Technological platforms for preimplantation genetic testing for aneuplody: comparative effectiveness of diagnosing chromosomal abnormalities
Objective. To analyze a range of chromosomal abnormalities in 5-6-day-old embryos using array comparative genomic hybridization (aCGH) and next-generation sequencing (NGS) methods in two centers for assisted reproductive technologies (ART) and to compare the results after applying two methods.Маlysheva О.V., Bichevaya N.K., Gzgzyan А.М., Glotov О.S., Kinunen А.А., Lobenskaya А.YU., Меkina I.D., Polyakova I.V., Puppo I.L., Saifitdinova А.F., Shcherbak S.G., Kоgan I.YU.
Materials and methods. Trophectoderm samples of 1041 embryos were studied. NSG was performed on the platform Illumina MiSeq with VeriSeq PGS Library Prep Kit (Illumina); aCGH was conducted using microarray kit G5963A, Agilent.
Results. Single chromosome aneuploidy rate starts increasing after 30 years of age, and aneuploidy involving two or more chromosomes after 37 years. In our sampling aCGH and NGS methods showed a similar effectiveness for performing PGT-A. Statistically significant differences in the incidence of chromosomal abnormalities between two ART centers were not revealed.
Conclusion. The decrease in the proportion of embryos with balanced chromosome complement is the most important factor which reduces reproductive potential of women of advanced reproductive age undergoing IVF. Both methods aCGH and NGS demonstrate a similar effectiveness for performing PGT-A. The incidence of chromosomal abnormalities of various types did not differ in embryos cultivated in two IVF centers which took part in the investigation.
Keywords
Preimplantationgenetictestingforaneuploidy(PGT-A) in IVF cycles significantly increases implantation rate, reduces the time necessary to achieve pregnancy (by the period of 6 months–1 year) and reduces the risk of pregnancy loss in the first trimester more than three times [1, 2]. Nowadays, PGT-A implies testing of all chromosomes and since 2012 centers for assisted reproductive technologies (ART) have introduced the practice of applying methods for comparative genomic hybridization on microarrays (aCGH) and massive parallel sequencing or the so-called next-generation sequencing (NGS). These methods have comparable resolving power and ability to reveal most types of known chromosomal abnormalities [3]. It is believed that the advantage of NGS is a better detection of mosaic variants, while some aCGH platforms demonstrate better resolving power in the detection of segmental aneuploidies [4, 5].
After the early versions of preimplantation diagnosis were applied (using the FISH method on polar bodies and blastomeres of 3-day-old embryos), an increased dependence of the incidence of chromosomal abnormalities in embryos on the mother’s age was revealed [6]. The proportion of aneuploid embryos begins to increase after 30 years and the chance of obtaining a euploid embryo from the own eggs cells of patients aged 43 years and older does not exceed 10% [7, 8]. According to the PGDIS recommendations, the age of a woman over 35 years is the leading reason for administering PGT-A.
The aim of this study was to analyze the spectrum of chromosomalabnormalitiesdetectedinthetrophectoderm of 5-day-oldembryosusingaCGHand NGSmethodsatthe department of D.O. Ott Research Institute of Obstetrics, Gynecology and Reproduction and International Center for Reproductive Medicine (St. Petersburg, Russia), to compare these methods and to evaluate the influence of different embryological protocols on the incidence of chromosomal abnormalities in embryos.
Materials and Methods
The materials for the study were the samples of trophectoderm biopsies of 5-day-old embryos obtained during IVF cycles, which were performed at the department of D.O. Ott Research Institute of Obstetrics, Gynecology and Reproduction (Ott RIOGR) and International Center for Reproductive Medicine (ICRM), St. Petersburg, Russia. A total of 1041 embryos obtained from 420 patients were included in the study. More detailed information about the distribution of patients by age is presented in Table 1. The egg donors were women aged 22–28 years. The exclusion criterion was the revealed carrier state of structural rearrangements and marker chromosomes in one of the parents. The study was approved by the Local Ethics Committee of D.O. Ott Research Institute of Obstetrics, Gynecology and Reproduction. All patients signed an informed consent to the participation in the study and processing of personal data, including medical history.
