Influence of controlled mechanical microvibration on embryo metabolomic profile
Objective. To assess the influence of controlled mechanical microvibration (CMMV) on the metabolomic profile of the 5-day embryo culture media (CM).Romanov A.Yu., Eldarov Ch.M., Frolova A.M., Makarova N.P., Bobrov M.Yu., Dolgushina N.V.
Materials and methods. This was a prospective cohort study of 62 CM samples collected from 44 patients. CM samples were obtained during IVF cycles on the fifth day of embryo development: 20 embryos were cultivated under CMMV conditions (microvibration group), and 42 embryos were cultivated under standard conditions (control group). In the microvibration group, the incubator was placed on an ArisTT180-s platform (K&S Advanced Systems Ltd, Israel) in an active vibration mode at a frequency of 40 Hz for 30 seconds with intervals of 30 minutes. The metabolites were extracted by adding three volumes of methanol and subsequently centrifuged. Chromatographic separation was performed using a reverse-phase chromatographic system on an Atlantis T3 C18 column (Waters, USA). Metabolites were detected on a hybrid quadrupole-time-of-flight mass spectrometer (Bruker Daltoniks, Germany).
Results. The analysis revealed significant differences in the metabolite profiles between the groups. The most significant changes were in regulatory molecules (progesterone, acetylcholine, oleamide, prostaglandin A2 and its glutathione conjugate, 2,3-dinor-thromboxane B2 and 20-hydroxy prostaglandin E2), amino acids and their metabolites (glutamine, hydroxypropyl glutamate, lysyl-gamma-glutamate).
Conclusions. There is a significant influence of controlled mechanical microvibration on the profile of metabolites in the culture media of the 5-day embryos. Further research should be conducted to analyze the impact of these differences on pregnancy rate, its course, and perinatal outcomes.
Keywords
The choice of the optimal parameters for the cultivation of human embryos is particularly important for the increase in the effectiveness of assisted reproductive technologies (ART) [1–4]. During cultivation in vitro, the embryo is constantly exposed to stress that it would not have experienced when developing in the mother’s body. These factors include changes in the pH of the culture medium (CM), temperature fluctuations, exposure to the atmospheric oxygen concentrations, natural and artificial light [5]. In order to improve the systems of human embryo cultivation, the optimal composition of the culture medium is selected [6–8], however, most cultivation systems represent a relatively small (up to 1 ml) and completely static volume of the medium [9–11]. These conditions are completely different from those where the human embryo is located in vivo, namely, constant dynamic interaction with its microenvironment due to peristaltic contractions of the fallopian tube muscle wall and the movement of the villi of its mucous membrane [12–14].
Besides the secretory epithelium, the fallopian tube mucosa is represented by villous epithelial cells; their villi constantly fluctuate with a frequency from 4.9 (0.2) Hz in the proliferative phase to 5.8 (0.3) Hz in the secretory phase of the menstrual cycle [15, 16]. According to Isachenko et al. [17], the embryo in natural environment is constantly influenced by the vibration with a frequency of up to 20 Hz. Ciliary contractions do not only have a direct effect on the embryo, but they also contribute to the diffusion of nutrients [18].
To achieve the improvement of the conditions for the cultivation of human embryos in ART programs, a new approach has been developed which combines the standard cultivation systems with microvibration [14, 17, 19, 20]. However, to date, the effect of controlled mechanical microvibration on the metabolism and development of human embryos has not been sufficiently studied. The aim of the study is to assess the influence of controlled mechanical microvibration on the metabolomic profile of the 5-day embryo culture media.
Materials and Methods
Patients’ characteristics
The prospective study included 44 married couples who did not have any contraindications to ART or any complications when performing ART programs. Before the patients entered the IVF program, they were examined in accordance with the Order of the Ministry of Health of the Russian Federation dated 30.08.2012 No. 107n “On the approval of the use of assisted reproductive technologies, contraindications and limitations to their use” [21]. The partners with the normal karyotype and women aged 18–45 years were eligible for inclusion in the study. There were the following non-inclusion criteria in the study: use of donor oocytes, planned cryopreservation of all received oocytes. Informed consent to participate in the study was obtained from all patients. The study was approved by the Ethics Committee of Biomedical Research at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russia.
