Для российского здравоохранения рак шейки матки сегодня представляет серьезную проблему и угрозу. Ежегодно в нашей стране выявляется около 15 тыс. новых случаев заболевания [1]. В России рак шейки матки занимает 2-е место по распространенности среди злокачественных новообразований у женщин до 45 лет и 1-е место по количеству потерянных лет жизни (продолжительность жизни заболевших женщин снижается в среднем на 26 лет). Заболеваемость неуклонно растет – за последние 10 лет увеличилась приблизительно на 26% [2].
Демографические потери по причине этого заболевания очень велики. Каждый год умирают около 8,5 тыс., а каждый день – около 17 россиянок [2, 3].
Больше трети (32%) первичных диагнозов рака шейки матки ставится уже на запущенных (III и IV) стадиях [4].
По прогнозу, сделанному на основании изучения динамики рака шейки матки в России в течение почти 30 лет (с 1989 по 2018 гг.), к 2028 г. в нашей стране будет диагностировано 29 новых случаев рака шейки матки на 100 тыс. населения.
В мире ежегодно фиксируется около 604 тыс. случаев цервикального рака и 349 тыс. случаев смертей, вызванных этим онкологическим заболеванием [1].
Данная проблема тем более серьезна, что распространенность вируса папилломы человека (ВПЧ) чрезвычайно высока. Достаточно сказать, что, по данным ВОЗ, именно ВПЧ считается самой частой инфекцией, передаваемой половым путем (ИППП) [5]. Общая инфицированность людей ВПЧ приближается к 700 млн.
Папилломавирусная инфекция (ПВИ) – единственная ИППП, которая постоянно инфицирует все большее число людей, живущих половой жизнью, и, по заключению ВОЗ, является проблемой номер один среди ИППП [6, 7].
Изучение ВПЧ позволило подтвердить его роль в развитии рака шейки матки (100%), рака полового члена у мужчин – в 40% случаев, рака ануса и у женщин, и у мужчин – в 90% случаев [1]. Поражение кожи, ротоглотки, головы и шеи диагностируется с каждым годом все чаще.
Врачам амбулаторно-поликлинического звена приходится решать проблемы, обусловленные ВПЧ-ассоциированными заболеваниями, ежедневно. Вопросы адекватной диагностики, лечения, профилактики рака и рецидивов заболеваний, обусловленных ВПЧ, имеют свои особенности [8, 9]. На этом пути множество подводных течений и сложностей, которые приходится преодолевать и пациентам, и врачам.
Попытаемся разобраться в сложных переплетениях реальных проблем и мифов, которыми переполнены информационные сервисы.
Что из себя представляет ВПЧ?
ВПЧ – это ДНК-вирусы, поражающие стратифицированный плоскоклеточный эпителий человека. Папилломавирусы – небольшие круглые ДНК-вирусы без оболочки, обладающие видовой и тканевой специфичностью, которые заражают млекопитающих, птиц и рептилий. Являются представителями одного из старейших, крупнейших и самых разнообразных из известных вирусных семейств. ВПЧ, как и все папилломавирусы, нацелены на стратифицированный плоскоклеточный эпителий. Некоторые типы ВПЧ также способны инфицировать железистый эпителий шейки матки. Современная статистика позволяет говорить о том, что инфекция чрезвычайно распространена, возможно, универсальна, в основном существует на скрытом уровне, не вызывая заметных изменений в тканях. ВПЧ распределяются по всему телу, но с различными анатомическими пристрастиями, что позволяет выделить три основные группы ВПЧ-ассоциированных заболеваний и вирусов: (1) обнаруживаемые в кожных бородавках, таких как подошвенные, общие и плоские бородавки; (2) ассоциированные с эпидермодисплазией, редким генодерматозом; (3) ассоциированные с поражениями гениталий или слизистых оболочек. Последняя группа типов ВПЧ и есть объект нашего внимания, в частности в этой статье.
Чем опасен ВПЧ? Канцерогенез
Основные принципы молекулярного и клеточного онкогенеза вируса папилломы были изучены к началу 1990-х гг. Это десятилетие ознаменовалось быстрым развитием многих эпидемиологических исследований, которые укрепили и дополнили доказательства того, что некоторые генитальные типы ВПЧ вызывают рак шейки матки. С более ограниченными, но растущими доказательствами к этому списку добавились и другие виды рака, такие как рак вульвы, влагалища, пениса, заднего прохода и ротоглотки.