Embryo culture and biopsy, ICRM
The embryos were fertilized by IVF or ICSI depending on the quality of the ejaculate and cultivated according to the above-mentioned protocol [9]. A visual qualitative assessment of pronucleui and polar bodies was performed in 16–18 hours after fertilization, after which the embryos were cultivated in COOK media for 5–6 days till the formation of blastocysts. Morphological assessment of blastocysts was conducted on the basis of blastocyst scoring system developed by Gardner [10, 11]. Trophectoderm biopsy was performed using laser in blastocysts of excellent and good quality, subsequent cryopreservation was done by vitrification in Kitazato media according to the manufacturer’s protocol (Kitazato Supply Co., Fujinomiya, Japan).
Embryo culture and biopsy, Ott RIOGR
The procedure of controlled ovarian stimulation was performed according to generally accepted methods. Cumulus-oocyte complexes were obtained by transvaginal puncture of follicles 35–36 hours after injecting the trigger. Fertilization was performed by IVF or ICSI depending on the quality of the ejaculate. The effectiveness of fertilization was evaluated after 17–18 hours and diploid zygotes were cultivated for 5-6 days till the stage of blastocyst. Cultivation was performed at 37°C in a CO2 incubator using commercial culture media (COOK, USA and Vitrolife, Sweden). Trophectoderm biopsy was performed using laser in embryos with quality not lower than 3BB [10, 11]. The fragment of trophectoderm containing 4-8 cells was placed in the buffer.
Whole genome amplification was performed using WGA-PCR PicoPlex SingleCell WGA Kit (Rubicon Genomics) and SmarTer PicoPLEX WGA kit (Takara Boi, USA) according to the manufacturer’s instructions. The amount of WGA product was assessed using the Qubit 4 fluorimeter (Invitrogen, Life technologies, USA) with the help of Qubit ds DNA BR Assay Kits (Invitrogen, Life technologies, USA) according to the manufacturer’s instructions and/or using 1.5% agarose gel electrophoresis. Whole genome amplification was done at Ott RIOGR and ICRM.
PGT-A was performed on two platforms. Oligonucleotide chips G5963A 8x60K produced by Agilent (USA) were used for aCGH; DNA samples were labeled using SureTag Complete DNA Labeling kit (Agilent (USA)). Hybridization, washing and scanning were performed in accordance with the manufacturer's recommendations. Agilent CytoGenomics program was used for the analysis of the results. According to the manufacturer's instructions, the resolving power of the platform starts with 5 million pairs of nucleotides for segmental aneuploidies and chromosomal mosaicism can be detected with the rate of more than 30%.
Preparation of libraries for sequencing was performed using VeriSeq PGS Library Prep Kit (Illumina, USA) with the subsequent assessment of the quality on the TapeStation 4200 (Agilent, USA). Sequencing was performed on Illumina MiSeq (Illumina, USA).
Analysis of numerical chromosomal abnormalities was performed using BluFuse Multi v4.3 software (Illumina, USA). The effective resolution of the method was 20 million pairs of nucleotides. Method aCGH was applied in the study at D.O. Ott Research Institute of Obstetrics, Gynecology and Reproduction; method NGS was used in City Hospital №40 and Cerbalab LTD.
Statistical analysis
Statistical processing of the obtained results was performed using software program GraphPadPrism v.6.0. In order to compare the incidence of chromosomal abnormalities in the groups, the analysis of 2x2 contingency tables was conducted using Fischer’s exact test. The results were considered statistically significant at a significance level of р<0.05.
Results and Discussion
At the first stage of the study the spectrum of chromosomal abnormalities detected in 5-day-old embryos was analyzed with aCGH.
Сhromosomal abnormalities were detected in 390 of 707 (55.2%) embryos, the proportion of embryos with a haploid set of chromosomes was 44.8%. The rest of the analyzed samples revealed a wide range of chromosomal abnormalities: aneuploidy involving one and several chromosomes (143 and 78 embryos, 20% and 12% of all embryos, respectively), segmental aneuploidies (36 embryos, 5%), numerical and structural abnormalities of sex chromosomes (13 embryos, 2%), mosaic variants (for whole chromosomes and segmental abnormalities, 44 embryos in total, 6%), triploid and chaotic embryos (16 embryos, 2%), and samples in which a combination of several types of chromosomal abnormalities was registered (for example, complete and segmental aneuploidy, aneuploidy and mosaicism, etc., totally 48 embryos, 7%).
