Assessment of the expression level of hsa_pir_020497 piRNA in the follicular fluid of patients with different in vitro fertilization outcomes
Objective: To determine the relationship between morphological and molecular biological criteria for assessing the quality of oocytes retrieved from the women on the day of transvaginal puncture of follicles.Shamina M.A., Timofeeva A.V., Fedorov I.S., Kalinina E.A.
Materials and methods: We used deep sequencing method with its subsequent validation by real-time RT-PCR to identify the spectrum of small non-coding RNAs (sncRNAs) present in the follicular fluid of the left and right ovaries of 25 patients; among them, three patients underwent IVF program again after a negative result of selective embryo transfer into the uterine cavity and preconception care (administration of myo-inositol in combination with folic acid, vitamin D and omega-3).
Results: We identified 86 piRNAs and 79 microRNAs by deep sequencing of sncRNA of the ovarian follicular fluid; expression level of hsa_pir_020497 piRNA was statistically significantly (p=0.01) higher in the group of patients with a positive ART treatment outcome (n=14) compared to the group of patients with a negative ART treatment outcome (n=8) according to real-time RT-PCR data. After preconception care, all three patients showed an increase in the expression level of hsa_piR_020497 in the follicular fluid in one or two ovaries to the expression level of hsa_piR_020497 which is characteristic of pregnant women. Protein products of potential target genes hsa_piR_020497 participate in signaling pathways mediated by the epidermal growth factor receptor (ErbB), mitogen-activated protein kinase (MAPK), transforming growth factor beta (TGFβ), insulin-like growth factor 1 (IGF-1), phosphatidylinositol-3-kinase/Akt pathway and in the regulation of cytosolic calcium and metabolism of vitamins and cofactors.
Conclusion: Since the protein products of the target genes hsa_piR_020497 are involved in the main signaling pathways responsible for follicle formation and egg maturation, it is relevant to conduct further studies on a large sample of couples to determine its impact on IVF outcomes.
Keywords
Infertility is one of the most relevant problems of our time in all developed countries. Although modern reproductive medicine helps most infertile couples to achieve pregnancy every year, the rate of successful in vitro fertilization (IVF) programs still remains at a low level [1].
Infertility can be caused by both male and female factors. The main factor which could influence the possibility of obtaining a good quality embryo capable of implantation and formation of a healthy fetus are competent female and male gametes. It is not technically difficult to determine the quality of the patient’s sperm, however, the assessment of the oocyte quality can be carried out only during transvaginal follicle puncture which is the part of IVF programs. Due to the fact that IVF programs are associated with hormonal drug therapy, potential intraoperative and postoperative complications, the development of ovarian hyperstimulation syndrome, scientists have been recently searching for biomarkers of oocyte quality, as well as ways to improve their quality.
Egg cells develop from primordial germ cells in the antenatal period and their quality depends on coordinated interaction with granulosa cells in the postnatal period through autocrine, paracrine and endocrine factors [2]. Therefore, the molecular composition of the follicular fluid reflects the quality of oogenesis in the antenatal and postnatal periods. This hypothesis was described in the review article of Qasemi M. and Amidi F. on the use of representatives of the class of small non-coding RNAs, microRNAs, present in the follicular fluid as biomarkers of possible disorders of folliculogenesis and oogenesis in polycystic ovary syndrome, premature ovarian failure and endometriosis [3].
Previous studies have demonstrated that myo-inositol combined with folic acid, omega-3 and vitamin D has a certain influence on the metabolomic, lipidomic, proteomic, transcriptomic profiles of follicular fluid; changes in these profiles were detected in women with reduced fertility [4, 5]. Myo-inositol is an important component of the follicular microenvironment and it plays a key role in both nuclear and cytoplasmic development of oocytes. Some studies have shown that the high content of myo-inositol in the follicular fluid of patients during IVF programs correlated with the high quality of oocytes, while decrease in the content of myo-inositol correlated with poor quality of oocytes [6]. In this regard, it can be assumed that the addition of myo-inositol in the process of preparation for IVF programs and for spontaneous pregnancy will be able to improve the quality of the obtained oocytes by reducing the number of degenerated and immature oocytes; the quality of the obtained embryos after fertilization may improve as well.
Previous studies have shown that the use of folates as well as omega-3 is an important aspect for the normal physiological development of the fetus. Folate deficiency impairs DNA methylation and leads to a functional insufficiency of omega-3 polyunsaturated fatty acids (PUFAs) in a pregnant woman, and omega-3 PUFA insufficiency stimulates disorders of folate metabolism [7]. Folate deficiency can also result in the impairment of cell growth and development and as a consequence an increase in the frequency of embryonic malformations [8]. The study published in 2017 showed that the use of myoinositol in preparation for IVF can increase the chance of obtaining mature oocytes, reduce the hormonal load and increase the effectiveness of the IVF program [9]. The research carried out in 2019 demonstrated the effect of prescribing inositol in combination with folic acid as a preconception care on the outcomes of the IVF program in patients with reduced ovarian reserve. It was found that a greater number of mature oocytes (5 (0–7) versus 2 (0–5); p=0.049) and good-quality embryos (42.2% versus 30.1%; p=0.049) were obtained in patients of the group with preconception care than in the group without preliminary treatment; there was also a higher pregnancy rate for embryo transfer into the uterine cavity (38.1% versus 30.0%; p=0.041) [10].