Активная фаза ВПЧ-инфицирования начинается с заражения «базальной или стволовой» клеточной популяции зоны трансформации шейки матки [10].
В клетках, приверженных плоскоклеточной дифференцировке, существует упорядоченная программа созревания по всей толщине эпителия как на морфологическом, так и на молекулярном уровне.
Канцерогенез шейки матки можно разделить на следующие этапы:
1) первичная инфекция ВПЧ;
2) персистенция генома ВПЧ в эписомальной форме и возможная продукция вирусных частиц;
3) интеграция ДНК ВПЧ в клеточный геном (на II и III стадиях начинают проявляться функции генов Е6, Е7, нарушающих контроль деления клеток);
4) индукция мутаций в клеточной ДНК, вызывающей нестабильность клеточного генома;
5) селекция клона клеток с мутантной ДНК, содержащих интегрированную ДНК ВПЧ;
6) активное размножение данного клона клеток и рост опухоли.
Многие аспекты клеточной иммунологии ВПЧ-инфекций плохо изучены. Данные литературы показывают, что по крайней мере 50% мужчин и женщин подвергаются воздействию ВПЧ в течение 3 лет после начала сексуальной активности и что даже моногамные женщины «низкого риска» обычно заражаются ВПЧ-инфекцией. Самоэлиминация ВПЧ возможна в 90% случаев. Этот механизм не совсем понятен, но он позволяет избежать онкотрансформации клеток в большинстве случаев. Для развития раковых процессов необходима длительная персистенция (2–3 года), причем именно высокоонкогенными типами ВПЧ.
ВПЧ высокого онкогенного риска могут иметь более длительную персистенцию по сравнению с ВПЧ низкого онкогенного риска, хотя очень длительная персистенция ВПЧ низкого онкогенного риска не является редкостью [11, 12].
Как выявить ВПЧ?
ПЦР-диагностика является на сегодня единственной возможностью определить наличие ВПЧ в мазках с кожи и слизистых [13].
При ПЦР-диагностике очень часто возникает вопрос и у пациентов, и у врачей: почему не выявляется ВПЧ при явных изменениях слизистой в виде остроконечных кондилом?
Необходимо понимать, что вирус персистирует локально и поражает клетки, рядом расположенные, а не переносится через кровь по всему организму. Поэтому, если наблюдается поражение слизистой вульвы, не обязательно будет иметь место поражение слизистой шейки матки [11].
Мазки принято брать с шейки матки, и если она не инфицирована, результат будет отрицательным. Если необходимо определить поражение ВПЧ в области роста кондилом, то и мазок необходимо брать из этой области [14]. Однако считается, что отсутствует необходимость определять тип вируса остроконечных кондилом, если нет подозрения на возможное озлокачествление (легкая ранимость, кровоточивость, участки некроза и т.д.) [15]. По данным различных исследователей, типы 6 и 11 ВПЧ в 90% случаев ответственны за развитие кондилом. В последнее время установлено, что причиной появления кондилом могут быть и такие типы ВПЧ, как 30, 42, 43, 44, 45, 51, 54, 55, 70, которые относятся к высокоонкогенным. Низкоонкогенные типы 6 и 11 нередко могут сочетаться с высокоонкогенными типами ВПЧ, поэтому при выявлении кондилом обязательно определение ВПЧ в эпителиальном покрове шейки матки [14].
Полимеразная цепная реакция (ПЦР) позволяет проводить качественно-количественный анализ ВПЧ. Знания о типе вируса позволяют врачу прогнозировать опасность поражения слизистых и необходимость проведения динамического наблюдения за пациенткой, расширенной диагностики и возможного лечения. Очень важными факторами в диагностике ВПЧ являются качество забора материала, правильная его транспортировка, условия хранения и качественное оборудование для ПЦР-диагностики.
В ходе наблюдения можно определять вирусную нагрузку и ее изменения, которые могут свидетельствовать о возможной элиминации вируса, а длительная персистенция может указать на высокий риск развития злокачественных процессов. Но бывают случаи, когда при явном ВПЧ-ассоциированном поражении слизистой вирус не выявляется.