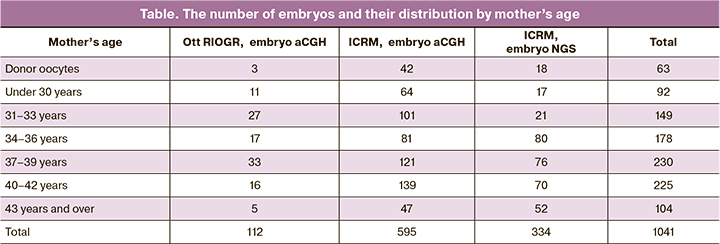
We revealed a dramatic increase in the number of aneuploid embryos depending on increasing maternal age (Figure 1A). The incidence of simple aneuploidies (tri and monosomies involving one chromosome) began to increase after 30 years, it reached a maximum in the group of 40–42 years, after which it began to decrease. Complex aneuploidies (tri and monosomies involving two or more chromosomes) were almost not found in patients up to 34–36 years (9 embryos out of 346, 2.6%), but starting from 37–38 years, their number began to increase progressively, reaching a maximum in patients 43 years and older. The proportion of other types of chromosomal abnormalities does not change with increasing maternal age.
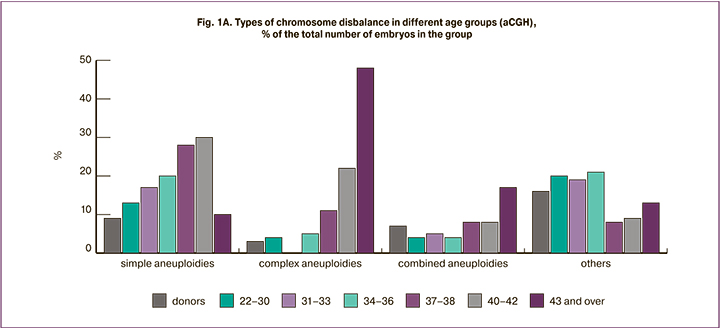
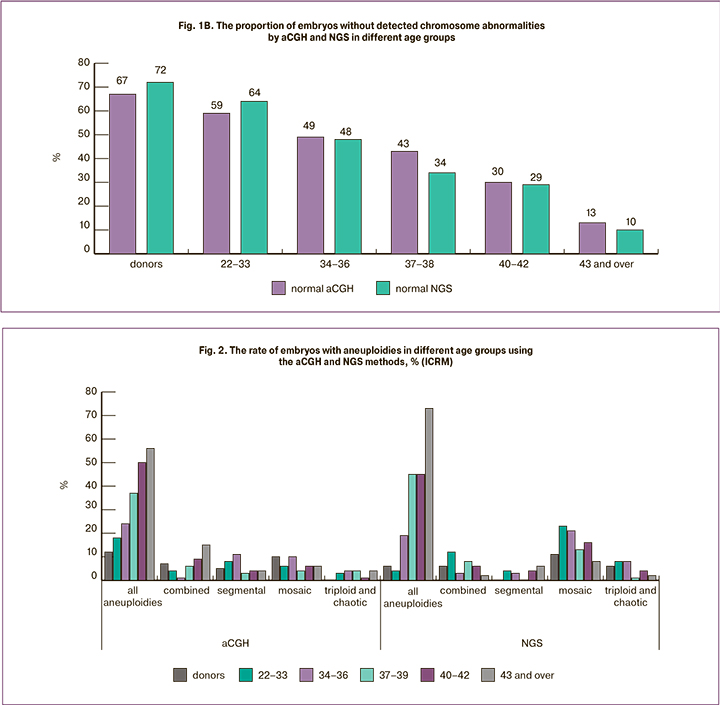
At the next stage, we compared the incidence and spectrum of chromosomal abnormalities detected by different methods. The analysis of 334 samples was carried out with NGS at the ICRM clinic and 206 embryos with chromosomal abnormalities were identified (62%). The results were compared with the data obtained using aCGH for 595 embryos from the same center. Since the incidence of chromosomal abnormalities increased with increasing maternal age, we analyzed the rate of embryos with detected chromosomal abnormalities separately in each age group. There were no significant differences in the rate of detection of chromosomal abnormalities when using different PGT-A platforms (Figure 1B) (the minimum value of p=0.2348 was noted in the group of patients aged 37-39 years, in other age groups it was p>0.7). The results of chromosomal abnormalities classified by different types are shown in Figure 2. Regardless of the method used in the study, the number of embryos with aneuploidies increased with increasing age of women (overall data on simple and complex aneuploidies are presented). The incidence of detection of aneuploidies, combined anomalies, segmental aneuploidies, triploid and chaotic embryos was the same when using both aCGH and NGS methods. However, mosaic abnormalities were more common in the NGS group (p<0.001). In a sample of 334 embryos, different variants of mosaicism were detected in 52 cases; mosaic embryos accounted for 15.6% of the total sample, compared to 4.2% in the aCGH group. It is worth noting that 29 of the 52 mosaic embryos detected by NGS (8.6% of all embryos analyzed using this method) had an abnormal clone proportion that did not exceed 30%. This level of mosaicism cannot be detected by aCGH or it is detected at the resolution limits of the method. However, according to the recommendations of PGDIS 2019 [12], embryo with a low mosaicism level (up to 40%) can be recommended for transfer and such embryos often have a good potential for implantation, pregnancy development, and the birth of a healthy child [13, 14]. According to our data, the frequency of mosaicism does not depend on maternal age.
