Impact of preimplantation genetic testing on assisted reproductive technology outcomes in couples with male factor infertility
Background: In recent years, considerable attention has been focused on the association between severe male factor (SMF) and the incidence of embryonic aneuploidy, including whether SMF should be considered an indication for preimplantation genetic testing for aneuploidy (PGT-A).Makarova N.P., Lobanova N.N., Kulakova E.V., Nepsha O.S., Ekimov A.N., Kalinina E.A.
Objective: To investigate the impact of men's age and form of male infertility on the rate of embryonic aneuploidy and the outcomes of assisted reproductive technology (ART).
Materials and methods: This retrospective study analyzed 2915 ART cycles (2225 stimulation cycles, including 371 cycles with PGT-A and 690 cryopreserved cycles). The ejaculate was evaluated based on the sperm quality criteria of the WHO reference values. The SMF group consisted of patients with oligoasthenoteratozoospermia and patients with testicular biopsy. Patients with teratozoospermia were divided into two groups, categorized by the percentage of morphologically abnormal spermatozoa. On day five after fertilization, the embryo trophectoderm was biopsied, followed by PGT-A.
Results: Comparison of ART outcomes in stimulation cycles and a fresh embryo transfer and in cryopreserved cycles with and without PGT-A showed statistically significantly lower pregnancy and birth rates in patients with the sperm morphology score of 0–2% and 3% in cryopreserved cycles without PGT-A. Patients with SMF undergoing ART with PGT-A showed a trend towards increasing pregnancy and birth rates.
Conclusion: PGT-A can improve pregnancy outcomes for couples with SMF with fewer embryos transferred due to reducing early pregnancy losses.
Keywords
Since its first introduction in 1992, intracytoplasmic sperm injection (ICSI) has dramatically changed the treatment for male factor infertility, making it possible for patients with severe male factor (SMF) to have their biological offspring [1]. However, according to the International Committee for Monitoring Assisted Reproductive Technologies world (ART) report, despite laboratory technology advances, the pregnancy and birth rate after ICSI is about 25–30% and 20%, respectively [2]. The strategy for selecting an embryo for transfer is usually based on the morphological characteristics of the embryo, while embryos of good or excellent morphological quality may be genetically abnormal. Today it is well known that aneuploidies are the most common genetic abnormalities that lead to implantation failure and early pregnancy loss in more than 50% of cases.
According to various sources, infertility associated with the male factor accounts for 20 to 50% of all cases [3]. Of these, slightly over 20% have severe male infertility [4]. It is also worth noting that delayed parenting is becoming more common for women and men worldwide [5], triggering growing interest in studying the effect of paternal age on male fertility, reproductive potential, and offspring health. It is known that the advanced male age adversely affects testicular function, hormonal balance of the male reproductive system, sperm parameters, and the integrity of the genome and epigenome of sperm. However, a systematic review of 12 studies showed that father age was not associated with ART outcomes [6], but the data regarding the embryological stage are less reliable and explicit.
Since the male factor is one of the most common indications for ART, the relationship between SMF and the incidence of embryonic aneuploidy is also in focus. The question of whether SMF should be considered as an indication for prenatal genetic testing for aneuploidy (PGT-A) is still an open one [7]. Early studies analyzing the incidence of aneuploidy showed that SMF might contribute to a higher prevalence of aneuploid embryos in ART. However, this conclusion is mainly based on studies using fluorescence in situ hybridization (FISH) analysis for a limited number of chromosomes in cleavage embryos [8].
This study aimed to investigate the impact of different forms of male infertility on reproductive outcomes, focusing on early embryonic development (fertilization rate and superior blastocyst formation rate), rate of embryonic aneuploidy, and ART results.
Materials and methods
This retrospective study was conducted at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility and the Laboratory of Molecular Genetics V.I. Kulakov NMRC for OG&P of Ministry of Health of Russia. The study analyzed 2915 ART cycles (2225 stimulation cycles, of which 371 cycles with PGT-A and 690 cryopreserved cycles) performed from January 2018 to June 2021. All couples signed voluntary informed consent. The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
The exclusion criteria were the age of women> 35 years old and altered karyotype in couples. We did not exclude couples using frozen sperm, donor sperm, and couples with donor oocytes, and the age of sex gamete donors did not exceed 35 years.
Ejaculate samples obtained after 3–5 days of sexual abstinence by masturbation on the day of transvaginal puncture were evaluated based on the sperm quality criteria of the WHO reference values [9]. Analyzed sperm parameters included sperm concentration (mln/ml), percentage of progressively motile sperm, and morphologically normal sperm. The study groups were formed based on ejaculate parameters. The control group included couples using IVF with donor sperm and having normozoospermia (concentration ≥15 million/ml, progressively motile sperm ≥32%, morphology ≥4%). The group with oligoasthenoteratozoospermia (OAT) consisted of patients with a concentration of <15 million/ml, progressively motile spermatozoa <32% and morphology <4%, including patients with azoospermia (concentration <1 million/ml in the native sample). Patients with obstructive and non-obstructive azoospermia, whose spermatozoa were obtained by aspiration or testicular biopsy, were included in the microTESE group. Additionally, we formed two groups with teratozoospermia categorized by the percentage of morphologically abnormal spermatozoa. Men with sperm morphology in the range from 0 to 2% made up the 0–2%T group, and those with 3% morphology were the 3%T group. The group of infertile men with SMF consisted of patients with OAT and patients with microTESE. These patient groups were pooled together due to the small number of observations.