ВПЧ может располагаться в клетке хозяина эписомально. В таких случаях вирус будет определяться при ПЦР-диагностике, но при цитологии и кольпоскопии изменения могут отсутствовать. Самостоятельная элиминация вируса позволяет полностью ликвидировать возможность развития тяжелых поражений эпителия. Большинство исследователей свидетельствуют именно о таком преимущественном течении ВПЧ-инфицирования (до 90%). Персистенция ВПЧ в 10% случаев приводит к развитию заболеваний, причем доминирующими являются кондиломатозные поражения, и только около 10% пациентов могут иметь предраковые и раковые поражения слизистых и кожи. Интегрирование ДНК ВПЧ в ДНК клеток хозяина провоцирует развитие патологических изменений эпителиального покрова [1, 14].
Активная ВПЧ-инфекция начинается с заражения базальных или стволовых клеток зоны трансформации шейки матки. Эти клетки обладают потенциалом дифференцировки по плоскоклеточным железистым или нейроэндокринным линиям и отвечают за физиологию эпителия в клетках. В базальных клетках, инфицированных ВПЧ, экспрессия генов папилломавируса подавляется до уровня, близкого к поддерживающему [11, 12].
Продуктивная экспрессия генов ВПЧ жестко регулируется и возможна только в клетках, начавших плоскоклеточное созревание с сопутствующей потерей пролиферативной способности [1].
В непосредственной парабазальной зоне происходят экспрессия ранних участков вирусного генома и по мере дифференцировки клеток – индукция всех вирусных генов, а также синтез вирусной ДНК. Это приводит к сборке и продуцированию вирионов в клетках промежуточных слоях эпителия.
В шейке матки такие поражения распознаются как низкодифференцированные плоскоклеточные интраэпителиальные поражения – биологический эквивалент того, что было названо легкой плоскоклеточной дисплазией (L-SIL), или койлоцитарной атипией.
Как можно заразиться ВПЧ? Защищает ли презерватив?
ВПЧ поражает базальные клетки эпителия. Микротравма, или микроскопические разрывы, эпителиального барьера обеспечивают доступ вируса к чувствительным клеткам. В шейке матки это происходит главным образом через проникающий половой акт. Для вульвовагинальной передачи инфекции может быть достаточно непроникающего абразивного контакта гениталий с гениталиями [16].
Последовательное использование презервативов обеспечивает относительную степень защиты от передачи ВПЧ в эпителий шейки матки, однако кумулятивная заболеваемость, связанная с последовательным использованием презервативов, все еще высока (37,8% в год). Оральное и анальное поражение ВПЧ, вероятно, приобретается путем прямого полового контакта (оральный и анальный секс).
Данные естественной истории свидетельствуют о том, что повторное заражение между партнерами встречается редко, вероятно, представляя собой развитие некоторого уровня естественного иммунитета против повторного заражения.
Этот вопрос чаще всего интересует пациенток, так как их волнует, не могут ли они постоянно заражаться от партнера или заразить его сами?
Очень много исследований последних лет показали значимость сопутствующих ИППП в прогрессивном воздействии ВПЧ на клетки эпителиального покрова влагалища и шейки матки. Уреа/микоплазменная инфекция, особенно хламидийная, усиливает риск развития предрака и рака шейки матки в 1,5–5 раз. Как следствие, применение презерватива для защиты от ко-факторов в виде ИППП, безусловно, актуально.
Как диагностировать ВПЧ-ассоциированные заболевания?
ВПЧ-ассоциированные заболевания: остроконечные кондиломы, поражения эпителиального покрова в виде L-SIL, H-SIL, рак шейки матки, VAIN, VIN, AIN.
Значимую роль в диагностике играет визуальный осмотр, при котором можно определить кондиломы, а иногда и онкоэкзофиты, отражающие злокачественное перерождение [17]. Отдельные очаги поражения обычно имеют размеры от нескольких миллиметров до 2 см в диаметре, но могут достигать и очень больших размеров, превышающих 10 см в диаметре, образуя гигантские кондиломы.