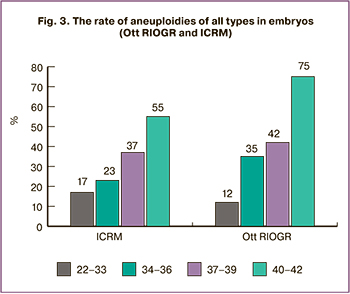 At the final stage of our study, we compared the incidence of chromosomal abnormalities in embryos obtained at different ART centers. Different scientists actively discuss the reasons for the high incidence of embryos with chromosomal abnormalities in humans, and, according to a number of opinions, ART protocols, conditions for embryo cultivation and biopsy methods can play an important role in the non-disjunction of chromosomes [15–17]. We compared the results of PGT-A with aCGH for the embryos obtained at the Department of Ott RIOGR (totally 112 embryos) and ICRM (totally 545 embryos) and received the following findings. A balanced set of chromosomes was found in 47 (42%) embryos at Ott RIOGR and in 270 (45%) embryos at ICRM. The rate of aneuploidies in both groups increased with the maternal age (Figure 3), there were no statistically significant differences in the rate of aneuploid embryos (the lowest value of p=0.0669 was in the group of patients aged 40 years and older, other groups showed p≥0.3616). Other types of chromosomal abnormalities which were not correlated with the age were revealed with the same rate in both groups of embryos. Thus, mosaicism was detected in 38 (6.9%) embryos at ICRM and in 6 (5.4%) embryos at Ott RIOGR (p=0.8323), segmental aneuploidies – in 34 (6.2%) embryos and 11(9%) embryos (p=0.1359), combined anomalies – in 34 (6.2%) embryos and 8 (7.2%) embryos (p=0.5047) and triploid/ chaotic variants – in 15 (2.7%) embryos and 2 (1.8%) (p=0.6412) embryos, respectively.
At the final stage of our study, we compared the incidence of chromosomal abnormalities in embryos obtained at different ART centers. Different scientists actively discuss the reasons for the high incidence of embryos with chromosomal abnormalities in humans, and, according to a number of opinions, ART protocols, conditions for embryo cultivation and biopsy methods can play an important role in the non-disjunction of chromosomes [15–17]. We compared the results of PGT-A with aCGH for the embryos obtained at the Department of Ott RIOGR (totally 112 embryos) and ICRM (totally 545 embryos) and received the following findings. A balanced set of chromosomes was found in 47 (42%) embryos at Ott RIOGR and in 270 (45%) embryos at ICRM. The rate of aneuploidies in both groups increased with the maternal age (Figure 3), there were no statistically significant differences in the rate of aneuploid embryos (the lowest value of p=0.0669 was in the group of patients aged 40 years and older, other groups showed p≥0.3616). Other types of chromosomal abnormalities which were not correlated with the age were revealed with the same rate in both groups of embryos. Thus, mosaicism was detected in 38 (6.9%) embryos at ICRM and in 6 (5.4%) embryos at Ott RIOGR (p=0.8323), segmental aneuploidies – in 34 (6.2%) embryos and 11(9%) embryos (p=0.1359), combined anomalies – in 34 (6.2%) embryos and 8 (7.2%) embryos (p=0.5047) and triploid/ chaotic variants – in 15 (2.7%) embryos and 2 (1.8%) (p=0.6412) embryos, respectively.