Vitamin D plays an important role in the proper development and functioning of the body. Activation of the vitamin D receptor can directly or indirectly regulate the expression of a very large number of genes [11]. A systematic review published in 2018 showed that the live birth rate in women with insufficient levels of vitamin D after IVF was significantly lower than in women with sufficient levels of vitamin D [RR 0.74, (95% CI: 0.58–0.90)] [12]. Despite the fact that some studies have shown an increase in pregnancy rate and live birth rate in women with the sufficient level of vitamin D, the reliable effect of vitamin D supplementation in patients with confirmed infertility has not been sufficiently studied yet.
In this regard, further studies on the effectiveness of the use of the above drugs before IVF treatment for improving the effectiveness of stimulated cycles continue to be relevant.
Materials and methods
Fifty-two couples were treated for infertility at the B.V. Leonov Department of Assistive Technologies in the Treatment of Infertility, V.I. Kulakov National Medical Research Centre for Obstetrics, Gynecology and Perinatology, Moscow, Russia. Among them, 25 couples were chosen for performing a selective transfer of one embryo into the uterine cavity on the 5th day of cultivation.
The couples who were included in this research underwent a preliminary full clinical and laboratory examination in accordance with the Order of the Russian Ministry of Health No. 107n dated 30.08.2012 “On approval of the use of assisted reproductive technologies, contraindications and limitations to their use”.
Ovarian stimulation of the examined women was performed according to the standard protocol from the 2nd-3rd day of the menstrual cycle using a gonadotropin-releasing hormone (GnRH) antagonist in combination with recombinant follicle-stimulating hormone (rFSH). For the final maturation of oocytes, human chorionic gonadotropin (hCG) was prescribed as an ovulation trigger at a dose of 8,000–10,000 IU/day once intramuscularly. Starting from the day of transvaginal follicle puncture, the luteal phase of the cycle was supported by micronized progesterone preparations 300 mg/day intravaginally. The doses of the administered drugs, as well as the duration of the stimulated cycle, did not differ statistically differ among the examined patients (the data are not presented). Then transvaginal follicle puncture was performed which was followed by a morphological assessment of the maturity of the obtained oocytes. A separate pool of the follicular fluid was collected from the group of follicles of the right and left ovaries, there was no blood in the obtained follicular fluid. On the day of transvaginal follicle puncture, men had semen analysis which was performed to assess the quality of sperm and to isolate the spermatozoa with the highest fertilizing capacity based on their visual morphological characteristics. All mature oocytes were fertilized by intracytoplasmic sperm injection (ICSI) into the oocyte. A visual assessment of the zygote development was performed 16-18 hours after fertilization. The quality of the obtained embryos was evaluated on the 4th-5th day of cultivation on the basis of morphological criteria for assessing the formation of embryos (developed by Gardner).
Molecular biological research methods
RNA was isolated from the obtaned follicular fluid using the miRNeasy Serum/Plasma Kit (Qiagen); it was analyzed with deep sequencing method using NEBNext Multiplex Small RNA Library Prep Set for Illumina (Set11, New England Biolab, Germany) on the NextSeq 500 platform (Illumina, USA); it was analyzed using quantitative real-time RT-PCR with miScript II RT Kit (Qiagen, Hilden, Germany) and miScript SYBR Green PCR Kit (Qiagen, Hilden, Germany) according to the protocols described in detail in previously published works by Timofeeva et al. [13, 14].
The relative level of cDNA expression was assessed by the fold change using the ΔΔCt method. M0s1/M0s2 = 2-CCt, where M0s1 and M0s2 are the initial amounts of cDNA in samples s1 and s2, ∆∆Ct=(Cts1-Ctnorm1)-(Cts2-Ctnorm2), Ct is the value of the amplification cycle at the crossing point of the kinetic curve of accumulation of the amplification product with the line of the threshold level of fluorescence, which is determined automatically by the software of the StepOnePlus amplifier; Cts1 and Cts2 are the values of the threshold cDNA amplification cycle of hsa_piR_020497 in two compared samples s1 and s2; Ctnorm1 and Ctnorm2 are the values of the threshold cDNA amplification cycle of the normalized endogenous hsa_piR_016945 in two compared samples s1 and s2.
Statistical analysis
We used scripts created in the R language and the RStudio program for statistical processing of the results. The analyzed parameters were assessed for normality using the Shapiro–Wilk test. The statistical analysis was carried out using the Mann–Whitney test, since the distribution of variables did not correspond to the law of normal distribution. The analyzed parameters were presented as medians (Me) and quartiles (Q1 and Q3) in the Me (Q1; Q3) format. Thresholds for the p-value statistical significance was 0.05.
