контроля и стабилизации микрососудистых осложнений [33]. При выборе контрацептива необходимо учитывать тип диабета, его продолжительность, имеющиеся осложнения, наличие сердечно-сосудистых факторов риска.
Современные гормональные контрацептивные методы признаны безопасными для женщин с неосложненным сахарным диабетом продолжительностью менее 20 лет. У пациенток с неконтролируемыми микрососудистыми осложнениями, такими как тяжелая ретинопатия, нефропатия с постоянной протеинурией, в ситуациях, когда негативное воздействие гормональных контрацептивов не может быть исключено, следует выбрать негормональные альтернативные варианты контрацепции [33, 44, 45].
Хороший профиль безопасности у женщин с различными заболеваниями, включая хорошо контролируемую гипертонию, неосложненный сахарный диабет и депрессию, показали низкодозированные КОК, содержащие дроспиренон [11, 29, 37, 46].
Предменструальный синдром (ПМС) является распространенной проблемой. Тяжелая форма называется предменструальным дисфорическим расстройством (ПМДР). Была изучена возможность применения КОК для лечения ПМС и ПМДР. Было показано, что противозачаточные таблетки с дроспиреноном дают лучший эффект, чем другие КОК. КОК с дроспиреноном и низким содержанием эстрогена были одобрены для лечения ПМДР, тяжелой формы ПМС у женщин, которые используют противозачаточные таблетки [29, 36, 37, 40].
Значимым этапом в развитии гормональной контрацепции явилось изменение режима приема КОК. Стандартной схемой назначения оральных контрацептивов является режим 21/7, имитирующий 28-дневный менструальный цикл (21 день приема активных таблеток + 7 дней безгормонального промежутка). Менструальноподобные кровянистые выделения из половых путей наступают в период безгормонального промежутка [45, 47–50].
Научно обоснованные данные об эффективности, безопасности и важности именно такого режима приема гормональной контрацепции отсутствуют, в связи с чем было предложено укорочение безгормонального промежутка приема КОК [24].
Многочисленные исследования в этой области позволили разработать режим приема КОК – 24/4 (24 дня приема таблеток, содержащих эстроген-гестагенные компоненты, и 4 таблетки плацебо). Менструальноподобное кровотечение наступает при приеме таблеток-плацебо. Данный режим приема КОК позволяет еще более эффективно снизить активность яичников [13], дает возможность укоротить дни кровянистых выделений из половых путей, уменьшить побочные явления и, соответственно, улучшить качество жизни женщины на фоне приема гормональной контрацепции [26].
Результаты ряда исследований показывают, что во время 7-дневного перерыва в стандартном режиме приема КОК (21/7 дней) гипофиз начинает секретировать гонадотропины. В ответ на гонадотропины в яичниках активно развиваются фолликулы и секретируются гормоны, в том числе эстрадиол и ингибин-Б [14, 15]. Фолликулостимулирующий гормон (ФСГ) и ингибин-Б начинают вырабатываться уже на 4-й и 5-й дни безгормонального интервала, затем повышается уровень лютеинизирующего гормона (ЛГ) и эстрадиола [14]. Синтез эстрогенов возобновляется в период безгормонального промежутка и продолжает расти на фоне КОК. После 7-дневного перерыва, когда возобновляется прием КОК, выработка гонадотропинов гипофизом недостаточно снижается для полноценного предотвращения фолликулогенеза и овуляции. В результате 7-дневный интервал может быть достаточен для восстановления полноценной фолликулярной фазы менструального цикла и функционирования яичников [15, 16, 51].
Это особенно важно при использовании препаратов с низким содержанием эстрогенного компонента КОК. Снижение уровня эндогенного эстрогена отмечается лишь через 2 недели приема гормонального контрацептива (КОК). По данным некоторых исследователей, именно в 7-дневный безгормональный промежуток возникают многие нежелательные явления, которые в литературе описываются как абстинентный синдром [16, 51].
Многочисленные исследования в области контрацепции позволили разработать еще более усовершенствованный режим приема КОК – пролонгированный режим, который подразумевает прием гормонально-активных таблеток на протяжении 84 дней + 7 дней перерыва или прием в этот период только этинилэстрадиола. Обоснованием для рекомендации именно такого режима приема КОК явилось максимально эффективное подавление функции яичников, еще большее укорочение дней кровянистых выделений из половых путей и более выраженный лечебный эффект при таких гинекологических заболеваниях, как эндометриоз, дисменорея, меноррагии и др. [17–19, 22, 30, 52, 53].
В настоящее время в России зарегистрировано более 40 препаратов для гормональной контрацепции. В связи с таким разнообразием контрацептивных средств, унифицированный подход к назначению контрацептивов и четкие критерии выбора препаратов являются необходимым условием.
В конце 2015 г. на российском рынке появилась инновационная линейка контрацептивов МОДЭЛЛЬ (табл. 1), которая отвечает всем основным потребностям женщин [54, 55].