Embryo selection and cultivation
Ovarian stimulation was performed according to the standard protocol involving gonadotropin-releasing hormone (GnRH) antagonists. Chorionic gonadotropin or GnRH agonist was used as an ovulation trigger. The embryos were cultured in separate drops of Irvine CSC culture medium (Fujifilm, USA) of equal volume (25 μl) for 5 days in a mixed solution N2/O2/CO2 (89/5/6%, respectively). The embryos were morphologically evaluated according to the Blastocyst Scoring system developed by Gardner (Gardner D.K., 1999) by an embryologist 120–122 hours after fertilization. Under the conditions of the mechanical microvibration during the cultivation, the incubator was placed on an ArisTT180-s platform (K&S Advanced Systems Ltd, Israel) in an active vibration mode at a frequency of 40 Hz for 30 seconds with intervals of 30 minutes [14]. Culturing under the microvibration condition was carried out throughout the entire period from receiving oocytes to the embryo transfer (or cryopreservation of the embryo); after that CM was selected and cryopreserved at -80°C. The built-in oscilloscope was used to estimate the real vibration frequency and amplitude [14].
In order to obtain a metabolomic profile, equal volumes of used CM were selected on the 5th day of embryo culturing and frozen until the analysis was performed at -80°C. A total of 62 CM samples of the human embryo on the fifth day of its development were analyzed: 20 embryos were cultured under the conditions of controlled mechanical microvibration (CMMV), and 42 embryos were cultured under standard conditions. All embryos were classified as high-quality embryos (classes 3 AA, 3AB, 4AA, 4AB, 5AA, according to Gardner’s classification).
Sample preparation and metabolite detection
The metabolites were extracted by adding three volumes of methanol to one volume of CM. The mixture was then vortexed, centrifuged at 14,000 g, and the supernatant was used for analysis. To perform high-performance liquid chromatography (HPLC) with mass spectrometry, 20 μl of extract from each sample was taken into chromatography vials. Sample separation was performed on an Atlantis T3 C18 column with a diameter of 1 mm, a length of 150 mm, and a particle size of 3 microns (Waters, USA) in the Ultimate 3000 Nano LC system (Thermo Scientific, USA) [22].
Elution of the sample components was performed in a gradient of mobile phase B (acetonitrile was added with 0.1% formic acid): 5% were eluded for the first 11 minutes, followed by a gradient of 5–95% of phase B for 10 minutes; then there was an elution for 5 minutes at 95% of phase B, and returning to 95–5% of phase B for 1 minute, followed by column balancing for 3 minutes at a concentration of phase B of 5%. The flow rate was 40 μl/ min, and the total chromatography time of one sample was 30 minutes. Metabolites were detected using a hybrid mass spectrometer Bruker Maxis Impact (Bruker Daltoniks, Germany) with the following parameters: capillary voltage – 4100 V, pressure of the nebulizer – 0.4 bar, drying gas – 4 L/min at a temperature of 180°C, mass – 100–1500 m/z [22].
Mass spectrum processing and statistical analysis
Peak detection and further processing of mass spectra were performed by the XCMS software package with the following parameters: centWave peak identification algorithm with a peak elution time from 10 to 45 seconds and an accuracy of 15 ppm; matched filter algorithm for peak grouping [23].
The MetaboAnalyst platform was used for the statistical analysis of the obtained data (www.metaboanalyst. ca) [24]. The method of orthogonal partial least squares discriminant analysis (OPLS-DA) was used for multivariate statistical analysis [25]. Student’s t-test was used for univariate analysis. The differences at the level of p<0.05 and the signal difference (integrated peak area) of at least two times were considered statistically significant [26, 27]. For the initial identification of the molecular ions selected this way, Human metabolome database (HMDB, www.hmdb.ca) was used; acceptable error of m/z is 15 ppm [28].
Results
As a result of processing the mass spectra in the CM from the human embryo on the fifth day of its development, 1146 different molecular ions were detected. Among them, there were 26 metabolites which significantly differed in the comparison groups with a multiplicity of signal intensity differences of more than two. To assess the clustering of samples and identify potential elution, a multivariate statistical analysis was performed using the OPLS-DA method, which revealed statistically significant differences between the study groups (Fig. 1).
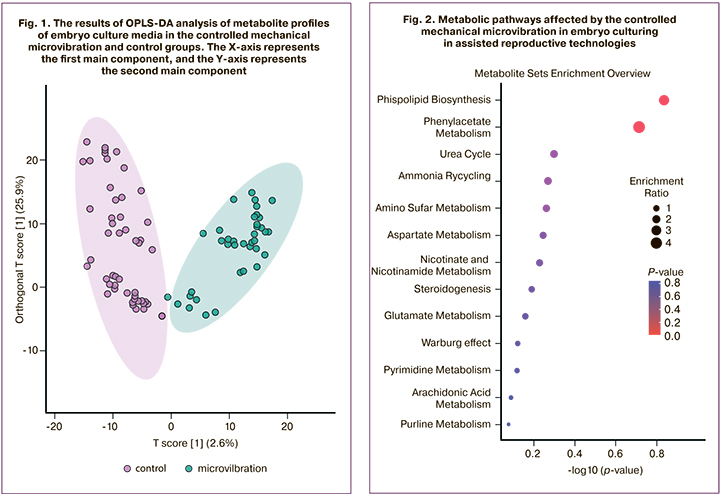
To identify the most significant metabolites that cause differences between the study groups, we selected metabolites that differ statistically significantly between groups with a difference in the total area of the corresponding peaks (proportional to the concentrations of substances) by two or more times between the groups. To identify the most significant metabolites that cause differences between the study groups, we selected metabolites that differ statistically significantly between groups in the total area of the corresponding peaks (proportional to the concentrations of substances) by two or more times between the groups. The initial identification of the molecular ions was performed using a search in the specialized HMDB database. Thus, a number of potential metabolites that change under the conditions of the CMMV were identified (Table).