Results
Clinical and anamnestic characteristics of couples
The analysis of clinical examination methods and laboratory tests of 22 couples did not identify any statistically significant differences (the data are not presented). The average age of the women included in the study was 31 years (23–35 years). The average age of men is 34 years (25–42 years). Secondary infertility was diagnosed in 14 couples, among them there were 8 couples who did not have pregnancies in their history. A combined infertility factor was revealed in 6 couples included in the study (tuboperitoneal factor in combination with the male factor). Infertility was caused by a male factor alone in 8 couples and by a female, namely tuboperitoneal factor, in 6 couples. Two patients included in the study underwent folliculometry every month during the last year prior to the IVF program; the results of this procedure revealed chronic anovulation. The study included 7 women with extragenital endometriosis; among them there were 2 patients diagnosed with a small ovarian endometrioid cyst that did not require surgical intervention. The average duration of infertility in couples included in the study was 4 years (1–7 years). Previous history of IVF attempts was noted in 8 couples; 2 or more unsuccessful attempts were made in 4 cases. This IVF attempt was the first one in 14 patients. The hormonal status of the patients was assessed on the 3rd day of the menstrual cycle in the cycle preceding stimulation. The status was within the range of normal values for this age group and the phase of the menstrual cycle. The average values of hormones in the blood serum were as follows: FSH 5.5 IU/L; LH 6.3 IU/L; AMH 2.8 ng/ml. The remaining indicators of hormonal status were also within the range of normal values. The IVF program and embryo transfer into the uterine cavity resulted in pregnancy in 14 patients; however, the outcome of the IVF program was negative in 11 patients. Among them, there were 3 couples with a negative outcome of the IVF program who did not have any cryopreserved embryos after the program; therefore, they were administered preconception care with a subsequent repeated IVF program. The clinical and anamnestic characteristics of these couples is presented in Table 1.
Effectiveness of the ART program before and after preconception care
The assessment of the morphological quality of the oocytes obtained from the patient of couple I revealed cytoplasmic anomalies (granularity of the cytoplasm, the presence of vacuoles in the cytoplasm), which were suggestive of the low quality of the oocytes; dark cytoplasm was also noted in the oocytes which is associated with the low quality of embryos according to some authors [15]. Diffuse peripheral granulation is connected with uneven formation of pronuclei. The presence of several vacuoles or one vacuole larger than 14 microns in diameter is a negative prognostic sign, since the fertilization rate of such oocytes is significantly reduced [16]. Moreover, patients with vacuoles in the oocyte had an increased rate of biochemical pregnancy which was followed by a decrease in the rate of clinical pregnancy. Due to the lack of an embryo of satisfactory quality suitable for transfer into the uterine cavity, the embryo transfer stage was canceled.
One embryo was transferred into the uterus on the 5th day of its development to patients from couples II and III under ultrasound guidance. Pregnancy test of one patient from couple III was negative on the 14th day after embryo transfer into the uterine cavity. A patient from couple II was diagnosed with missed abortion at 8 weeks’ gestation during ultrasound examination; therefore, vacuum aspiration of the gestational sac was performed. The cytogenetic study showed 46,XX karyotype of abortus.
Given the unsatisfactory outcomes of the IVF program in these patients, they were prescribed treatment to improve the quality of the obtained oocytes, the quality of the embryos obtained after fertilization, and, accordingly, the outcomes of the IVF program. The patients were prescribed the following medical preparations: 25 mcg (1000 IU) Ultra D vitamin D3 twice a day, 600 mg omega-3 once a day, 1000 mg myo-inositol, 0.1 mg folic acid twice a day.
One patient from couple I presented to the department for the repeated IVF program after 3 months of taking medications, one patient from couple II presented after 7 months and one patient from couple III received IVF treatment after 4 months of therapy. Ovulation stimulation of the above-mentioned women was carried out according to the protocol similar to the previous stimulated cycle. In 35–36 hours after the introduction of the ovulation trigger, we performed transvaginal follicle puncture with a subsequent embryological stage which included: assessment of the degree of oocyte maturity, as well as their morphological characteristics; assessment of spermogram indicators on the day of transvaginal follicle puncture with spermatozoa selection; fertilization of oocytes and further cultivation of the obtained embryos.
The selective transfer of one embryo into the uterine cavity was performed in couples I and II on the 5th day after transvaginal follicle puncture under ultrasound guidance. Couple III had a transfer of two embryos into the uterine cavity on the 4th day after performing transvaginal follicle puncture due to infertility history and desire of the couple. Pregnancy test of a patient from couple II was negative on the 14th day after embryo transfer into the uterine cavity. A patient from couple III was diagnosed with missed abortion at 10 weeks’ gestation during ultrasound examination; therefore, the gestational sac was instrumentally removed and there was curettage of the uterine cavity. The cytogenetic study showed 46,XX karyotype of abortus. A patient from couple I had a full-term spontaneous delivery and a birth of a healthy fetus after this IVF cycle.
The detailed characteristics of the embryological stage of three patients included in the study are shown in Table 2.