У большинства пациенток присутствует от 3 до 8 поражений, но общее число может иногда превышать 50, особенно у иммунодискордантных пациентов [18]. Отдельные поражения могут также объединяться в бляшки. Генитальные бородавки имеют цвет окружающих тканей с просветлением на гиперкератизированных участках, различную степень гиперпигментации. Откровенно пигментированные бородавки заслуживают особого внимания, поскольку на самом деле они могут быть интраэпителиальными неоплазиями, связанными с онкогенными типами ВПЧ.
Пациенту с генитальными бородавками может быть рекомендовано аноскопическое исследование при наличии перианальных бородавок, анамнезе анального рецептивного полового акта или наличии анальных симптомов.
Три четверти пациентов с аногенитальными бородавками не испытывают никаких симптомов. Наиболее частыми проявлениями могут быть зуд, жжение, иногда боль, болезненность или дискомфорт, которые лишь изредка мешают половому акту [18]. Психологическое воздействие болезни несет наибольшую нагрузку по крайней мере у половины больных.
Дифференциальная диагностика наружных генитальных бородавок должна осуществляться с множеством заболеваний: папилломатозные поражения, контагиозный моллюск, кондиломы Латум (сифилис) эпидермоидные кисты, обычные бородавки, плоскоклеточный рак, лимфогранулема венерическая, плоские папулы, пигментные поражения, невусы, себорейный кератоз, интраэпителиальная неоплазия (включая боуэноидный папулез, болезнь Боуэна).
Остроконечные кондиломы диагностируются большей частью визуально и редко требуют использования кольпоскопии и биопсии, особенно когда речь идет о поражении вульвы и перианальной области [18, 1]. Определение кондилом на поверхности шейки матки требует использования кольпоскопа, так как свежие мелкие папиллярные образования видны только при уксусной пробе. Учитывая, что женщины, у которых наиболее часто определяются кондиломы шейки матки, находятся в молодом возрасте (±20 лет), у большинства из них впоследствии наблюдается спонтанный регресс. При необходимости абляционную терапию (криотерапию, лазерную абляцию) можно проводить в случаях стойкого симптоматического заболевания.
Трудность заключается в степени воздействия на многие складки стенки влагалища, которые могут оставить часть кондилом недоступными. Ограниченный доступ в сочетании со склонностью к рецидивированию этих поражений у лиц с ослабленным иммунитетом приводит к необходимости проведения скорее иммуноподдерживающей терапии, чем постоянного удаления кондилом.
Цитология, гистология
Цитология – обязательное скрининговое исследование.
Первый этап – взятие цитологического мазка с экзо/эндоцервикса (при необходимости с боковых стенок влагалища, ануса, мазков-отпечатков с вульвы).
Очень важно обратить внимание на методику забора материала для цитологического исследования состояния эпителия. Забор материала необходимо производить цитобрашем (цервикс-браши) или щеточками для ПЦР, но ни в коем случае не шпателями. Шпатели не позволяют осуществить полноценный забор материала и, кроме того, нанести забранный материал на стекла [19]. Забор материала шпателем переносит менее 10% отобранных клеток, а эффективность забора цервикс-брашем приближается к 95%.
Более информативным методом цитологического исследования является жидкостная цитология, поскольку собранный материал практически полностью будет оценен цитологом [20]. Жидкостная цитология улучшила забор клеток для исследования, тем самым уменьшив ошибку отбора проб в 4 раза по сравнению с традиционным методом.
Цитологическая классификация Bethesda, модифицированная в 2014 г. (табл. 1), дает градацию клеточных изменений постадийно [21].
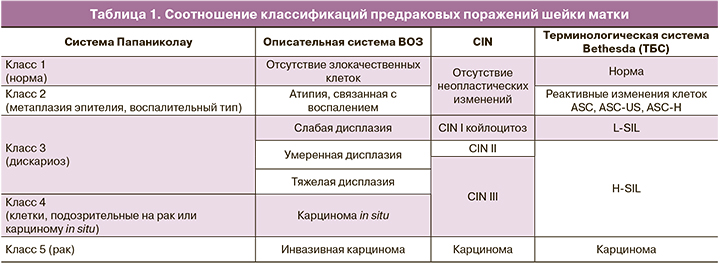
ASC-US демонстрирует неэффективный (в смысле клеточного деления онкобелков E6/E7) и опосредованный синтез ДНК хозяина, который производит увеличенные ядра и увеличенное ядерно-цитоплазматическое соотношение, что распознается как аномальное. Если процесс не полностью развит или, возможно, регрессирует, то клетки, полученные с поверхности, часто имеют меньшую ядерную аномалию (атипичные плоскоклеточные клетки неопределенного значения или ASC-US), чем те, которые наблюдаются при классической дисплазии [22].