The most significant changes in the study groups were in regulatory molecules, namely progesterone, glutamine, hydroxyprolyl glutamate, lysyl-gamma-glutamate, acetylcholine, oleamide, prostaglandin A2 and its glutathione conjugate, 2,3-dinor-thromboxane B2 and 20-hydroxy prostaglandin E2.
Progesterone is the most important endogenous steroid that affects the menstrual cycle, pregnancy, and development of human embryos [29]. According to the data obtained, the progesterone content in the CM in the CMMV group was 2.8 times higher than in the control group.
Glutamine is one of the 20 standard amino acids that make up protein. Amino acids play an important role in the preimplantation development of the embryo, serve as energy sources, biosynthetic precursors, buffers of intracellular pH, antioxidants, and regulators of cell differentiation [30–32]. Amino acid consumption is one of the developing methods for evaluating the quality and implantation potential of embryos [33, 34]. The consumption of glutamine in the CM of embryos is known to be associated with the development of embryos in the first three days of the development [35]. In addition, the consumption of aspartate and glutamine is connected with the function of the mitochondria of the developing embryo [36]. According to the data obtained, the content of glutamine in the CM in the CMMV group was 2.3 times lower than in the control group. It should be noted that amino acids spontaneously deaminate and release ammonium at 37°C, with glutamine being the most heat labile. The accumulation of ammonium inhibits the development of the embryo, changes their metabolism and gene expression [37].
Another important amino acid for embryo metabolism is glutamate. Thus, 2–3-day-old embryos that develop further to the blastocyst stage are characterized by lower consumption of glutamine, arginine, and methionine, as well as lower release of alanine and asparagine, compared to embryos that do not form a blastocyst [38]. Reduced levels of glycine and leucine, increased levels of asparagine and glutamate in the CM are associated with an increase in the rate of pregnancy and live birth [9, 39]. The study revealed a positive effect of CMMV on the metabolism of glutamate in a developing embryo: the level of hydroxyprolyl-glutamate was 4.8 times higher, and the level of lysyl-gamma-glutamate was 6.9 times higher than one in the control group.
Acetylcholine is an important excitatory neurotransmitter that can depolarize or hyperpolarize the cell membrane depending on the type of receptor. Addition of choline (a precursor of acetylcholine) in the CM of bovine embryos is known to increase the proportion of embryos developing to the blastocyst stage, the number of blastocyst cells, and the proportion of embryos capable of spontaneous hatching [40]. According to the obtained data, the level of acetylcholine in the CM in the CMMV group was 3.1 times higher than in the control group.
Oleamide is an oleic acid amide and it probably interacts with several neurotransmitter systems. An extremely important property of oleamide is the separation of intercellular gap junctions [41, 42]. The separation of gap junctions occurs normally only at the stage of decompacted morula and is not typical for other stages of embryo development. According to the obtained data, the content of oleamide in the CM in the CMMV group was 2.2 times lower than in the control group.
There were also four metabolites which were different and they were related to eicosanoids or their derivatives. Eicosanoids consist of prostaglandins (PG), thromboxanes (TX), leukotrienes (LT), and lipoxins (LX). All eicosanoids act locally at the site of synthesis via the receptor-mediated signaling pathways.
Prostaglandin A2 or medullin (PGA2) is an endogenous metabolite derived from arachidonic acid. In high concentrations, it shows antiproliferative activity. The findings of the study showed that the content of prostaglandin A2 in the CM in the CMMV group was 3.0 times higher than in the control group, and the content of its metabolite (S-(PGA2)-glutathione) was 3.8 times lower.
2,3-dinor-thromboxane B2 is a metabolite of thromboxane B2. Its urinary excretion has been studied in patients in ART programs and has an important prognostic value in relation to pregnancy [43, 44], but the effect of thromboxane and its metabolites on the developing embryo has not been sufficiently studied yet [45, 46]. According to the obtained results, the content of 2,3-dinor-thromboxane B2 in the CM in the CMMV group was 2.5 times higher than in the control group.