Change in the expression level of hsa_piR_020497 in ovarian follicular fluid in patients before and after preconception care
To identify small non-coding RNAs which could differentiate the samples of follicular fluid of patients with different outcomes of the ART program, deep sequencing of RNA samples of six patients was performed: five patients had a negative outcome of the ART program (patients 1, 4, 5, 6, 7) and one patient had a positive outcome of the ART program (patient 8). Negative outcome of the ART program could be caused by the following: low morphological quality of oocytes in combination with a low MII/OCC ratio and the absence of blastocyst formation by the 5th day after fertilization of the egg (patients 1 and 4) or normal morphology of oocytes in combination with a ratio of MII/OCC equal to 1 and the absence of blastocyst formation (patient 5) or normal morphology of oocytes in combination with a ratio of MII/OCC equal to 0.8 and the prevalence of embryo formation of unsatisfactory quality over blastocysts by the 5th day after fertilization (patient 7) or normal morphological parameters of the eggs and the obtained embryos (patient 6). The ovarian follicular fluid of patient 8 was used as a control one due to the excellent morphological quality of the obtained egg cells, the ratio of MII/OCC equal to 0.9, the formation of blastocysts of satisfactory, good and excellent quality according to the morphological criteria developed by Gardner for assessing the development of embryos when there are no embryos of unsatisfactory quality. The partners of patients 1, 4, 5, 6 and 7 were diagnosed with teratozoospermia, and therefore the ICSI method was chosen for fertilization of egg cells. The partner of patient 8 had normozoospermia. Taking into account the history of unsuccessful IVF attempts, as well as the desire of the couple, fertilization of oocytes was performed using the ICSI method.
We identified 86 piRNAs and 79 microRNAs in all 6 samples using deep sequencing; among them, we selected hsa_piR_020497 piRNA which differentiates all 5 samples of the follicular fluid with a negative outcome of the ART program from the control sample and we selected hsa_piR_016945 piRNA as a reference RNA for subsequent studies due to the stable level of expression in all analyzed samples (Table 3).
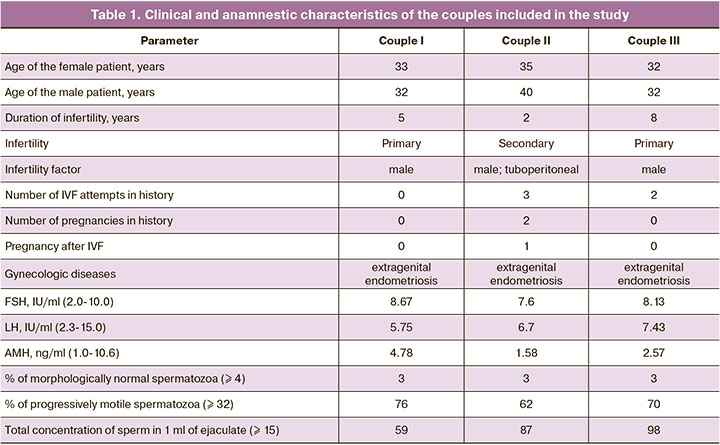
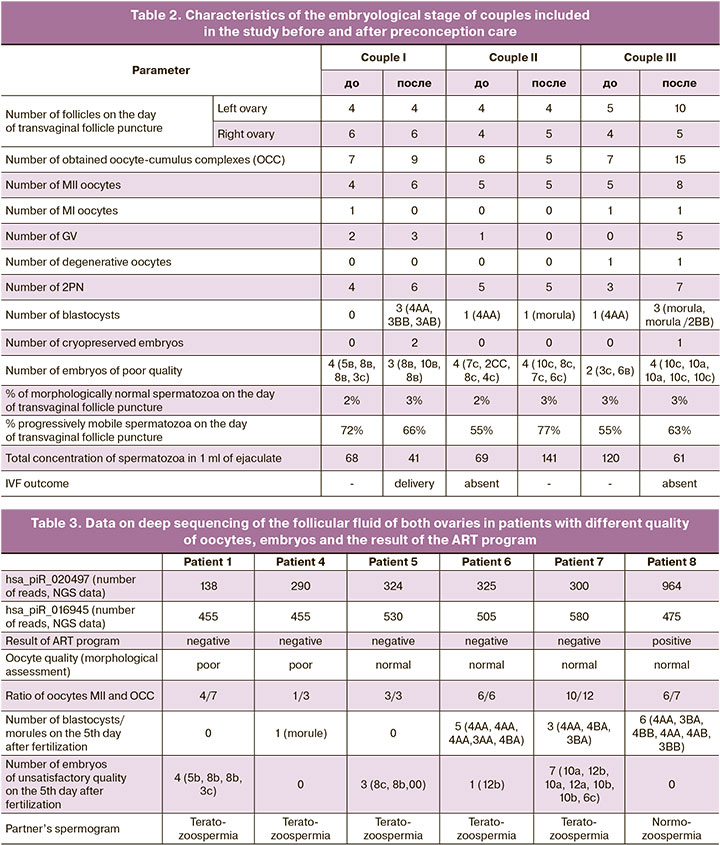
In order to validate the revealed differences in the expression level of hsa_piR_020497, the method of quantitative real-time RT-PCR was used. We analyzed 44 samples of the follicular fluid from the left and right ovaries of 22 patients; among them, 8 patients did not become pregnant and 14 patients had a positive outcome of the ART program. The samples of the follicular fluid of the left and right ovaries of patients from above-mentioned couples I–III before and after preconception care were compared to the given sample for the expression level of hsa_piR_020497. The data of the patient from couple I are presented in Figure 1, the data of the patient from couple II are shown in Figure 2, and findings of the patient from couple III are shown in Figure 3.