All patients started controlled ovarian stimulation on day 2–5 of their menstrual cycle using the ovarian stimulation protocol with gonadotropin-releasing hormone antagonists and recombinant follicle-stimulating hormone or human menopausal gonadotropin. Ovulation was triggered by a human chorionic gonadotropin injection of 6,000–10,000 IU intramuscularly when the follicles reached ≥ 17 mm diameter. The transvaginal ovarian puncture to retrieve oocytes was performed 36 hours after the ovulation trigger injection, followed by oocyte quality assessment. Immediately after follicular fluid aspiration, oocyte-cumulus complexes (COC) were identified, and oocyte maturity was evaluated under a stereomicroscope on the heated surface of a sterile laminar box.
Fertilization of oocytes was carried out by intracytoplasmic sperm injection (ICSI), after which the fertilized cells were transferred to the culture medium (CSCM, Irvine Sc., USA) for further embryo culture. The onset of the stage of two pronuclei (zygote formation) was assessed 14–16 h after fertilization. All stages of embryo culture were carried out in the SOOK MultiGas Incubator (Ireland) in 25 μL drops under oil (Irvine Sc., USA). The medium CSCM, Irvine Sc., USA) was not changed during five days of embryo culture. One embryo of good or excellent quality was transferred on day 5 or 6 of the stimulation cycle.
In several cycles, a trophectoderm biopsy was performed on day 5 or 6 after fertilization, and embryos were cryopreserved. Blastocysts suitable for genetic analysis were assessed according to the classification adopted by the Istanbul consensus workshop on embryo assessment and corresponded to grade 3BB and higher [10]. The obtained trophectoderm cells were transferred into Eppendorf tubes containing lysis buffer for PGT-A using next-generation sequencing (NGS). The PGT-A procedure consisted of several stages. At the first stage, whole-genome amplification and preparation of the library for application to the chip were carried out. Special molecular barcode labels unique for each sample were attached to the DNA fragments to create a library. Then ion semiconductor sequencing was performed, followed by bioinformatic analysis of the results and preparation of a conclusion based on the obtained data according to the standard PGT-A.
Patients received cyclic hormonal therapy during the transfer of cryopreserved embryos, including estradiol valerate (6 mg/day) starting on day 4–5 of the menstrual cycle and vaginal micronized progesterone (400–600 mg/day) from day 15-16 of the menstrual cycle. Patients underwent ultrasound monitoring of the changes in endometrial growth on day 9–10 of the menstrual cycle and on day 15–16 of the cycle for gestagens administration. Embryo transfer was carried out on days 20–21of the menstrual cycle using a Wallace (Germany) or Cook (Australia) soft embryo transfer catheter. The preliminary thawing of embryos and the management of the post-transfer period were carried out according to the protocols accepted in clinical practice.
The number of COC, mature oocytes, fertilization, and blastulation rates were assessed as the main embryological indicators of stimulation cycles. Blastulation rate was determined as the ratio of the number of blastocysts of good and excellent quality (the number of frozen blastocysts + the number of transferred embryos) to the number of zygotes with two pronuclei. Primary clinical outcomes for ART were the pregnancy rate, the pregnancy loss rate before 12 weeks of gestation, and the birth rate. On day 14, after the embryo transfer, patients donated blood for the beta-subunit of human chorionic gonadotropin (β-hCG) for pregnancy diagnosis. A positive result was determined as β-hCG level > 35 ΜE/L. In the case of a positive β-hCG test for the diagnosis of clinical pregnancy, a gestational sac was visualized on transvaginal ultrasound on day 21 after an embryo transfer.
Cases of ectopic and anembryonic pregnancies and termination of pregnancy before 12 weeks of gestation were included in the group with early losses. The birth rate was calculated by the number of transfers.
Statistical analysis
Statistical analysis was performed using the IBM SPSS Statistics software version 23.0 (USA) and Microsoft Excel. The distribution of continuous variables was tested for normality using the Kolmogorov–Smirnov test. Categorical variables were presented as counts (n) and percentages (%). Continuous variables were expressed as the median (Me) with interquartile range (Q1; Q3). The Kruskal–Wallis test was used to compare numerical data between groups, followed by pairwise comparison using the Mann–Whitney U-test. Categorical variables were compared by the χ2 test. Bonferroni correction was applied for multiple comparisons. The odds ratio (OR) was calculated with 95% confidence interval (CI) of 95% to assess the relationship between some outcomes and a risk factor. CIs not including 1 signified a statistically significant relationship between the factor and the outcome at p <0.05. The critical level of significance when interpreting the results of statistical analysis was considered at p<0.05.
Results
Analysis of the association between ejaculate parameters and the embryological stage included 2225 ART cycles (161 cycles with donor oocytes and 87 cycles with donor sperm). All analyzed parameters (age, COC number, number of mature oocytes, fertilization, and blastulation rates) were not normally distributed (Kolmogorov–Smirnov test, p<0.001). Nonparametric tests were used to compare groups. The median age of men (n=2138) was Me 34 (31; 38), excluding sperm donors. The median age of women (n=2064) was Me 31 (29; 34), excluding oocyte donors.
To assess the effect of the older reproductive age of men (> 40 years) on the embryological stage, two groups were formed. One included 1918 programs with men aged ≤40 years and 307 programs with men aged> 40 years. No significant differences were found between groups of men of different ages: the fertilization rate 100.0 (80.0; 100.0) in the group of men ≤40 versus 92.3 (75.0; 100.0) in the group of men> 40; blastulation rate 50.0 (33.3; 75.0) in the group of men ≤40 versus 50.0 (33.3; 75.0) in the group of men> 40. (Table 1).