20-hydroxy prostaglandin E2 is a metabolite of prostaglandin E2. The significant role of prostaglandin E2 in resuming oocyte meiosis and cumulus expansion is described in the literature. Its positive effect on glucose consumption by the developing embryo, the quality of the blastocyst, and its antioxidant effect have been studied [47–49]. The data obtained showed that the content of 20-hydroxy prostaglandin E2 in the CM in the CMMV group was 2.5 times higher than in the control group.
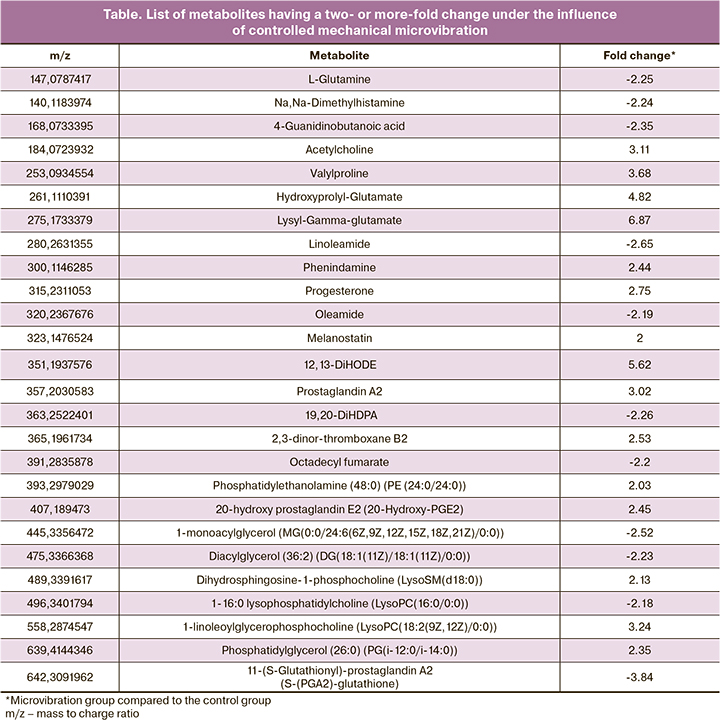
The analysis revealed two of the most significant metabolic pathways: phospholipid biosynthetic pathway (phospholipids are part of all cell membranes); and phenylacetate metabolism pathway (phenylacetate is primarily responsible for the removal of nitrogenous bases during amino acid metabolism). These metabolic pathways are presented in Figure 2.
Embryo culturing under the conditions of controlled mechanical microvibration is a new approach to the improvement of human embryo culture conditions in ART programs, aimed at bringing the cultivation conditions closer to natural ones. However, the effect of CMMV on preimplantation development of the human embryo and long-term outcomes remains understudied. The most favorable outcome of the ART program is the selective transfer of one embryo into the uterine cavity, but morphological evaluation may not be enough to select the embryo with the maximum implantation potential.
Metabolic profiling is a promising tool for further evaluation of the quality and implantation potential of the human embryo in ART programs [33]. In this work, we studied the influence of CMMV on the CM metabolic profile of human embryos and identified 30 different metabolites, namely: lipids and lipid-like molecules (17), organic acids and their derivatives (6), nitrogencontaining organic compounds (2), and benzenoids (1).
The identified molecules were mainly regulatory molecules (progesterone, acetylcholine, oleamide, prostaglandin A2 and its conjugate with glutathione, 2,3-dinor-thromboxane B2 and 20-hydroxyprostaglandin E2), amino acids and their metabolites (glutamine, hydroxyprolyl-glutamate, lysyl-gamma-glutamate). Some of them have a proven effect on the development of embryos in humans and mammals, while the role of others remains poorly understood.
The analysis of metabolic pathways confirms the influence of CMMV on phospholipid biosynthesis and phenylacetate metabolism. Phospholipids are part of all cell membranes, so the activation of their synthesis is extremely important for the constantly fragmenting and developing embryo. The metabolism of phenylacetate is primarily responsible for the excretion of nitrogenous bases during the metabolism of amino acids, which are the main source of energy during embryo cultivation in the ART laboratory
Conclusion
There is a significant influence of controlled mechanical microvibration on the profile of metabolites in the culture media of the 5-day embryos. Further research should be conducted to analyze the impact of the differences on pregnancy rate, its course, and perinatal outcomes.
References
- Shafei R.A., Syrkasheva A.G., Romanov A.Y., Makarova N.P., Dolgushina N. V., Semenova M.L. Blastocyst hatching in humans. Russ. J. Dev. Biol. 2017; 48(1): 5- 15. https://dx.doi.org/10.1134/S1062360417010106.