Увеличение ядра и гиперхромазия, признанные патологами атипией, являются прямым результатом опосредованной активации синтеза ДНК хозяина онкобелками Е6/Е7. При ASC-US ни цитологически, ни кольпоскопически мы не можем четко определить, насколько вероятно дальнейшее патологическое изменение эпителия у конкретной пациентки. Наличие ВПЧ высокоонкогенных типов говорит о большей вероятности дальнейшего развития патологии.
В связи с этим современные изыскания, направленные на поиск маркеров для неинвазивных методов определения рисков развития рака для каждой конкретной пациентки, весьма актуальны [23].
Все белки ВПЧ являются иммуногенными и способны вызывать как гуморальные, так и клеточные иммунные реакции. В естественном течении ВПЧ контроля вирусной экспрессии генов нет, также отсутствует и возможность свести к минимуму воздействие антигенов в инфицированных клетках. L-SIL обычно регрессирует в течение короткого времени и редко сохраняется в течение длительного периода. Но персистенция вируса и клеточные изменения, обусловленные ими, могут привести к развитию H-SIL [11].
Клеточный иммунный ответ имеет специфичность, о чем свидетельствует наличие у пациентов с ВПЧ-поражением лимфопролиферативного ответа, системного и локального, на вирусные белки, особенно Е6 и Е7. Цитотоксический ответ также может быть обнаружен. Эти наблюдения послужили основой для разработки лечебных терапевтических препаратов. Естественные клетки-киллеры также присутствуют в CIN, но важность их роли неизвестна.
Гуморальный ответ на ВПЧ-инфекцию лучше понятен. Ранние вирусные белки генерируют очень слабый иммунный ответ, который не обнаруживается у большинства пациентов. Однако около половины пациентов с инвазивным раком шейки матки имеют антитела к белкам Е6 и Е7. Самый сильный иммунный ответ во время естественной инфекции направлен на белок L1 в его нативной конформации. Он выявляется примерно у 50–70% пациентов и является хорошим маркером прошлой или настоящей инфекции.
В структуре патологии шейки матки CIN составляют 17–29%. Распространенность в мире CIN I степени, по данным ВОЗ, составляет 30 млн случаев, а CIN II–III степени – 10 млн. Наиболее часто диагностируют CIN II степени, частота перехода CIN в карциному in situ (CIS) варьирует от 40 до 64% [1].
На сегодня сложность представляет возможность воспроизводимого определения дифференцировки стадий поражения эпителия цитологически, гистологически и кольпоскопически. Поэтому для полноценной диагностики необходимо проведение максимального набора возможно доступных методов обследования: визуальный, цитология, ВПЧ-типирование, кольпоскопия, биопсия, гистология, определение маркеров р16/ki67 [24].
В марте 2012 г. Колледж американских патологов и Американское общество по кольпоскопии и цервикальной патологии в сотрудничестве с 35 организациями заинтересованных сторон созвали консенсусную конференцию под названием «Проект по аномальной плоскоклеточной терминологии (LAST)» [25]. Рекомендации этого проекта включают использование единой двухуровневой терминологии для описания гистологии, связанной с ВПЧ-ассоциированными заболеваниями во всех тканях аногенитального тракта: вульвы, влагалища, шейки матки, пениса, перианальной области и ануса, вместо трехуровневой, которой пользуются сегодня.
Рекомендуемая терминология – «низкосортная», или «полноценная плоскоклеточная интраэпителиальная патология (SIL)». Она соответствует терминологии цитологических отчетов Bethesda System.