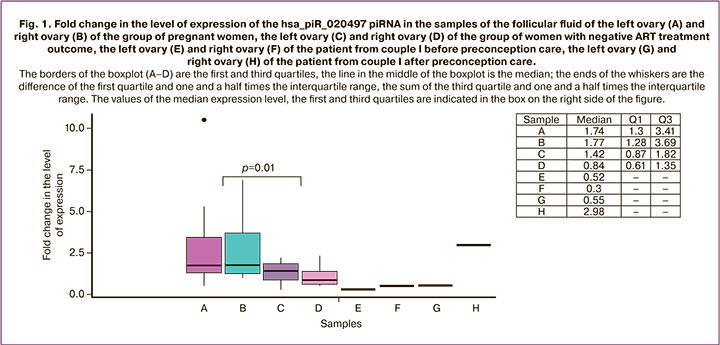
We found a statistically significant difference (p=0.01) between the group of pregnant women and the group of women who could not become pregnant: the expression level of hsa_piR_020497 in the follicular fluid of the left and right ovaries was higher in the group of patients with a positive ART outcome ((1.74 (1.3;3.41) and 1.77 (1.28;3.69), respectively)) compared to that in the follicular fluid of the left and right ovaries in the group of patients with a negative ART outcome ((1.42 (0.87;1.82) and 0.84 (0.61;1.35), respectively)). The statistically significant difference was revealed by the Mann–Whitney U-test.
It is noteworthy that there is an increase in the expression level of hsa_piR_020497 in the follicular fluid of the right ovary of the patient from couple I after the treatment (2.98 (2.98;2.98), Fig. 1 (H)) to the values of the expression level of hsa_piR_020497 typical for the left and right ovaries in the group of pregnant patients (1.74 (1.3;3.41), Fig. 1 (A) and 1.77 (1.28;3.69), Fig. 1 (B), respectively) in comparison with one in the follicular fluid of the left and right ovaries of this patient before the treatment (0.52 (0.52; 0.52), Fig. 1 (E) and 0.3 (0.3; 0.3), Fig. 1 (F)). The absence of changes in the expression level of hsa_piR_020497 in the follicular fluid of the left ovary after the treatment (0.55 (0.55;0.55), Fig. 1 (G)) may be due to the presence of an endometrioid cyst and a possible long-term negative effect on folliculogenesis in this ovary. The quality of the obtained oocytes as well as embryological parameters improved after the treatment (Table 2) and this improvement resulted in pregnancy and full-term delivery.
The patient from couple II had an increase in the expression level of hsa_piR_020497 in the follicular fluid of both ovaries after the treatment (Fig. 2 (K) versus (I), (L) versus (J)); the expression level of hsa_piR_020497 in the right ovary (2.38 (2.38;2.38), Fig. 2 (L)) reached the values typical for ones in the left and right ovaries in the group of pregnant patients (1.74 (1.3;3.41), Fig. 2 (A) and 1.77 (1.28; 3.69), Fig. 2 (B), respectively). However, the patient did not become pregnant even after preconception care possibly due to the pelvic inflammatory process after the history of miscarriage of dichorionic triplet pregnancy, complicated by placental abruption, vaginal bleeding and subsequently by obstruction of the fallopian tubes.
The patient from couple III had a significant increase in the expression level of hsa_piR_020497 in the follicular fluid of both ovaries after the treatment (Fig. 3 (O) versus (M), (P) versus (N)); the expression levels of hsa_piR_020497 in the left ovary (1.97 (1.97;1.97), Fig. 3 (O)) and in the right ovary (4.41 (4.41;4.41), Fig. 3 (P)) reached the values of ones typical for pregnant women (1.74 (1.3;3.41), Fig. 3 (A) and 1.77 (1.28;3.69), Fig. 3 (B), respectively). After the treatment, there was an increase in the number of OCC and the number of embryos suitable for transfer, compared with the ART cycle before treatment (Table 2). After the transfer of two embryos on the 4th day after fertilization, one of the embryos was implanted, but missed abortion was diagnosed at 10 weeks’ gestation.
Functional significance of potential target genes hsa_piR_020497
The analysis of potential target genes hsa_mir_020497 identified using the algorithm described in the article by Timofeeva et al. [14] revealed their participation in the following biological processes: regulation of nucleic acid metabolism (paired-homeodomain transcription factor PAX4; JUNB proto-oncogene; NHLH1, a basic helix-loop-helix transcription factor), cell growth (protein phosphatase SSH1, which regulates dynamic changes of actin filaments), substance transportation (purinergic receptor P2RX1, which forms ATP-regulated ion channels, providing fast and selective permeability for cations), protein metabolism (TRIM10, a member of the E3 ubiquitin ligase complex), metabolic and energetic processes in the cell (nicotinamide adenine dinucleotide (NAD) synthetase-1 (NADSYN1), which is a coenzyme in redox reactions, a precursor for some cellular signaling molecules and a substrate for posttranslational modifications of proteins) (Fig. 4).