- Романов А.Ю., Ковальская Е.В., Макарова Н.П., Сыркашева А.Г., Долгушина Н.В. Использование цейтраферной съемки для оценки качества эмбрионов человека в программах экстракорпорального оплодотворения. Цитология. 2017; 59(7): 462-6. [Romanov A.Yu., Kovalskaya E.V.,Makarova N.P., Syrkasheva A.G., Dolgushina N.V. Using time-lapse photography to assess the quality of human embryos in in vitro fertilization programs. Tsitologiya/Cytology. 2017; 59(7): 462-6. (in Russian)]. Available at: https://elibrary.ru/download/elibrary_29773536_76509530.pdf
- Ибрагимова Э.О., Долгушина Н.В., Сыркашева А.Г., Романов А.Ю., Языкова О.И., Макарова Н.П. Роль вспомогательного хетчинга в программах лечения бесплодия методами вспомогательных репродуктивных технологий: обзор литературы. Гинекология. 2016; 18(2): 44-7. [Ibragimova E.O., Dolgushina N.V., Syrkasheva A.G., Romanov A.Yu., Yazykova O.I., Makarova N.P. The role of assisted hatching in infertility treatment programs using assisted reproductive technologies: a literature review. Ginekologiya/Gynecology. 2016; 18(2): 44-7. (in Russian)]. Available at: https://elibrary.ru/item.asp?id=27719470
- Долгушина Н.В., Ибрагимова Э.О., Романов А.Ю., Макарова Н.П., Довгань А.А., Сыркашева А.Г., Калинина Е.А. Роль проназного хетчинга в повышении эффективности программ вспомогательных репродуктивных технологий. Акушерство и гинекология. 2018; 3: 70-5. [Dolgushina N.V., Ibragimova E.O., Romanov A.Yu., Makarova N.P., Dovgan A.A., Syrkasheva A.G. et al. The role of pronase hatching in improving the effectiveness of assisted reproductive technology programs. Obstetrics and gynecology. 2018; 3: 70-5. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.3.70-74.
- Ковальская Е.В., Сыркашева А.Г., Романов А.Ю., Макарова Н.П., Долгушина Н.В. Современные представления о компактизации эмбрионов человека в условиях in vitro. Технологии живых систем. 2017; 1: 25-35. [Kovalskaya E.V., Syrkasheva A.G., Romanov A.Yu., Makarova N.P., Dolgushina N.V. Modern concepts of human embryo compaction in vitro. Technologies of living systems. 2017; 1: 25-35. (in Russian)]. https://elibrary.ru/item.asp?id=29715173
- Biggers J.D., Summers M.C. Choosing a culture medium: making informed choices. Fertil. Steril. 2008; 90(3): 473-83. https://dx.doi.org/10.1016/j.fertnstert.2008.08.010.
- Loutradis D., Drakakis P., Kallianidis K., Sofikitis N., Kallipolitis G., Milingos S. et al. Biological factors in culture media affecting in vitro fertilization, preimplantation embryo development, and implantation. Ann. N. Y. Acad. Sci. 2000; 900: 325-35. https://dx.doi.org/10.1111/j.1749-6632.2000.tb06245.x.
- Chronopoulou E., Harper J.C. IVF culture media: past, present and future. Hum. Reprod. Update. 2015; 21(1): 39-55. https://dx.doi.org/10.1093/humupd/dmu040.
- Brison D.R., Houghton F.D., Falconer D., Roberts S.A., Hawkhead J., Humpherson P.G. et al. Identification of viable embryos in IVF by non-invasive measurement of amino acid turnover. Hum. Reprod. 2004; 19(10): 2319-24. https://dx.doi.org/10.1093/humrep/deh409.
- Thompson J.G. Culture without the petri-dish. Theriogenology. 2007; 67(1): 16-20. https://dx.doi.org/10.1016/j.theriogenology.2006.09.016.
- Gardner D.K., Lane M. Ex vivo early embryo development and effects on gene expression and imprinting. Reprod. Fertil. Dev. 2005; 17(3): 361-70. https://dx.doi.org/10.1071/rd04103.
- Isachenko V., Maettner R., Sterzik K., Strehler E., Kreinberg R., Hancke K. et al. In-vitro culture of human embryos with mechanical micro-vibration increases implantation rates. Reprod. Biomed. Online. 2011; 22(6): 536-44. https://dx.doi.org/10.1016/j.rbmo.2011.02.006.
- Muglia U., Motta P.M. A new morpho-functional classification of the Fallopian tube based on its three-dimensional myoarchitecture. Histol. Histopathol. 2001; 16(1): 227-37. https://dx.doi.org/10.14670/HH-16.227.