Трехуровневая классификация внутриэпителиальной неоплазии (CIN), используемая для гистопатологии связанных с ВПЧ плоских поражений (CIN I, CIN II, CIN III), иногда представляет некоторые проблемы в интерпретации результатов. Хотя CIN II и CIN III считаются высокосортными поражениями, диагноз промежуточной категории CIN II имеет гораздо более низкую воспроизводимость среди патологов, чем СIN III.
Экспертная группа рекомендовала использовать термины, знакомые клиницистам, и приняла решение о двухуровневой системе, аналогичной той, которая используется для отчетности по цервикальной цитологии. Поражения будут относиться к категории высокого или низкого класса, за которыми следует фраза «плоское внутриэпителиальное поражение». Используются такие же сокращения, как в системе Bethesda (L-SIL и H-SIL).
При переходе на новую терминологию и по просьбе клинициста диагноз может быть дополнен текущей терминологией «(СIN)». Если используется спецификатор СIN, он будет указан в скобках после основного диагноза. Например, биопсия шейки матки, ранее определяемая как «CIN II», теперь будет обозначена как «H-SIL» или «H-SIL (CIN II)».
Если клетки имеют правильные количество и форму экспрессии цитокератин-связывающего белка ВПЧ E4, то они выглядят как кoйлоциты. Клетки с хорошо развитой койлоцитарной атипией классифицируются как производные от легкой дисплазии (L-SIL) [25].
Выделяя пациентов с более низким риском развития рака (p16-отрицательный CIN II) и позволяя им избежать потенциального вреда от ненужного лечения, пересмотренная терминология делает важный шаг на пути к оптимизации лечения и улучшению результатов лечения.
Консервативное ведение молодых женщин с H-SIL имеет некоторый риск прогрессирования поражения (как и консервативное управление CIN II или CIN II–III), поэтому решение возможного лечения и наблюдения потребует индивидуализации и клинического обсуждения. Клиницист должен сбалансировать потенциал потерь от последующего наблюдения с опасностью чрезмерных повреждений ткани шейки матки.
Консультация патоморфолога может дать клиницисту дополнительную информацию для принятия окончательного решения.
Важной составляющей наблюдения пациентов с ВПЧ-ассоциированными заболеваниями является цитологический скрининг.
Цитологический скрининг основывается на возрасте пациенток (табл. 2).
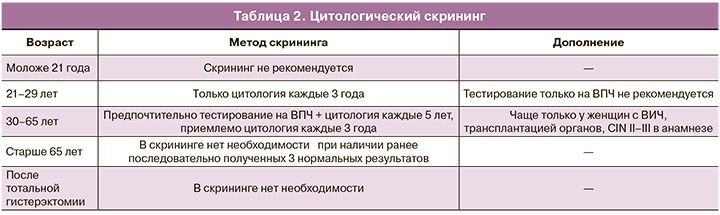
Однако в последние годы предлагается заменить скрининговое проведение цитологии у молодых женщин с 25 до 29 лет ПЦР-диагностикой на определение ВПЧ.
«Нет ВПЧ – нет рака шейки матки!»
Кроме того, как оказывается, достоверность и информативность цитологии не столь велики, и даже гистология биоптата шейки матки не является однозначно золотым стандартом. Все они могут зависеть от множества сопутствующих факторов.
В нашей стране цитология рекомендована 1 раз в год.
Выявление ВПЧ методом ПЦР является важным моментом, особенно для понимания наличия реактивных изменений, обусловленных воспалительным процессом; для решения вопроса об активной тактике ведения пациенток с ASC-US, L-SIL или возможном динамическом наблюдении. Определение ВПЧ в динамике определит наличие персистенции вируса, рост вирусной нагрузки или ее снижение. Эти параметры помогут в диагностике, прогнозировании и оценке эффективности лечения ВПЧ-ассоциированных заболеваний.