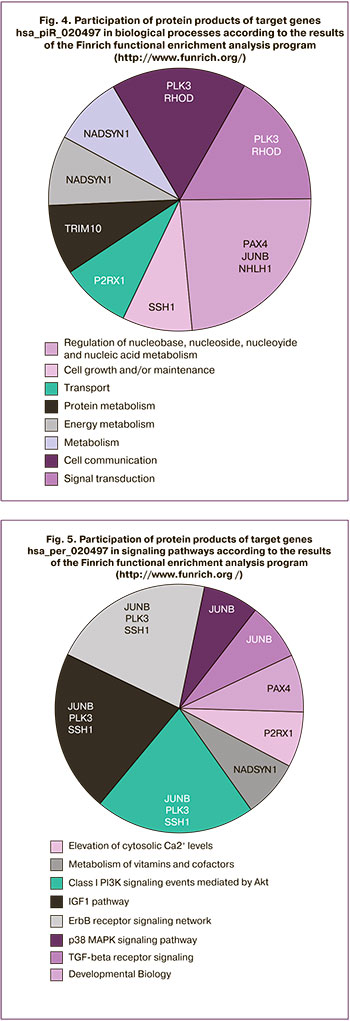
When considering the signaling pathways which involve the protein products of the target genes hsa_piR_020497, one should pay attention to the participation of JUNB, PLK3, SSH1 in the main signaling pathways responsible for follicle formation and egg cell maturation, namely: signaling pathways mediated by the epidermal growth factor receptor (ErbB), mitogen-activated protein kinase (MAPK), transforming growth factor beta (TGFβ), insulin-like growth factor 1 (IGF-1), phosphatidylinositol-3-kinase/Akt pathway[17, 18]. Moreover, the participation of protein products of other target genes hsa_piR_020497, P2RX1 and NADSYN1 in the regulation of cytosolic calcium concentration and metabolism of vitamins and cofactors, respectively, suggests the mutual participation of myo-inositol and hsa_piR_020497 in oogenesis. Myo-inositol is known to be formed from glucose-1-phosphate in a NAD-catalyzed reaction and participates in the phosphoinositide signaling pathway regulating the concentration of intracellular calcium which plays an important role in oocyte maturation, fertilization and embryonic development [19–21].
Discussion
Assisted reproduction programs are mainly aimed at providing infertile couples with personalized and optimal treatment based on their clinical and anamnestic characteristics in order to achieve a positive result and minimize the risk of canceling the ART program. Insufficient quantity, as well as poor quality of retrieved oocytes during follicle puncture and/or low level of fertility after ICSI are considered to be the main reasons for a negative result during ART programs. The use of oral contraceptives or corticosteroids for improving the ovarian response to stimulation often does not lead to the desired result or shows statistically insignificant improvements [22–24].
In this study we assessed the effect of combined preconception care in the form of combined administration of myo-inositol, folic acid, vitamin D and omega-3 to three women with unsuccessful IVF attempts. The effectiveness of the treatment was evaluated according to the morphological criteria of the obtained oocytes and embryos, as well as the expression level of hsa_piR_020497 piRNA in the follicular fluid. The expression level was statistically significantly higher in the group of patients with a positive ART outcome compared to the group of patients who did not become pregnant after ART treatment.
The comparative analysis of stimulated cycles of the patients included in this study before and after the treatment showed that there were no differences in the duration of stimulation, as well as the total dose of gonadotropins. The comparative analysis of the embryological stages in a patient from couple I demonstrated that a greater number of OCC was obtained after the treatment and the percentage of mature MII oocytes was also high. The ratio of the number of zygotes at stage 2PN and the number of mature oocytes, as well as the number and quality of the obtained embryos was higher after the treatment, which allowed the specialists to transfer the best embryo into the uterine cavity. It should be noted that the quality of all the embryos obtained before the treatment was unsatisfactory.
The analysis of the two embryological stages of the patient from couple II before and after the treatment did not show any significant differences. However, the IVF cycle before the treatment resulted in pregnancy, which was terminated due to a missed abortion at 8 weeks’ gestation and was followed by vacuum aspiration of the fetus; during the IVF cycle after the treatment, the embryo implantation into the uterine cavity did not occur.
The patient from couple III received a higher number of OCC after the treatment, the percentage of mature oocytes at stage MII and the number of 2PN zygotes were also higher. The morphological evaluation of the obtained embryos revealed that the number of embryos suitable for transfer into the uterine cavity or for cryopreservation was higher after the treatment. However, after the transfer of two embryos into the uterine cavity, the pregnancy resulted in a missed abortion at 10 weeks’ gestation.
Despite the differences in morphological parameters of oocytes and obtained embryos in three patients after the treatment with myo-inositol in combination with folic acid, omega-3 and vitamin D, all patients showed an increase in the expression level of hsa_piR_020497 in the follicular fluid of one or two ovaries to the values of the expression level of hsa_piR_020497 typical for the group of pregnant patients. Since the protein products of the target genes hsa_mir_020497 are involved in the main signaling pathways responsible for follicle formation and oocyte maturation, one can assume that the therapy is effective. But in order to understand the causes of infertility in couples, it is necessary to conduct further studies on a large sample with the selection of the dosage of these or other drugs, duration of their use and evaluation of their efficacy using the molecular biological profile of the follicular fluid.
Conclusion
This study shows how relevant the problem of poor oocyte quality is in young patients who seek IVF treatment to overcome infertility. The low quality of oocytes is one of the main factors affecting the possibility of obtaining a good quality embryo. Preconception care which was provided to these patients had a positive effect on the characteristics of the embryological stage. In this regard, it is necessary to continue searching for drugs used as a preliminary therapy for women undergoing IVF/ICSI treatment in order to improve reproductive outcomes.
References
- Liang J., Wang S., Wang Z. Role of microRNAs in embryo implantation. Reprod. Biol. Endocrinol. 2017; 15(1): 90. https://dx.doi.org/10.1186/s12958-017-0309-7.