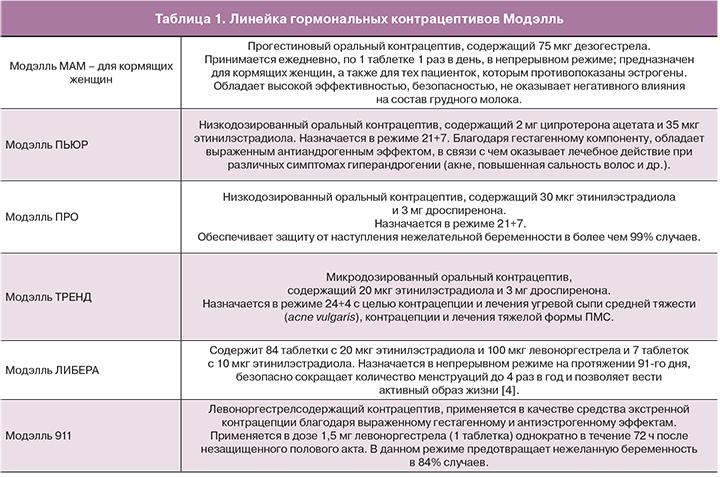
Особого внимания в вышеперечисленной линейке препаратов заслуживают низкодозированные КОК, содержащие дроспиренон (ДРСП).
Исследования, в которых оценивали влияние различных доз ДРСП как отдельно, так и в сочетании с ЭЭ, показали, что ДРСП ингибирует функцию шейки матки, повышая вязкость цервикальной слизи независимо от дозы, таким образом, предоставляя дополнительные контрацептивные эффекты. Активность яичников сравнивали между двумя группами с использованием ДРСП 3 мг/ЭЭ 20 мкг в режиме 24/4 и режиме 21/7. Обе схемы дозирования эффективно подавляли активность яичников, однако режим 24/4 был связан с большим подавлением, отражая возможную более высокую контрацептивную эффективность этой дозированной формулировки. Комбинация EE 20 мкг/ ДРСП 3 мг приводила к меньшему колебанию эндогенного эстрогена [38].
Было выявлено, что длительное применение (13 циклов) ДРСП 3 мг и ЭЭ 30 мкг в режиме дозы 21/7 оказывает выраженное антипролиферативное действие на эндометрий [39], что обусловлено главным образом прогестагеном.
ДРСП не проявляет андрогенной, эстрогенной или антиглюкокортикоидной активности, и его рецепторсвязывающий профиль очень похож на профиль прогестерона, однако он проявляет меньшую антиандрогенную активность и не обладает глюкокортикоидной активностью. Дроспиренон обладает высокой антиминералокортикоидной (в восемь раз выше, чем у спиронолактона) и антиандрогенной активностью, что объясняет ряд эффектов при его применении. В частности, КОК способен эффективно противодействовать эстрoгензависимой задержке натрия и жидкости в организме женщины [42].
В режиме 24/4 препарат ЭЭ 20/ДРСП 3 мг не оказывал существенного влияния на артериальное давление в течение 13 циклов лечения у 1027 женщин, которые участвовали в многонациональном многоцентровом исследовании [22].
Комбинация ЭЭ с ДРСП представляет собой высокоэффективный низкодозированный контрацептивный препарат, обладающий рядом дополнительных преимуществ в силу особенности гестагенного компонента. Неконтрацептивные преимущества этого противозачаточного средства включают улучшение состояния при дисменорее, возможность применения при лечении акне, применение при ПМС. 24-дневный режим активного приема таблеток с последующей 4-дневной паузой оказался эффективным для лечения эмоциональных и физических симптомов у женщин с ПМДР как наиболее тяжелой формой предменструального синдрома.
Заключение
Индивидуальное консультирование по подбору комбинированных гормональных контрацептивов должно учитывать не только характеристику препарата – дозу эстрогена, тип прогестина, режим приема, но и наличие возможных противопоказаний у женщины. Кроме того, довольно часто необходимо использовать неконтрацептивные эффекты КОК с лечебной целью.
Значимую роль при выборе контрацептива играет режим дозирования. Укорочение безгормонального интервала несет ряд преимуществ (уменьшение менструальной кровопотери, менструальной боли и др.) и помогает повысить приемлемость выбранного препарата. Удлиненный, в течение 24 дней, режим приема способен дать дополнительный контрацептивный эффект, поскольку связан с усилением подавления овариальной активности, благодаря непосредственному влиянию продолжительного применения прогестина на фолликулогенез. Также именно с пролонгированным режимом применения связано сокращение числа ошибок (пропуска таблетки) применения, что особенно актуально у юных и молодых женщин.
Возможность индивидуального подбора контрацептива требует от врача необходимости хорошо ориентироваться в современных методах контрацепции. Модэлль — это коллекция контрацептивов, которая учитывает индивидуальные потребности женщины и защищает ее от незапланированных перемен в течение всей сексуальной жизни.
Каждый препарат в линейке помогает найти оптимальное решение для женщины в соответствии с ее потребностями и особенностями организма. Представленные контрацептивы дают возможность врачу легко ориентироваться при индивидуальном подборе гормональной контрацепции в соответствии с желанием и потребностями женщины, с учетом рекомендаций ВОЗ, позволяя врачу и пациентке находить индивидуальное решение вопроса контрацепции. Основываясь на объективных критериях выбора, легко ориентироваться при переходе на другой препарат при изменении образа жизни и потребностей, связанных со здоровьем (период лактации, акне, проблемы с репродуктивным здоровьем и т.д.).