Conclusion
We have confirmed that the proportion of embryo abnormalities increases with maternal age. It should be noted that up to 70% of embryos produced by young female donors have a normal chromosomal structure, 48% of embryos are suitable for transfer in ART patients of 34–36 years old, in patients of 40–42 years there are 30% of embryos, and after 43 years no more than 10–13% of embryos are suitable. The decrease in genetic quality is primarily due to the increase in the number of simple aneuploidies, which begins in patients over 30 years and reaches its maximum at the age of 40–42 years, as well as due to aneuploidies involving two or more chromosomes. This type of chromosomal abnormality is almost never detected in the embryos of patients under 36 years of age; after a woman reaches 37 years of age, the proportion of embryos with complex aneuploidies increases progressively. In patients aged 43 years and older, this type of chromosomal abnormality was detected in 48% of embryos.
Both methods aCGH and NGS demonstrate a similar effectiveness for performing PGT-A. The NGS method detects a larger number of mosaic embryos, but this advantage is achieved primarily due to better detection of low percentage mosaicism (20–30%); according to the recommendations of PGDIS 2019, the transfer of such embryos can be performed in most cases.
Finally, we have not detected any statistically significant differences in the incidence of chromosomal abnormalities between the two centers since stimulation schemes and embryo culture conditions there meet the appropriate standards.
References
- Chen M., Wei S., Hu J., Quan S. Can comprehensive chromosome screening technology improve IVF/ICSI Outcomes? A meta-analysis. PLoS One. 2015; 10(10): e0140779. https://dx.doi.org/10.1371/journal.pone.0140779.
- Lee C.I., Wu C.H., Pai Y.P., Chang Y.J., Chen C.I., Lee T.H., Lee M.S. Performance of preimplantation genetic testing for aneuploidy in IVF cycles for patients with advanced maternal age, repeat implantation failure, and idiopathic recurrent miscarriage. Taiwan. J. Obstet. Gynecol. 2019; 58(2): 239-43. https://dx.doi.org/10.1016/j.tjog.2019.01.013.
- Александрова Н.В., Шубина Е.С., Екимов А.Н., Кодылева Т.А., Мукосей И.С., Макарова Н.П., Кулакова Е.В., Левков Л.А., Барков И.Ю., Трофимов Д.Ю.,Сухих Г.Т. Сравнение результатов преимплантационного генетического скрининга, проведенного методами CGH и NGS. Молекулярная биология. 2017; 51(2): 308-13. [Aleksandrova N.V., Shubina E.S., Ekimov A.N., Kodyleva T.A., Mukosey I.S., Makarova N.P., Kulakova E.V., Levkov L.A., Barkov I.Y., Trofimov D.Y., Sukhikh G.T. Comparative results of preimplantation genetic screening by array comparative genomic hybridization and new-generation sequencing.Molecular Biology/Molekulyarnaya biologiya. 2017; 51(2): 269-73. (in Russian).] https://dx.doi.org/10.7868/S0026898417010025.
- Fiorentino F., Biricik A., Bono S., Spizzichino L., Cotroneo E., Cottone G. et al. Development and validation of a next-generation sequencing-based protocol for 24-chromosome aneuploidy screening of embryos. Fertil. Steril. 2014; 101(5): 1375-82. https://dx.doi.org/10.1016/j.fertnstert.2014.01.051.
- Munné S., Wells D. Detection of mosaicism at blastocyst stage with the use of high-resolution next-generation sequencing. Fertil. Steril. 2017; 107(5): 1085-91. https://dx.doi.org/10.1016/j.fertnstert.2017.03.024.
- Kuliev A., Cieslak J., Verlinsky Y. Frequency and distribution of chromosome abnormalities in human oocytes. Cytogenet. Genome Res. 2005; 111(3-4): 193-8.
- Ubaldi F.M., Cimadomo D., Capalbo A., Vaiarelli A., Buffo L., Trabucco E. et al. Preimplantation genetic diagnosis for aneuploidy testing in women older than 44 years: a multicenter experience. Fertil. Steril. 2017; 107(5): 1173-80. https://dx.doi.org/10.1016/j.fertnstert.2017.03.007.