- Senbon S., Hirao Y., Miyano T. Interactions between the oocyte and surrounding somatic cells in follicular development: lessons from in vitro culture. J. Reprod. Dev. 2003; 49(4): 259-69. https://dx.doi.org/10.1262/jrd.49.259.
- Qasemi M., Amidi F. Extracellular microRNA profiling in human follicular fluid: new biomarkers in female reproductive potential. J. Assist. Reprod. Genet. 2020; 37(8): 1769-80. https://dx.doi.org/10.1007/s10815-020-01860-0.
- Luti S., Fiaschi T., Magherini F., Modesti P.A., Piomboni P., Governini L. et al. Relationship between the metabolic and lipid profile in follicular fluid of women undergoing in vitro fertilization. Mol. Reprod. Dev. 2020; 87(9): 986-97. https://dx.doi.org/10.1002/mrd.23415.
- Pla I., Sanchez A., Pors S.E., Pawlowski K., Appelqvist R., Sahlin K.B. et al. Hum. Reprod. 2021; 36(3): 756-70. https://dx.doi.org/10.1093/humrep/deaa335.
- Akbari Sene A., Tabatabaie A., Nikniaz H., Alizadeh A., Sheibani K., Mortezapour A. et al. The myo-inositol effect on the oocyte quality and fertilization rate among women with polycystic ovary syndrome undergoing assisted reproductive technology cycles: a randomized clinical trial. Arch. Gynecol. Obstet. 2019; 299(6): 1701-7. https://dx.doi.org/10.1007/s00404-019-05111-1.
- Громова О.А., Торшин И.Ю., Тетруашвили Н.К., Рейер И.А. Синергизм между фолатами и докозагексаеновой кислотой в рамках раздельного приема микронутриентов во время беременности. Акушерство и гинекология. 2018; 7: 12-9. [Gromova O.A., Torshin I.Yu., Tetruashvili N.K., Reier I.A. Synergy between folates and docosahexaenoic acid when taking the micronutrients separately during pregnancy. Obstetrics and Gynecology. 2018; 7: 12-9. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.7.12-19.
- Ших Е.В., Махова А.А. Вопросы выбора формы фолата для коррекции фолатного статуса. Акушерство и гинекология. 2018; 8: 33-40. [Shikh E.V., Makhova A.A. Problems in the choice of a folate formulation for correction of folate status. Obstetrics and Gynecology. 2018; 8: 33-40. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.8.33-40.
- Владимирова И.В., Донников А.Е., Макарова Н.П., Калинина Е.А. Применение миоинозитола в лечении женского бесплодия в программах вспомогательных репродуктивных технологий у пациенток с высоким риском получения незрелых гамет. Акушерство и гинекология. 2017; 7: 146-9. [Vladimirova I.V., Donnikov A.E., Makarova N.P., Kalinina E.A. The use of myo-inositol in the treatment of female infertility in assisted reproductive technology programs in patients at high risk for immature gametes. Obstetrics and Gynecology. 2017; 7: 146-9. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.7.146-9.
- Вартанян Э.В., Цатурова К.А., Девятова Е.А., Михайлюкова А.С., Левин В.А., Сагамонова К.Ю., Громенко Д.С., Овсянникова Т.В., Эрлихман Н.М., Колосова Е.А., Сафронова Е.В., Фотина О.В., Красновская Е.В., Пожарищенская Т.Г., Аутлева С.Р., Гзгзян А.М., Нуриев И.Р., Воропаева Е.Е., Пестова Т.И., Здановский В.М., Ким Н.А., Котельников А.Н., Сафронов О.В. , Назаренко Т.А., Ионова Р.М. Подготовка к лечению бесплодия методом экстракорпорального оплодотворения при сниженном овариальном резерве. Акушерство и гинекология. 2019; 8: 134-42. [Vartanyan E.V., Tsaturova K.A., Devyatova E.A., Mikhailyukova A.S., Levin V.A., Sagamonova K.Yu., Gromenko D.S. et al. Preparation for the in vitro fertilization treatment of infertility in diminished ovarian reserve. Obstetrics and Gynecology. 2019; 8: 134-42. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.8.134-142.
- Paffoni A., Somigliana E., Sarais V., Ferrari S,. Reschini M., Makieva S. et al. Effect of vitamin D supplementation on assisted reproduction technology (ART) outcomes and underlying biological mechanisms: protocol of a randomized clinical controlled trial. The “supplementation of vitamin D and reproductive outcome” (SUNDRO) study. BMC Pregnancy Childbirth. 2019; 19(1): 395. https://dx.doi.org/10.1186/s12884-019-2538-6.
- Ciepiela P., Dulęba A.J., Kowaleczko E., Chełstowski K., Kurzawa R.Vitamin D as a follicular marker of human oocyte quality and a serum marker of in vitro fertilization outcome. J. Assist. Reprod. Genet. 2018; 35(7): 1265-76. https://dx.doi.org/10.1007/s10815-018-1179-4.
- Timofeeva A.V., Chagovets V.V., Drapkina Yu.S., Makarova N.P., Kalinina E.A., Sukhikh G.T. Cell-free, embryo-specific sncRNA as a molecular biological bridge between patient fertility and IVF efficiency. Int. J. Mol. Sci. 2019; 20(12): 2912. https://dx.doi.org/10.3390/ijms20122912.