- Романов А.Ю., Фролова А.М., Макарова Н.П., Долгушина Н.В. Первый российский опыт применения управляемой механической микровибрации при культивировании эмбрионов человека в программах вспомогательных репродуктивных технологий. Акушерство и гинекология. 2019; 12: 120-5. [Romanov A.Yu., Frolova A.M., Makarova N.P., Dolgushina N.V. The first Russian experience of using controlled mechanical microvibration in the cultivation of human embryos in programs of assisted reproductive technologies. Obstetrics and gynecology. 2019; 12: 120-5. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.12.120-125.
- Lyons R.A., Djahanbakhch O., Mahmood T., Saridogan E., Sattar S., Sheaff M.T. et al. Fallopian tube ciliary beat frequency in relation to the stage of menstrual cycle and anatomical site. Hum. Reprod. 2002; 17(3): 584-8. https://dx.doi.org/10.1093/humrep/17.3.584.
- Lyons R.A., Saridogan E., Djahanbakhch O. The reproductive significance of human Fallopian tube cilia. Hum. Reprod. Update. 2006; 12(4): 363-72. https://dx.doi.org/10.1093/humupd/dml012.
- Isachenko E., Maettner R., Isachenko V., Roth S., Kreienberg R., Sterzik K. Mechanical agitation during the in vitro culture of human pre-implantation embryos drastically increases the pregnancy rate. Clin. Lab. 2010; 56(11-12): 569-76.
- Matsuura K., Hayashi N., Kuroda Y., Takiue C., Hirata R., Takenami M. et al. Improved development of mouse and human embryos using a tilting embryo culture system. Reprod. Biomed. Online. 2010; 20(3): 358-64. https://dx.doi.org/10.1016/j.rbmo.2009.12.002.
- Romanov A.Y., Silachev D.N., Makarova N.P., Dolgushina N.V. Effect of mechanical microvibration on the quality of human embryos during in vitro culturing and outcomes of assisted reproduction technologies. Bull. Exp. Biol. Med. 2018; 165(4): 544-7. https://dx.doi.org/10.1007/s10517-018-4211-x.
- Романов А.Ю., Силачев Д.Н., Макарова Н.П., Долгушина Н.В. Влияние механической микровибрации на качество эмбрионов человека при культивировании in vitro и исходы программ вспомогательных репродуктивных технологий. Клеточные технологии в биологии и медицине. 2018; 2: 86-90. [Romanov A.Yu., Silachev D.N., Makarova N.P., Dolgushina N.V. Effect of Mechanical Microvibration on the Quality of Human Embryos during In Vitro Culturing and Outcomes of Assisted Reproduction Technologies. Cell technologies in biology and medicine. 2018; (2):86-90. (in Russian)]. https://elibrary.ru/item.asp?id=35040131
- Министерство здравоохранения Российской Федерации. Приказ Минздрава России от 30.08.2012 N 107н (ред. от 11.06.2015) «О порядке использования вспомогательных репродуктивных технологий, противопоказаниях и ограничениях к их применению». [Ministry of Health of the Russian Federation. Order of the Ministry of Health of the Russian Federation of 30.08.2012 N 107n (ed.from 11.06.2015) "On the procedure for using assisted reproductive technologies, contraindications and restrictions to their use".(in Russian)].
- Токарева А.О., Чаговец В.В., Чжихао Ван, Родионов В.В., Кометова В.В.,Родионова М.В., Кононихин А.С., Стародубцева Н.Л., Чингин К., Франкевич В.Е., Хуаньвэнь Чэнь, Сухих Г.Т. Прямая масс-спектрометрия как метод экспресс-идентификации опухолевой ткани у больных раком молочной железы. Акушерство и гинекология. 2017; 4: 119-25. [Tokareva A.O., Chagovets V.V., Chzhihao V., Rodionov V.V., Kometova V.V.,Rodionova M.V. et al. Direct mass spectrometry as a method for rapid identification of tumor tissue in patients with breast cancer. Obstetrics and gynecology. 2017; 4:119-25. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.4.119-25.
- Smith C.A., Want E.J., O’Maille G., Abagyan R., Siuzdak G. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006; 78(3): 779-87. https://dx.doi.org/10.1021/ac051437y.
- Chong J., Wishart D.S., Xia J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinforma. 2019; 68(1): e86. https://dx.doi.org/10.1002/cpbi.86.
- Bylesjö M., Rantalainen M., Cloarec O., Nicholson J.K., Holmes E., Trygg J. OPLS discriminant analysis: combining the strengths of PLS-DA and SIMCA classification. J. Chemometr. 2006; 20(8-10): 341-51. https://dx.doi.org/10.1002/cem.1006.