Кольпоскопия
Очень важное место в диагностике патологии шейки матки, слизистой вульвы, влагалища и ануса занимает кольпоскопия. Но при всей своей значимости она субъективна. Эффективность кольпоскопии зависит от очень многих факторов: наличия оборудования, врача, хорошо владеющего данной методикой и знающего все современные достижения в цитологии, гистологии, молекулярных методах диагностики и последние достижения в проблеме канцерогенеза шейки матки. Важно разбираться в роли множества факторов и ко-факторов, позволяющих максимально достоверно интерпретировать кольпоскопическую картину у пациенток разных возрастов [26, 27]. Необходимо понимать, что кольпоскопических признаков, специфичных только для ВПЧ, нет. При кольпоскопии мы оцениваем совокупность изменений и предполагаем, что они могут быть обусловлены ВПЧ, хотя это могут быть хронические воспалительные процессы различной этиологии, нарушения гормонального гомеостаза [28]. На сегодняшний день необходимо понимать, что кольпоскопически выявленная атипия не всегда будет подтверждена гистологической атипией. Поэтому для полноценного обследования пациентки необходима совокупность всех исследований и особенно цитологии, ПЦР, кольпоскопии, морфологии и цито/гистохимии [29],Кольпоскопия зависит от достижений морфологии, и в зависимости от ее понимания меняется интерпретация кольпоскопических картин и, соответственно, классификация кольпоскопических терминов.
Последняя классификация была принята в 2011 г. и дополнена в 2017 г. [30, 31], сейчас она выглядит, как в таблице 3.
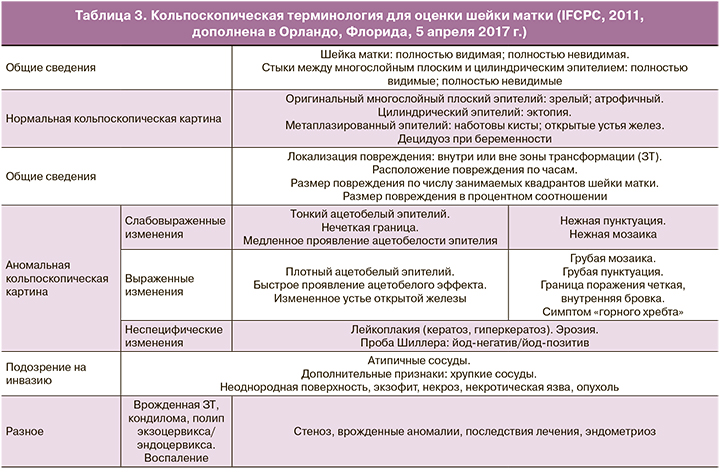
Обязательным в протоколе кольпоскопического исследования должно быть исследование слизистой влагалища по тому же алгоритму, что и шейки матки.
Кольпоскопия не дает возможности поставить диагноз, но позволяет сделать заключение о степени выраженности тяжести поражения эпителия, поэтому в протоколе заключение может быть: «нормальная кольпоскопическая картина», «слабовыраженные изменения», «выраженные изменения», «подозрение на инвазию» и «разное».
Основная роль кольпоскопии заключается в выявлении наиболее пораженного эпителия, чтобы затем провести прицельную биопсию. Очень важно, чтобы место взятия биоптата было правильно определено и затем качественно проведен забор материала для исследования. Если полученное морфологическое исследование биоптата не дает уверенной оценки изменений эпителия, необходимо их дополнить определением маркеров р16/ki67.
Особая группа пациентов – беременные. Остроконечные кондиломы в I триместре у беременных женщин могут разрастаться. Объясняется это естественной иммуносупрессией, направленной на сохранение плода. Со II триместра, как правило, отмечается уменьшение роста кондилом и даже полное их исчезновение к родам. В противном случае при необходимости проводят криодеструкцию, лазерную вапоризацию, химическую коагуляцию (нельзя использовать препараты на основе подофилотоксина).
Кольпоскопическая, цитологическая картина могут демонстрировать реактивные изменения на уровне и L-SIL, и H-SIL. Беременных наблюдают с использованием цитологии (1 раз в триместр), ПЦР для определения уровня вирусной нагрузки, определение маркеров р16/ki67. Биопсия и особенно цервикальный кюретаж не показаны, если речь не идет о раке. Первый осмотр после родов рекомендован через 6 недель после родов.
Вопросы, которые беспокоят беременных
1. Опасен ли ВПЧ для ребенка?
Крайне редко бывает респираторный кондиломатоз у новорожденных. В нашей стране не описан ни один случай.
2. Обязательно ли родоразрешение путем кесарева сечения, если есть ВПЧ, остроконечные кондиломы?