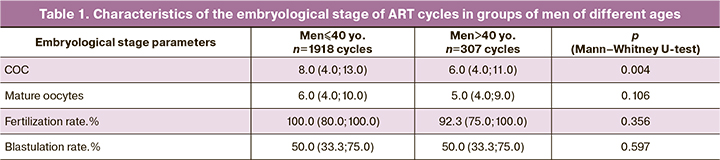
Based on findings of semen analysis, five groups were formed: the comparison group consisted of 257 couples with normozoospermia (including couples with donor sperm), 214 couples with OAT, 105 couples with testicular biopsy (microTESE), 1061 couples with sperm morphology 0–2% and 588 couples with 3% sperm morphology. It is worth noting that the comparison group included couples with tubal-peritoneal factor, couples with a male factor, or single women without a partner who used donor sperm (87 couples), as well as couples with infertility with normal sperm counts. The median age of men did not differ significantly between the groups (p=0.057). Analysis of the number of COC and mature oocytes between the groups did not reveal significant differences in the stimulation of the patients (Table 2). When comparing parameters of the embryological stage in groups with different male pathologies using the Kruskal–Wallis test, it was shown that there were significant differences between the groups concerning fertilization and blastulation rates. A post-hoc analysis and pairwise comparison of the analyzed groups with the control group were performed using the Mann–Whitney test with Bonferroni correction. As a result, a new critical level of significance, p=0.0125, was determined, which was used to interpret the results. There were statistically significant differences in the blastulation rates between the OAT group and the comparison group (p=0.007) and the group with 0–2% morphology and the comparison group (p=0.005). Groups of men with OAT and sperm morphology 0–2% showed a statistically significant decrease in blastulation rate compared with the control group. Pairwise comparison of fertilization rates of groups with different parameters of semen analysis with the control group yielded p < 0.0125. However, it should be noted that the fertilization rates in the OAT and microTESE groups were on average lower than in the groups with sperm morphology of 0–2% and 3%. Perhaps, due to the insufficient number of observations, these differences did not reach statistical significance. Still, a tendency was noted, which manifested itself when comparing all analyzed groups using the Kruskal-Wallis test (p=0.03) (Table 2).
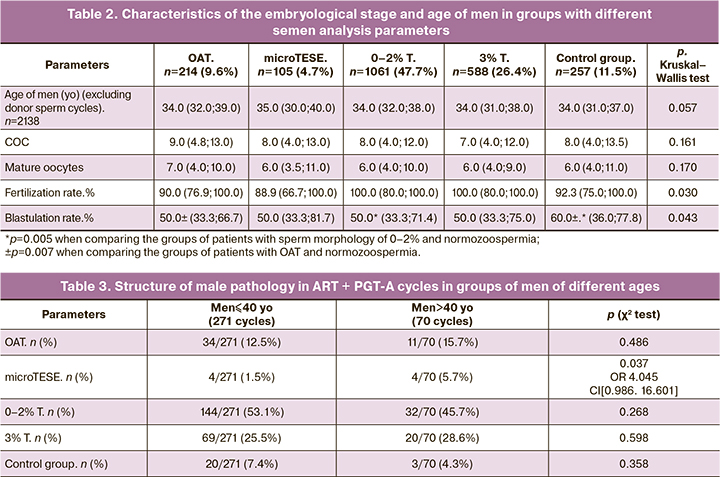
To assess the effect of male age on the incidence of aneuploidies, 341 ART programs with PGT-A (1022 embryos) were analyzed. The patient cohort was divided into two groups, including men aged ≤40 and > 40. In the group of men ≤40 (n=271), the median age of men was Me 34 (32; 36) years, the median age of women was Me 32 (29.75; 34) years. In the cohort of men > 40 (n=70), the median age of men was Me 44 (42; 48) years, the median age of women was Me 33 (31; 34). The structure of male pathology based on semen analysis did not differ in the groups of men aged ≤40 and> 40: group OAT – 34/271 (12.5%) versus 11/70 (15.7%); group with morphology 0–2% – 144/271 (53.1%) versus 32/70 (45.7%); group with 3% morphology – 69/271 (25.5%) versus 20/70 (28.6%); control group – 20/271 (7.4%) versus 3/70 (4.3%), respectively (Table 3). However, patients with testicular biopsy (microTESE) were more likely to be > 40 years [4/70 (5.7%)] than ≤40 years [4/271 (1.5%)], respectively (p=0.037).
Comparison of embryological stage parameters did not reveal significant differences in the groups of men of different ages: fertilization rate [88.9 (77.8; 100.0) in the group of men aged≤40 versus 87.5 (75.0; 100.0) > 40 years old] and blastulation rate [50.0 (33.3; 66.7) among men aged ≤40 versus 42.9 (26.7; 66.7) > 40 years] were comparable in analyzed groups and had no statistically significant differences (table. 4).