- Некрасова М.Е., Чаговец В.В., Стародубцева Н.Л., Кононихин А.С., Салимова Д.Ф., Токарева А.О., Лагутин В.В., Наумов В.А., Назарова Н.М., Франкевич В.Е., Сухих Г.Т. Липидные маркеры неопластической трансформации эпителия шейки матки при заболеваниях, ассоциированных с вирусом папилломы человека. Акушерство и гинекология. 2018; 4: 64-70. [Nekrasova M.E., Chagovets V.V., Starodubtseva N.L., Kononikhin A.S., Salimova D.F., Tokareva A.O. et al. Lipid markers of neoplastic transformation of the cervical epithelium in diseases associated with the human papillomavirus. Obstetrics and gynecology. 2018; 4: 64-70. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.4.64-70.
- Worley B., Powers R. Multivariate analysis in metabolomics. Curr. Metabolomics. 2012; 1(1): 92-107. https://dx.doi.org/10.2174/2213235X11301010092.
- Wishart D.S., Feunang Y.D., Marcu A., Guo A.C., Liang K., Vázquez-Fresno R. et al. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 2018; 46(D1): D608-17. https://dx.doi.org/10.1093/nar/gkx1089.
- Puscheck E.E., Awonuga A.O., Yang Y., Jiang Z., Rappolee D.A. Molecular biology of the stress response in the early embryo and its stem cells. Adv. Exp. Med. Biol. 2015; 843: 77-128. https://dx.doi.org/10.1007/978-1-4939-2480-6_4.
- Crosby I.M., Gandolfi F., Moor R.M. Control of protein synthesis during early cleavage of sheep embryos. Reproduction. 1988; 82(2): 769-75. https://dx.doi.org/10.1530/jrf.0.0820769.
- Edwards L.J., Williams D.A., Gardner D.K. Intracellular pH of the mouse preimplantation embryo: amino acids act as buffers of intracellular pH. Hum. Reprod. 1998; 13(12): 3441-8. https://dx.doi.org/10.1093/humrep/13.12.3441.
- Martin P.M. Amino acid transport regulates blastocyst implantation. Biol. Reprod. 2003; 69(4): 1101-8. https://dx.doi.org/10.1095/biolreprod.103.018010.
- Зорина И.М., Смольникова В.Ю., Бобров М.Ю. Изучение продуктов метаболизма эмбрионов в культуральных средах как инструмент определения потенциала к имплантации. Акушерство и гинекология. 2017; 2: 11-6. [Zorina I.M., Smolnikova V.Yu., Bobrov M.Yu. Studying the products of embryo metabolism in culture media as a tool for determining the potential for implantation. Obstetrics and gynecology. 2017; 2: 11-6. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.2.11-6.
- Зорина И.М., Смольникова В.Ю., Эльдаров Ч.М., Ярыгина С.А., Горшинова В.К., Макарова Н.П., Калинина Е.А., Бобров М.Ю. Анализ потребления глюкозы и глутамата в питательных средах как метод оценки качества эмбрионов человека пятых суток развития. Акушерство и гинекология. 2018; 5: 64-9. [Zorina I.M., Smolnikova V.Yu., Eldarov Ch.M., Yarygina S.A., Gorshinova V.K., Makarova N.P. et al. Analysis of glucose and glutamate consumption in nutrient media as a method for assessing the quality of human embryos of the fifth day of development. Obstetrics and gynecology. 2018; (5): 64-9. (in Russian)].https://dx.doi.org/10.18565/aig.2018.5.64-69.
- Drábková P., Andrlová L., Hampl R., Kanďár R. Amino acid metabolism in human embryos. Physiol. Res. 2016; 65(5): 823-32. https://dx.doi.org/10.33549/physiolres.933240.
- Picton H.M., Elder K., Houghton F.D., Hawkhead J.A., Rutherford A.J., Hogg J.E. et al. Association between amino acid turnover and chromosome aneuploidy during human preimplantation embryo development in vitro. Mol. Hum. Reprod. 2010; 16(8): 557-69. https://dx.doi.org/10.1093/molehr/gaq040.
- Gardner D.K., Kelley R.L. Impact of the IVF laboratory environment on human preimplantation embryo phenotype. J. Dev. Orig. Health Dis. 2017; 8(4): 418-35. https://dx.doi.org/10.1017/S2040174417000368.
- Houghton1 F.D. Non-invasive amino acid turnover predicts human embryo developmental capacity. Hum. Reprod. 2002; 17(4): 999-1005. https://dx.doi.org/10.1093/humrep/17.4.999.
- Seli E., Botros L., Sakkas D., Burns D.H. Noninvasive metabolomic profiling of embryo culture media using proton nuclear magnetic resonance correlates with reproductive potential of embryos in women undergoing in vitro fertilization. Fertil. Steril. 2008; 90(6): 2183-9. https://dx.doi.org/10.1016/j.fertnstert.2008.07.1739.