- Ubaldi F.M., Cimadomo D., Vaiarelli A., Fabozzi G., Venturella R., Maggiulli R. et al. Advanced maternal age in IVF: still a challenge? The present and the future of its treatment. Front. Endocrinol. (Lausanne). 2019; 10: 94. https://dx.doi.org/10.3389/fendo.2019.00094.
- Корсак В.С., ред. Руководство по клинической эмбриологии. 2-е изд. М.: СИМК; 2019. 224 с. [Korsak V.S., ed. Rukovodstvo po klinicheskoi embriologii. 2nd ed. Moscow: SIMK; 2019. 224 p. (in Russian).]
- Gardner D.K., Schoolcraft W.B. In vitro culture of human blastocysts. In: Jansen R., Mortimer D., eds. Toward reproductive certainty: fertility and genetics beyond. London: Parthenon Publ.; 1999: 378-88.
- Gardner D.K., Schoolcraft W.B. Culture and transfer of human blastocysts. Curr. Opin. Obstet. Gynecol. 1999; 11(3): 307-11. https://dx.doi.org/10.1097/00001703-199906000-00013.
- Cram D.S., Leigh D., Handyside A., Rechitsky L., Xu K., Harton G. et al. PGDIS position statement on the transfer of mosaic embryos. Reprod. Biomed. Online. 2019; 39(Suppl. 1): e1-4. https://dx.doi.org/10.1016/j.rbmo.2019.06.012.
- Zhang L., Wei D., Zhu Y., Gao Y., Yan J., Chen Z.J. Rates of live birth after mosaic embryo transfer compared with euploid embryos. J. Assist. Reprod. Genet. 2019; 36(1): 165-72. https://dx.doi.org/10.1007/s10815-018-1322-2.
- Victor A.R., Tyndall J.C., Brake A.J., Lepkowsky L.T., Murphy A.E., Griffin D.K. et al. One hundred mosaic embryo transferred prospectively in a single clinic: exploring when and why they result in pregnancies. Fertil. Steril. 2019; 111(2): 280-93.
- Palmerola K.L., Vitez S.F., Amrane S., Fischer C.P., Forman E.J. Minimizing mosaicism: assessing the impact of fertilization method on rate of mosaicism after next-generation sequencing (NGS) preimplantation genetic testing for aneuploidy (PGT-A). J. Assist. Reprod. Genet. 2019; 36(1): 153-7. https://dx.doi.org/10.1007/s10815-018-1347-6.
- Swain J.E. Controversies in ART: can the IVF laboratory influence preimplantation embryo aneuploidy? Reprod. Biomed. Online. 2019; 39(4): 599-607. https://dx.doi.org/10.1016/j.rbmo.2019.06.009.
- Omidi M., Khalili M.A., Halvaei I., Montazeri F., Kalantar S.M. Quality of blastocysts created by embryo splitting: a time-lapse monitoring and chromosomal aneuploidy study. Cell J. 2020; 22(3): 367-74. https://dx.doi.org/10.22074/cellj.2020.6717.
Received 29.01.2020
Accepted 07.02.2020
About the Authors
Olga V. Malysheva, senior researcher, laboratory of genomics, FSBSI «The Research Institute of Obstetrics, Gynecology, and Reproductology named after D.O. Ott»,199034, Mendeleyevskaya liniya, 3, St.Petersburg, Russia, tel. +7(812) 3289809; senior researcher, laboratory of neuroendocrinology, FSBSI Pavlov Institute of Phisioligy,
199034, 3, Makarova enb, St.Petersburg, Russia, tel. +7(812) 7(812) 328-07-01. E-mail: omal99@mail.ru. ORCID 0000-0002-8626-5071.
Natalia K. Bichevaya, Head of the Laboratory of Assisted Reproductive Technologies, International Centre for Reproductive Medicine. Tel.: +7(812)327-19-50.
E-mail: bichevaya@mcrm.ru. ORCID 0000-0001-8866-5821. 53/1 Komendantskij prospect, St. Petersburg, 197350, Russian Federation.
Alexander M. Gzgyan, Head of ART Department, FSBSI «The Research Institute of Obstetrics, Gynecology, and Reproductology named after D.O. Ott». .
Tel.: +7(812) 328-9-822. E-mail: agzgzyan@gmail.com. ORCID 0000-0003-3916-9493. 3 Mendeleyevskaya Line, St. Petersburg, 199034, Russian Federation.