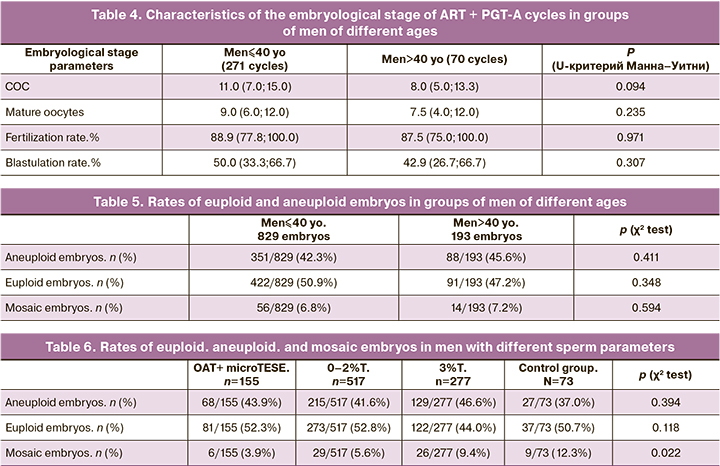
Analysis of euploid, aneuploid, and mosaic embryos in the groups of men aged≤40 and> 40 years showed no influence of the age on the embryo genetic status: the rate of having euploid embryos in the group of men aged ≤40 was 422/829 (50.9%), and 91/193 (47.2%) among men aged > 40 (Table 5). The rate of having mosaic embryos also did not differ significantly between the groups of men aged ≤40 and> 40 [56/829 (6.8%) versus 14/193 (7.2%), respectively].
Considering the limited impact of male age on the incidence of aneuploidies, we decided to evaluate the effect of SMF (OAT and microTESE group) and other ejaculate parameters (sperm morphology 0–2% and 3%) on the incidence of aneuploidies in ART + PGT-cycles and analyzed the results of genetic analysis of embryos in groups of patients with different semen analysis parameters. The results showed that the rate of aneuploid and euploid embryos was not associated with male pathology's structure (Table 6). It should be noted that the rate of obtaining mosaic embryos significantly differed in the compared groups from 6/155 (3.9%) in the group with SMF to 9/73 (12.3%) in the control group with normal semen analysis parameters (p=0.022). When correcting for multiple comparisons, a new critical level of significance p=0.0125 was determined. However, pairwise comparison of the study and control groups yielded p<0.017, 0.029 and 0.456 when comparing OAT, 0–2% T and 3% T versus the control group, respectively.
To assess the effect of ejaculate parameters on the outcomes of ART programs, the study included all couples who underwent one embryo transfer on days 4 and 5 in the stimulation cycle or transfer of one embryo on days 5 and 6 of culture with and without PGT-A in a cryopreserved cycle, except for pairs with donor oocytes.
The primary outcomes of ART based on semen analysis were the pregnancy rate per number of transfers, early pregnancy loss rate before 12 weeks of gestation, and delivery rate per number of transfers. It should be noted that information for endpoints is not available for all patients since some of them are currently pregnant.
Comparison of the outcomes of ART programs at control time points between groups of men with different semen analysis parameters showed that the structure of male pathology did not affect the outcomes of infertility treatment, neither in the stimulation cycle during fresh embryo transfer, nor in the cryopreserved cycle with freeze-thaw cycles with and without PGT-A (Tables 7–9).
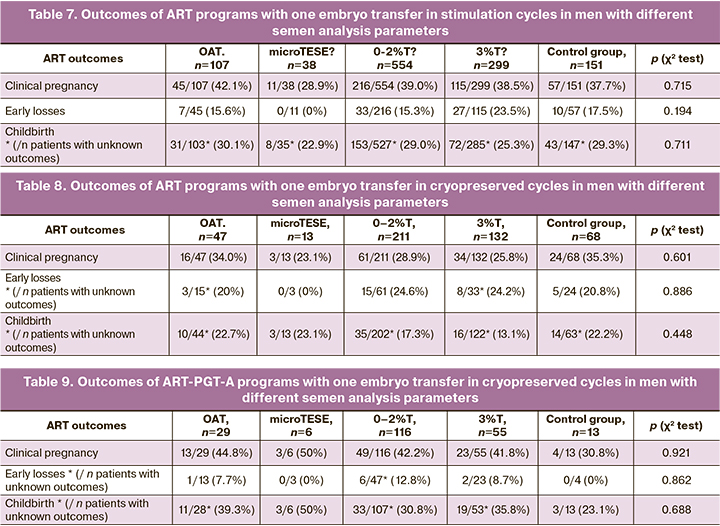
Comparing ART outcomes in stimulation cycles after fresh embryo transfer and cryopreserved cycle without PGT-A showed lower pregnancy and birth rates in cryopreserved cycles for all groups of patients with different semen analysis parameters. However, a statistically significant decrease in the pregnancy rate and birth rate was found for patients with sperm morphology of 0–2%T and 3%T. The pregnancy rate after fresh embryo transfer in the group with morphology 0–2% was 216/554 (39%) versus 61/211 (28.9%) in cryopreserved cycle (p=0.01, χ2), OR 0.636, CI [0.452; 0.897], birth rate 153/527 (29.0%) versus 35/202 (17.3%), respectively (p=0.002, χ2), OR 0.512, CI [0.340; 0.772]. In the group with 3% morphology, the pregnancy rate after fresh embryo transfer was 115/299 (38.5%) versus 34/132 (25.8%) in the cryopreserved cycle (p=0.01, χ2), OR 0.555, CI [032; 0.874]; birth rate - 72/285 (25.3%) versus 16/122 (13.1%), respectively (p=0.007, χ2), OR 0.447, CI [0.248; 0.805].