- Estrada-Cortés E., Negrón-Peréz V.M., Tríbulo P., Zenobi M.G., Staples C.R., Hansen P.J. Effects of choline on the phenotype of the cultured bovine preimplantation embryo. J. Dairy Sci. 2020; 103(11): 10784-96. https://dx.doi.org/10.3168/jds.2020-18598.
- Togashi K., Kumagai J., Sato E., Shirasawa H., Shimoda Y., Makino K. et al. Dysfunction in gap junction intercellular communication induces aberrant behavior of the inner cell mass and frequent collapses of expanded blastocysts in mouse embryos. J. Assist. Reprod. Genet. 2015; 32(6): 969-76. https://dx.doi.org/10.1007/s10815-015-0479-1.
- Ehrlich H.P., Sun B., Saggers G.C., Kromath F. Gap junction communications influence upon fibroblast synthesis of Type I collagen and fibronectin. J. Cell. Biochem. 2006; 98(4): 735-43. https://dx.doi.org/10.1002/jcb.20852.
- van der Weiden R.M., Helmerhorst F.M., Keirse M.J. Which prostanoid metabolites should be determined for the study of reproductive processes? Prostaglandins Leukot. Essent. Fatty Acids. 1998; 58(3): 205-7. https://dx.doi.org/10.1016/s0952-3278(98)90115-6.
- van der Weiden R.M., Helmerhorst F.M., Keirse M.J. Prostanoid excretion before in vitro fertilization relates to the likelihood of pregnancy. Prostaglandins Leukot. Essent. Fatty Acids. 1995; 53(6): 419-21. https://dx.doi.org/10.1016/0952-3278(95)90106-x.
- van der Weiden R.M., Noort W.A., Naaktgeboren N., Helmerhorst F.M., Keirse M.J. Prostanoid levels in in vitro fertilization culture medium are not related to the likelihood of implantation. Fertil. Steril. 1994; 62(6): 1217-20. https://dx.doi.org/10.1016/s0015-0282(16)57188-x.
- Geissler F.T., Kuzan F.B., Faustman E.M., Henderson W.R. Lipid mediator production by post-implantation rat embryos in vitro. Prostaglandins. 1989; 38(2):145-55. https://dx.doi.org/10.1016/0090-6980(89)90078-6.
- Boruszewska D., Kowalczyk-Zieba I., Suwik K., Staszkiewicz-Chodor J., Jaworska J., Lukaszuk K. et al. Prostaglandin E2 affects in vitro maturation of bovine oocytes. Reprod. Biol. Endocrinol. 2020; 18(1): 40. https://dx.doi.org/10.1186/s12958-020-00598-9.
- Talukder A.K., Yousef M.S., Rashid M.B., Awai K., Acosta T.J., Shimizu T. et al. Bovine embryo induces an anti-inflammatory response in uterine epithelial cells and immune cells in vitro: possible involvement of interferon tau as an intermediator. J. Reprod. Dev. 2017; 63(4): 425-34. https://dx.doi.org/10.1262/jrd.2017-056.
- Rodrigues S.A.D., Pontelo T.P., Kussano N.R., Kawamoto T.S., Leme L.O., Caixeta F.M.C. et al. Effects of prostaglandins E2 and F2α on the in vitro maturation of bovine oocytes. Domest. Anim. Endocrinol. 2020; 72: 106447. https://dx.doi.org/10.1016/j.domaniend.2020.106447.
Received 21.10.2020
Accepted 27.10.2020
About the Authors
Andrey Yu. Romanov, postgraduate student, researcher of R&D Department, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation. Tel.: +7(903)158-94-00. E-mail: romanov1553@yandex.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.Chupalav M. Eldarov, senior researcher of the Laboratory of Molecular Pathophysiology, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation. Tel.: +7(495)438-77-00. E-mail: ch_eldarov@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Alexandra M. Frolova, embryologist of IVF Department, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,
Ministry of Healthcare of Russian Federation. E-mail: i.a.m.frolova@mail.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Nataliya P. Makarova, PhD, Researcher of the IVF Department, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,
Ministry of Healthcare of Russian Federation. E-mail: np_makarova@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Mikhail Yu. Bobrov, MD, Head of the Laboratory of Molecular Pathophysiology, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation. E-mail: mbobr@mail.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Nataliya V. Dolgushina, M.D., Ph.D., M.P.H., Head of R&D Department, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,
Ministry of Healthcare of Russian Federation. E-mail: n_dolgushina@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
For citation: Romanov A.Yu., Eldarov Ch.M., Frolova A.M., Makarova N.P., Bobrov M.Yu., Dolgushina N.V. Influence of controlled mechanical microvibration on embryo metabolomic profile.Akusherstvo i Ginekologiya / Obstetrics and gynecology. 2020; 11: 131-138 (in Russian).
https://dx.doi.org/10.18565/aig.2020.11.131-138