Oleg S. Glotov, Head of the Genetic Laboratory, City Hospital №40, St.-Petersburg, 9 Borisova Street, Sestrorezk, St. Petersburg, 197706, Russia; tel. +9(812)4374075, senior researcher, laboratory of genomics, FSBSI «The Research Institute of Obstetrics, Gynecology, and Reproductology named after D.O. Ott», 199034, Mendeleyevskaya liniya, 3, St.Petersburg, Russia, tel. +7(812) 3289809; e-mail: olglotov@mail.ru. ORCID 0000-0002-0091-2224
Anna A. Kinunen, geneticist physician of St. Petersburg Centre for Medical Genetics, Tobolskaya St, 5, St. Petersburg, 194044, Russia, geneticist physician of the International centre of reproductive medicine, Komendantskij prospect, 53k1, St. Petersburg, 197350, Russia; tel. (812) 327-19-50 kinunen@mcrm.ru. ORCID 0000-0001-6030-125X
Anastasia Yu. Lobenskaya, Head of the Laboratory, Genetic Laboratory CERBALAB LCC/. Tel.: +7(812)602-93-38. E-mail: alobenskaya@cerbalab.ru.
ORCID 0000-0002-6399-2962. 90-2 letter «Z», Bolshoy Ave. V. O., St. Petersburg, 199106, Russian Federation.
Irina D. Mekina, Senior Researcher of ART Department, FSBSI «The Research Institute of Obstetrics, Gynecology, and Reproductology named after D.O. Ott»,
Tel.: +7(812) 328-98-22. E-mail: irendf@mail.ru. ORCID 0000-0002-0813-5845. 3 Mendeleyevskaya Line, St. Petersburg, 199034, Russian Federation.
Irina V. Poliakova, Biologist of the Genetic Laboratory, City Hospital №40. Tel.: +7(812)437-40-75. E-mail: irena.88@inbox.ru. ORCID 0000-0002-5738-8443.
9 Borisova Street, Sestrorezk, St. Petersburg, 197706, Russian Federation.
Irina L. Puppo, Associate Professor, Department of Laboratory Medicine and Genetics, Almazov National Medical Research Centre of the Ministry of Health of the Russian Federation, 2 Akkuratova Street, St. Petersburg, 197341, Russia; Biologist, Laboratory of Assisted Reproductive Technologies, International Centre for Reproductive Medicine, 53/1 Komendantskij prospect, St. Petersburg, 197350, Russia; tel. +7(812)3271950 il_trofimova@list.ru. ORCID 0000-0001-8538-3845
Alsu F. Saifitdinova, Associate Professor, Department of Human and Animal Anatomy and Physiology, Herzen State Pedagogical University of Russia, 48 Moyka Embankment, St. Petersburg, 191186, Russia; Deputy Head of the Laboratory of Assisted Reproductive Technologies, International Centre for Reproductive Medicine, 53/1 Komendantskij prospect, St. Petersburg, 197350, Russia; tel. +7(812)3271950 saifitdinova@mail.ru. ORCID 0000-0002-1221-479X
Sergei G. Scherbak, Chief Physician of the City Hospital №40. Tel.: +7(812) 437-40-75. E-mail: sgsherbak@mail.ru. ORCID 0000-0001-5047-2792.
9 Borisova Street, Sestrorezk, St. Petersburg, 197706, Russian Federation.
Igor.Yu. Kogan, Director of FSBSI «The Research Institute of Obstetrics, Gynecology, and Reproductology named after D.O. Ott». Tel.: +7(812) 328-98-22.
E-mail: ikogan@mail.ru. ORCID 0000-0002-7351-6900. 3 Mendeleyevskaya Line, St. Petersburg, 199034, Russian Federation.
For citation: Маlysheva О.V., Bichevaya N.K., Gzgzyan А.М., Glotov О.S., Kinunen А.А., Lobenskaya А.YU., Меkina I.D., Polyakova I.V., Puppo I.L., Saifitdinova А.F., Shcherbak S.G., Kоgan I.YU. Technological platforms for preimplantation genetic testing for aneuplody: comparative effectiveness of diagnosing chromosomal abnormalities.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 4: 65-71. (In Russian).
https://dx.doi.org/10.18565/aig.2020.4.65-71