When comparing the outcomes of ART programs in cryopreserved cycle with PGT-A and without genetic testing for aneuploidy, an increase in the pregnancy rate during euploid embryo transfer, a decrease in the number of early losses, and an increase in the frequency of births were noted, which is due to the transfer of a genetically normal embryo according to screening results. However, statistically significant differences were also observed for patients with sperm morphology of 0–2% and 3%. The pregnancy rate after euploid embryo transfer in the group with morphology of 0–2% was 49/116 (42.2%) versus 61/211 (28.9%) in cryopreserved cycle without PGT-A (p = 0.015, χ2), OR 1.798, CI [1.120; 2.888]; birth rate - 33/107 (30.8%) versus 35/202 (17.3%), respectively (p = 0.007, χ2), OR 2.128, CI [1.229; 3.683]. In the group with 3% morphology, the pregnancy rate in the cryopreserved cycle after euploid embryo transfer was 23/55 (41.8%) versus 34/132 (25.8%) in cryopreserved cycle without PGT-A (p = 0.03, χ2) , OR 2.072, CI [1.068; 4.019]; birth rate 19/53 (35.8%) versus 16/122 (13.1%), respectively (p <0.001, χ2), OR 3.702, CI [1.716; 7.989]. Thus, the pregnancy rate and the birth rate in the groups with the morphology of 0–2% and 3% are almost twice lower in the cryopreserved cycle than after a fresh embryo transfer in stimulation cycle and a euploid embryo transfer in a cryopreserved cycle, in the absence of a difference in the rates of euploid embryos in groups with altered semen analysis parameters and men older and younger than 40 years.
Discussion
To date, the data on the association of the male factor (age, SMF) with ART results, such as embryo viability, early embryonic development, the incidence of aneuploidy, and the pregnancy and birth rates, are somewhat controversial. The present study aimed to investigate the impact of older men's age (>40 years) and the presence of SMF on the rate of embryonic aneuploidy, the outcomes of IVF/ICSI, and the embryological stage of infertility treatment. Taking into account that most embryonic aneuploidies are the result of maternally inherited aberrations, the prevalence of which increases from 30% in women aged 30 years to almost 90% in women aged 44 years [11], we excluded all ART cycles in which age women over 35 to neutralize the influence of the maternal factor on the incidence of aneuploidies and the outcomes of ART.
The age of men is believed to adversely affect ART outcomes, possibly due to the sperm DNA damage resulting from oxidative stress [11]. We found no significant differences in the characteristics of the embryological stage in the groups of men aged over and under 40; fertilization and blastulation rates were comparable in both groups, which is consistent with the data of Kasman A.M. et al. [13], who showed that father's age (≥40 years) is not associated with either the embryological stage or ART outcomes. Although the study by Dviri M. et al. [14] noted a statistically significant decrease in the fertilization rate from 80% to 76% in men ≥50 compared with younger men, which of course is not such clinically significant, while Hanson B.M. et al. in their work in the analysis of 4058 cycles showed a decrease in the percentage of blastulation in men ≥40 years old [15], and Morris G. et al. in the 2021 study showed that regardless of the cause of infertility, an increase in male age (≥50 years) is associated with a decrease in the number of live births and pregnancy rate, but does not affect the early pregnancy losses [16]. Further research is required to investigate the possible mechanisms of this effect, and it is also necessary to improve existing methods of sperm selection that can mitigate these effects.
Comparing the embryological stage parameters of men with different ejaculate parameters showed a decrease of high-quality blastocysts compared with men with normozoospermia. There was a statistically significant difference between men with OAT and sperm morphology of 0–2%, which may be attributed to the impact of the male factor on early embryonic development. Mazzilli R. et al. analyzed 1219 cycles and reported that men with SMF had a lower fertilization rate than men with normozoospermia, while the rate of top-quality blastocysts was significantly lower only in the group with non-obstructive azoospermia [17]. Analysis of 1266 ICSI cycles showed that a decrease in concentration (<5 million / ml) is associated with a reduction in fertilization rate but does not affect the rate of obtaining blastocysts of excellent quality and ongoing pregnancy rate [18].
Sperm plays an essential role in the stages of embryogenesis, such as fertilization, epigenetic control, and cell division, which can affect ICSI results. There is an opinion that the adverse effect of severe OAT on the blastulation rate may be associated with increased fragmentation of sperm DNA [19]. A recent study in which embryos were monitored using time-lapse imaging demonstrated that SMF is associated with a lower percentage of blastocysts available for transfer compared to other causes of infertility. However, early and late morphokinetic parameters of embryos remained similar regardless of the severity of male infertility [20]. Still, this fact does not confirm the high reproductive potential of the transferred blastocysts. Therefore, the presence of SMF primarily affects the ability of fertilized oocytes to reach the blastocyst stage, which leads to a decrease in the number of top-quality blastocysts. However, this does not fully prove whether the reproductive potential of the transferred blastocysts is preserved.
As early studies using FISH have shown, the number of aneuploid embryos at the cleavage stage may depend on the severity of male infertility. However, the likelihood that cleavage aneuploid embryos reach the blastocyst stage may negatively correlate with SMF. Thus, the rate of euploid embryos at the blastocyst stage may be the same for men with reduced sperm counts, as shown by recent studies [17, 21]. This can be explained by the advantage of survival of euploid cleavage stage embryos to the stage of blastocysts. Another possible explanation is that the timing of embryo biopsy affects the incidence of aneuploidy since the embryo ploidy status is more accurately determined at the blastocyst stage than at the cleavage stage [22].