- Timofeeva A., Drapkina Yu., Fedorov I., Chagovets V., Makarova N., Shamina M., Kalinina E., Sukhikh G. Small noncoding RNA signatures for determining the developmental potential of an embryo at the morula stage. Int. J.. Mol Sci. 2020; 21(24): 9399. https://dx.doi.org/10.3390/ijms21249399.
- Esfandiari N., Burjaq H., Gotlieb L., Casper R.F. Brown oocytes: implications for assisted reproductive technology. Fertil. Steril. 2006; 86(5): 1522-5. https://dx.doi.org/10.1016/j.fertnstert.2006.03.056.
- Горшкова А.Г., Долгушина Н.В., Макарова Н.П., Ковальская Е.В., Калинина Е.А. Факторы риска развития дисморфизмов ооцитов в программах вспомогательных репродуктивных технологий. Акушерство и гинекология. 2015; 5: 66-73. [Gorshkova A.G., Dolgushina N.V., Makarova N.P., Kovalskaya E.V., Kalinina E.A. Risk factors for oocyte dysmorphisms in assster reproductive technology programs. Obstetrics and Gynecology. 2015; 5: 66-73. (in Russian)].
- Alexandri C., Daniel A., Bruylants G., Demeestere I. The role of microRNAs in ovarian function and the transition toward novel therapeutic strategies in fertility preservation: from bench to future clinical application. Hum. Reprod. Update. 2020; 26(2): 174-96. https://dx.doi.org/10.1093/humupd/dmz039.
- Santonocito M., Vento M., Guglielmino M.R., Battaglia R., Wahlgren J., Ragusa M. et al. Molecular characterization of exosomes and their microRNA cargo in human follicular fluid: bioinformatic analysis reveals that exosomal microRNAs control pathways involved in follicular maturation. Fertil. Steril. 2014; 102(6): 1751-61.e1. https://dx.doi.org/10.1016/j.fertnstert.2014.08.005.
- Di Paolo G., De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006; 443(7112): 651-7. https://dx.doi.org/10.1038/nature05185.
- Vitale S.G., Rossetti P., Corrado F., Rapisarda A.M., La Vignera S., Condorelli R. et al. How to achieve high-quality oocytes? The key role of myo-inositol and melatonin. Int. J. Endocrinol. 2016; 2016: 4987436. https://dx.doi.org/10.1155/2016/4987436.
- Wakai T., Mehregan A., Fissore R.A. Ca2+ signaling and homeostasis in mammalian oocytes and eggs. Cold Spring Harb. Perspect. Biol. 2019; 11(12): a035162. https://dx.doi.org/10.1101/cshperspect.a035162.
- Farquhar C., Rombauts L., Kremer J.A., Lethaby A., Ayeleke R.O. Oral contraceptive pill, progestogen or oestrogen pretreatment for ovarian stimulation protocols for women undergoing assisted reproductive techniques. Cochrane Database Syst. Rev. 2017; (5): CD006109. https://dx.doi.org/10.1002/14651858.CD006109.pub3.
- Kalem M.N., Kalem Z., Gurgan T. Effect of metformin and oral contraceptives on polycystic ovary syndrome and IVF cycles. J. Endocrinol. Invest. 2017; 40(7): 745-52. https://dx.doi.org/10.1007/s40618-017-0634-x.
- Kalampokas T., Pandian Z., Keay S., Bhattacharya S. Glucocorticoid supplementation during ovarian stimulation for IVF or ICSI. Cochrane Ddatabase Syst. Rev. 2017; (3): CD004752. https://dx.doi.org/10.1002/14651858.CD004752.pub2.
Received 23.09.2021
Accepted 19.11.2021
About the Authors
Maria A. Shamina, PhD student at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P,Ministry of Health of Russia, +7(916)096-60-90, mariashamina@mail.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Angelika V. Timofeeva, PhD, Chief of the Laboratory of Applied Transcriptomics at the Department of Systems Biology in Reproduction, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-13-41, avtimofeeva28@gmail.com, 117997, Russia, Moscow, Ac. Oparina str., 4.
Ivan S. Fedorov, Junior Researcher at the Laboratory of Applied Transcriptomics at the Department of Systems Biology in Reproduction V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-13-41, is_fedorov@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Elena A. Kalinina, Dr. Med. Sci., Professor, Head of the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-13-41, e_kalinina@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Authors’ contributions: Kalinina E.A., Timofeeva A.V. – developing the concept and design of the study; Shamina M.A. – collecting material and creating a clinical database of samples; Timofeeva A.V., Fedorov I.S. – obtaining experimental data; Fedorov I.S. – statistical data processing; Timofeeva A.V., Shamina M.A.– writing the text of the article; Kalinina E.A. – editing the text of the article.
Conflicts of interest: The authors declare that they have no competing interests.
Funding: The study was performed without any financial support.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Shamina M.A., Timofeeva A.V., Fedorov I.S., Kalinina E.A.
Assessment of the expression level of hsa_pir_020497 piRNA
in the follicular fluid of patients with different in vitro fertilization outcomes.
Akusherstvo i Ginekologiya / Obstetrics and gynecology. 2021; 11: 134-153 (in Russian)
https://dx.doi.org/10.18565/aig.2021.11.143-153