Previous research has shown that maternal age is the leading cause of embryonic aneuploidies [11]. There is no research evidence regarding the association of father's age and ejaculate parameters with aneuploidies. It is known that advanced male age is associated with spermatozoa point mutations responsible for the inheritance of certain diseases (schizophrenia, autism). Paternally inherited mutations double every 16.5 years [23], while evidence of the genesis of paternal aneuploidies remains unclear. Our findings suggest that neither abnormal sperm parameters (OAT, microTESE, sperm morphology) nor the father's age over 40 is associated with an increased incidence of embryo aneuploidy, which is consistent with earlier studies [17,21]. Carrasquillo R.J. et al. analyzed 1202 cycles with donor oocytes and PGT-A and reported that the older reproductive age of the father did not affect the embryo's genetic status [24]. Similar results were obtained when analyzing 3118 embryos for aneuploidy in groups of men of different ages (≤39, 40–49, ≥50 years) in programs with donor oocytes: no relationship was found between the father's age and the level of aneuploidy [14]. However, Coates A. et al. reported an increased frequency of sex chromosome abnormalities in embryos in men with oligozoospermia than in men with normal sperm [25]. A lower incidence of euploidy, a higher incidence of mosaicism, and a higher incidence of abnormal morphokinetic development have been described in ICSI with testicular sperm in women ≤35 years, compared with normal sperm [26].
The analysis of the outcomes of the ART programs showed that during the transfer of both a fresh embryo in the stimulation cycle and a thawed embryo in a cryopreserved cycle, the endpoints (pregnancy rate, early-stage losses, birth rate) did not differ statistically significantly in groups with different semen analysis parameters. Thus, it can be concluded that factors of male infertility associated with changes in ejaculate parameters do not affect ART outcomes. It should be noted that the best outcomes in terms of the total pregnancy rate (42.0%), early pregnancy losses (10%), and birth rate (33.3%) were observed in ART programs with PGT-A, which is due to the transfer of euploid embryos, in contrast to programs without PGT-A. A minimum total pregnancy rate of 29.3%, a maximum number of early losses of 22.8%, and a minimum birth rate of 17.6% were observed after a frozen-thawed embryo transfer in cryopreserved cycles without PGT-A.
Comparing ART outcomes for couples with normozoospermia after the transfer of both fresh and thawed embryos in cycles with and without PGT-A showed no significant differences in all endpoints. The birth rate was 29.3%, 22.2%, and 23.1%, respectively. Therefore, for couples with normozoospermia and women aged ≤35, PGT-A does not improve ART outcomes. In the group with SMF (OAT and microTESE pooled group), there was an increase in pregnancy rate, a decrease in early loss, and an increase in birth rate compared to thawed embryo transfer without PGT-A: 45.7% versus 31.7%; 6.3% versus 16.7% and 41.2% versus 22.8%, respectively. However, these differences were not statistically significant, though there was a trend in the delivery rate (p = 0.064). Similar conclusions were made by Xu R. et al. [27], who showed that NGS-based PGT-A could improve pregnancy outcomes for couples with SMF by significantly reducing the rate of early miscarriage (6.7% versus 21.6%, p = 0.02), but no effect on the cumulative incidence of ongoing pregnancy (54.9% versus 55.8%, p=0.90) versus ICSI without PGT-A.
Comparison of ART outcomes of men with the morphology of 0–2% and 3% showed a statistically significant decrease in pregnancy rate and birth rate when transferring frozen-thawed embryos with unknown genetic status, as compared to fresh embryo transfer in a stimulation cycle in a similar group and comparison with embryo transfer in cryopreserved cycles with PGT-A. This is consistent with work showing that the presence of teratozoospermia in men increases the risk of ART failure [28]. Teratozoospermia is one of the most common variants of abnormalities in the semen analysis in the general population of patients, and the morphological assessment of spermatozoa is highly subjective and largely depends on the embryologist. Implantation failures are associated by 1/3 with the embryo and 2/3 with the endometrium [29]. In these couples, infertility is mainly attributed to female infertility factors. The decrease in the pregnancy rate and birth rate in cryopreserved cycles in such couples is probably associated with the insufficient examination of the woman. In couples with SMF (OAT, microTESE), as a rule, women do not have a complicated medical history, which is confirmed by the absence of statistically significant differences in the pregnancy and birth rates, both after embryo transfer during the stimulation cycle and thawed embryo transfer in cryopreserved cycles.
Conclusion
The present study has demonstrated that in couples with SMF, NGS-based PGT-A can increase the birth rate with fewer embryos transferred by reducing early pregnancy losses. Our findings showed that the frequency of obtaining euploid and aneuploid embryos in groups of men with different sperm parameters, including those with OAT and microTESE, did not differ from that in the control group and amounted to about 50%. This approach can be recommended for couples with SMF. It is also worth noting that severe forms of male infertility and advanced father’s age (≥50 years) may adversely affect fertilization and blastulation rates, but not the pregnancy rate in ICSI cycles. However, well-designed and sufficiently powerful randomized controlled trials are still needed to confirm these results.
References
- Palermo G., Joris H., Devroey P., Van Steirteghem A.C. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992; 340(8810): 17-8. https://dx.doi.org/10.1016/0140-6736(92)92425-f.
- de Mouzon J., Chambers G.M., Zegers-Hochschild F., Mansour R., Ishihara O., Banker M. et al. International Committee for Monitoring Assisted Reproductive Technologies world report: assisted reproductive technology 2012† Hum. Reprod. 2020; 35(8): 1900-13. https://dx.doi.org/10.1093/humrep/deaa090.
- Brugh V.M., Lipshultz L.I. Male factor infertility: evaluation and management. Med. Clin. North Am. 2004; 88(2): 367-85. https://dx.doi.org/10.1016/S0025-7125(03)00150-0.
- Punab M., Poolamets O., Paju P., Vihljajev V., Pomm K., Ladva R. et al. Causes of male infertility: a 9-year prospective monocentre study on 1737 patients with reduced total sperm counts. Hum. Reprod. 2017; 32(1): 18-31. https://dx.doi.org/10.1093/humrep/dew284.
- Bergh C., Pinborg A., Wennerholm U.B. Parental age and child outcomes. Fertil. Steril. 2019; 111(6): 1036-46. https://dx.doi.org/10.1016/j.fertnstert.2019.04.026.
- Sagi-Dain L., Sagi S., Dirnfeld M. Effect of paternal age on reproductive outcomes in oocyte donation model: a systematic review. Fertil. Steril. 2015; 104(4): 857-65.e1. https://dx.doi.org/10.1016/j.fertnstert.2015.06.036.
- Carvalho F., Coonen E., Goossens V., Kokkali G., Rubio C., Meijer-Hoogeveen M. et al. ESHRE PGT Consortium good practice recommendations for the organisation of PGT. Hum. Reprod. Open. 2020; 2020(3): hoaa021. https://dx.doi.org/10.1093/hropen/hoaa021.
- Magli M.C., Gianaroli L., Ferraretti A.P., Gordts S., Fredericks V., Crippa A. Paternal contribution to aneuploidy in preimplantation embryos. Reprod. Biomed. Online. 2009; 18(4): 536-42. https://dx.doi.org/10.1016/s1472-6483(10)60131-9.
- Cooper T.G., Noonan E., von Eckardstein S., Auger J., Baker H.W.G., Behre H.M. et al. World Health Organization reference values for human semen characteristics. Hum. Reprod. Update. 2010; 16(3): 231-45. https://dx.doi.org/10.1093/humupd/dmp048.
- Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum. Reprod. 2011; 26(6): 1270-83. https://dx.doi.org/10.1093/humrep/der037.
- Franasiak J.M., Forman E.J., Hong K.H., Werner M.D., Upham K.M., Treff N.R., Scott R.T. Jr. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil. Steril. 2014; 101(3): 656-63.e1. https://dx.doi.org/10.1016/j.fertnstert.2013.11.004.
- Смольникова В.Ю., Агаджанян Д.С., Красный А.М. Активные формы кислорода и компоненты системы антиоксидантной защиты как маркеры прогнозирования качества эмбрионов у супружеских пар с различными типами бесплодия. Акушерство и гинекология. 2020; 11: 55-60. [Smolnikova V.Yu., Agadzhanyan D.S., Krasnyi A.M. Reactive oxygen species and components of the antioxidant defense system as markers for prediction of embryo quality in married couples with different infertility types. Obstetrics and Gynegology. 2020; 11: 55-60. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.11.55-60.
- Kasman A.M., Li S., Zhao Q., Behr B., Eisenberg M.L. Relationship between male age, semen parameters and assisted reproductive technology outcomes. Andrology. 2021; 9(1): 245-52. https://dx.doi.org/10.1111/andr.12908.
- Dviri M., Madjunkova S., Koziarz A., Antes R., Abramov R., Mashiach J. et al. Is there a correlation between paternal age and aneuploidy rate? An analysis of 3,118 embryos derived from young egg donors. Fertil. Steril. 2020; 114(2): 293-300. https://dx.doi.org/10.1016/j.fertnstert.2020.03.034. Erratum in: Fertil. Steril. 2020; 114(5): 1122.
- Hanson B.M., Kim J.G., Osman E.K., Tiegs A.W., Lathi R.B., Cheng P.J. et al. Impact of paternal age on embryology and pregnancy outcomes in the setting of a euploid single-embryo transfer with ejaculated sperm: retrospective cohort study. F S Rep. 2020; 1(2): 99-105. https://dx.doi.org/10.1016/j.xfre.2020.06.004.
- Morris G., Mavrelos D., Odia R., Viñals Gonzalez X., Cawood S., Yasmin E. et al. Paternal age over 50 years decreases assisted reproductive technology (ART) success: A single UK center retrospective analysis. Acta Obstet. Gynecol. Scand. 2021; 100(10): 1858-67. https://dx.doi.org/10.1111/aogs.14221.
- Mazzilli R., Cimadomo D., Vaiarelli A., Capalbo A., Dovere L., Alviggi E. et al. Effect of the male factor on the clinical outcome of intracytoplasmic sperm injection combined with preimplantation aneuploidy testing: observational longitudinal cohort study of 1,219 consecutive cycles. Fertil. Steril. 2017; 108(6): 961-72.e3. https://dx.doi.org/10.1016/j.fertnstert.2017.08.033.
- Bartolacci A., Pagliardini L., Makieva S., Salonia A., Papaleo E., Viganò P. Abnormal sperm concentration and motility as well as advanced paternal age compromise early embryonic development but not pregnancy outcomes: a retrospective study of 1266 ICSI cycles. J. Assist. Reprod. Genet. 2018; 35(10): 1897-903. https://dx.doi.org/10.1007/s10815-018-1256-8.
- Sedó C.A., Bilinski M., Lorenzi D., Uriondo H., Noblía F., Longobucco V. et al. Effect of sperm DNA fragmentation on embryo development: clinical and biological aspects. JBRA Assist. Reprod. 2017; 21(4): 343-50. https://dx.doi.org/10.5935/1518-0557.20170061.
- Sacha C.R., Dimitriadis I., Christou G., James K., Brock M.L., Rice S.T. et al. The impact of male factor infertility on early and late morphokinetic parameters: a retrospective analysis of 4126 time-lapse monitored embryos. Hum. Reprod. 2020; 35(1): 24-31. https://dx.doi.org/10.1093/humrep/dez251.
- Tarozzi N., Nadalini M., Lagalla C., Coticchio G., Zacà C., Borini A. Male factor infertility impacts the rate of mosaic blastocysts in cycles of preimplantation genetic testing for aneuploidy. J. Assist. Reprod. Genet. 2019; 36(10): 2047-55. https://dx.doi.org/10.1007/s10815-019-01584-w.
- Liñán A., Lawrenz B., El Khatib I., Bayram A., Arnanz A., Rubio C. et al. Clinical reassessment of human embryo ploidy status between cleavage and blastocyst stage by Next Generation Sequencing. PLoS One. 2018; 13(8): e0201652. https://dx.doi.org/10.1371/journal.pone.0201652.
- Kong A., Frigge M.L., Masson G., Besenbacher S., Sulem P., Magnusson G. et al. Rate of de novo mutations and the importance of father's age to disease risk. Nature. 2012; 488(7412): 471-5. https://dx.doi.org/10.1038/nature11396.
- Carrasquillo R.J., Kohn T.P., Cinnioglu C., Rubio C., Simon C., Ramasamy R., Al-Asmar N. Advanced paternal age does not affect embryo aneuploidy following blastocyst biopsy in egg donor cycles. J. Assist. Reprod. Genet. 2019; 36(10): 2039-45. https://dx.doi.org/10.1007/s10815-019-01549-z.
- Coates A., Hesla J.S., Hurliman A., Coate B., Holmes E., Matthews R. et al. Use of suboptimal sperm increases the risk of aneuploidy of the sex chromosomes in preimplantation blastocyst embryos. Fertil. Steril. 2015; 104(4): 866-72. https://dx.doi.org/10.1016/j.fertnstert.2015.06.033.
- Kahraman S., Sahin Y., Yelke H., Kumtepe Y., Tufekci M.A., Yapan C.C. et al. High rates of aneuploidy, mosaicism and abnormal morphokinetic development in cases with low sperm concentration. J. Assist. Reprod. Genet. 2020; 37(3): 629-40. https://dx.doi.org/10.1007/s10815-019-01673-w.
- Xu R., Ding Y., Wang Y., He Y., Sun Y., Lu Y., Yao N. Comparison of preimplantation genetic testing for aneuploidy versus intracytoplasmic sperm injection in severe male infertility. Andrologia. 2021; 53(6): e14065. https://dx.doi.org/10.1111/and.14065.
- Долгушина Н.В., Сокур С.А., Глинкина Ж.И., Калинина Е.А. Исходы программ вспомогательных репродуктивных технологий у супружеских пар с различными видами патозооспермии у мужчин. Акушерство и гинекология. 2013; 10: 69-75. [Dolgushina V.F., Sokur S.A., Glinkina Zh.I., Kalinina E.A. Outcomes of assisted reproductive technology programs in married couples with different types of pathozoospermia in men. Obstetrics and Gynegology. 2013; 10: 69-75. (in Russian)].
- Achache H., Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum. Reprod. Update. 2006; 12(6): 731-46. https://dx.doi.org/10.1093/humupd/dml004.
Received 12.10.2021
Accepted 28.10.2021
About the Authors
Natalia P. Makarova, Dr. Biol. Sci., Senior Researcher at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, np_makarova@oparina4.ru, https://orcid.org/0000-0003-8922-2878, 117997, Russia, Moscow, Academician Oparin str., 4.Nataliya N. Lobanova, Junior Researcher at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia, n_lobanova@oparina4.ru, https://orcid.org/0000-0002-0818-4073, 117997, Russia, Moscow, Academician Oparin str., 4.
Elena V. Kulakova, Ph.D., Senior Researcher at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia, e_kulakova@oparina4.ru, https://orcid.org/0000-0002-4433-4163, 117997, Russia, Moscow, Academician Oparin str., 4.
Oksana S. Nepsha, Ph.D. (Biol. Sci.), Researcher at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, nepsha@oparina4.ru, https://orcid.org/0000-0002-9988-2810, 117997, Russia, Moscow, Academician Oparin str., 4.
Alexey N. Ekimov, Clinical Geneticist at the Molecular Genetics Laboratory, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, a_ekimov@oparina4.ru,
https://orcid.org/0000-0001-5029-0462, 117997, Russia, Moscow, Academician Oparin str., 4.
Elena A. Kalinina, Dr. Med. Sci., Professor, Head of the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, e_kalinina@oparina4.ru, https://orcid.org/0000-0002-8922-2878, 117997, Russia, Moscow, Academician Oparin str., 4.
Corresponding author: Oksana S. Nepsha, nepsha@oparina4.ru
Authors' contributions: Makarova N.P. – embryo culture, embryo trophectoderm biopsy, embryo cryopreservation, manuscript editing and approval; Nepsha O.S., Lobanova N.N. – collection and review of the relevant literature, manuscript preparation; Kulakova E.V. – manuscript editing; Ekimov A.N. – c preimplantation genetic testing for aneuploidy by next-generation sequencing (NGS); Kalinina E.A. – manuscript editing and approval.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Makarova N.P., Lobanova N.N., Kulakova E.V., Nepsha O.S., Ekimov A.N., Kalinina E.A. Impact of preimplantation genetic testing on assisted reproductive technology outcomes in couples with male factor infertility.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 11: 154-164 (in Russian)
https://dx.doi.org/10.18565/aig.2021.11.154-164