Показанием к кесареву сечению является только наличие крупных кондилом, которые могут мешать прохождению плода через естественные родовые пути.
3. Нужно ли прерывать беременность, если выявили поражения эпителия шейки матки на уровне L-SIl и H-SIL?
Нет, возможно даже родоразрешение через естественные родовые пути, что решается в каждом конкретном случае индивидуально.
Анальная интраэпителиальная неоплазия
Серьезной проблемой сегодняшнего дня становится анальная интраэпителиальная неоплазия (АИН). Частота АИН в последнее время нарастает, особенно у женщин. Симптомы сходны с симптомами анальных кондилом, и, соответственно, оценка состояния пациента такая же. Как и в случае с шейкой матки, поражения могут быть обнаружены с помощью анальной цитологии, с использованием кольпоскопа (аноскопия высокого разрешения), при необходимости – в сочетании с биопсией, позволяющей оценить тяжесть поражения. Женщины чаще всего обращаются к гинекологу. Поэтому при подозрении на поражение ануса (анальный секс, кондиломы в около-анальной области, зуд, наличие H-SIL) возникает необходимость проведения аноскопии высокого разрешения. Взятие цитологического мазка, мазка для выявления ВПЧ не представляет больших трудностей для врача, который занимается кольпоскопией. А вот биопсия и дальнейшее хирургическое лечение – прерогатива проктолога.
Лечение
Самый сложный вопрос на сегодня – вопрос лечения ВПЧ-ассоциированных заболеваний. Современные реалии хорошо продемонстрировали, что препаратов направленного действия на вирусы в мире не существует. Основная идея борьбы с вирусами – общественный иммунитет. Для ВПЧ показательными являются достижения Австралии, где вакцинировано более 70% мужского, женского и детского населения, резко снизилось число раков, обусловленных ВПЧ, а также практически исчезли остроконечные кондиломы. Минздрав Австралии считает, что к 2030 г. полностью избавит население страны от ВПЧ [32].
Но сегодня такой уровень вакцинации от ВПЧ практически недостижим, поскольку страны несут большие финансовые затраты на вакцинацию от COVID-19. А пациентов необходимо лечить от ВПЧ-ассоциированных заболеваний здесь и сейчас. Врач должен быть вооружен эффективными, безопасными препаратами с большой долей доказательности их действия.
Одним из таких препаратов является инозин пранобекс, широко известный как Гроприносин, Инозин ацедобен димепранол, Изопринозин или Метизопринол. Представляет собой синтетическое соединение п-ацетамидобензоатной соли N-N-диметиламино-2-пропанол с инозином в молярном соотношении 3:1, обладающий иммуномодулирующими и противовирусными свойствами. Препарат был первоначально разрешен в 1971 г. и в настоящее время продается более чем в 70 странах мира для лечения вирусных заболеваний.
Инозин пранобекс – синтетическое иммуномодулирующее соединение, предназначенное для применения при заболеваниях, ассоциированных с ВПЧ [33].
Множественные исследования показали, что иммуномодулирующая активность инозина пранобекса характеризуется усиленной пролиферацией лимфоцитов, продукцией цитокинов и цитотоксичностью NK-клеток. Активация передачи сигналов NKG2D на NK-клетках, CD8+ Т-клетках и γδ Т-клетках также приводит к этим результатам. Инозин пранобекс изменяет клеточный иммунитет за счет индукции экспрессии лиганда NKG2D на клетках-мишенях, тем самым усиливая активацию иммунных клеток через рецептор NKG2D.
В августе 2019 г. группа чешских исследователей опубликовала аналитический обзор с говорящим названием «Инозин пранобекс: ключевой игрок в игре против широкого спектра вирусных инфекций и неинфекционных заболеваний» [34]. Авторы заключили, что это вещество усиливает пролиферацию Т-лимфоцитов и повышает активность NK-клеток, что наряду с повышением выработки провоспалительных цитокинов позволяет преодолеть иммуносупрессию. Клинический эффект – подавление репликации некоторых вирусов, в том числе ВПЧ.
Естественные киллерные клетки – это тип врожденных лимфоидных клеток, которые играют важную роль в 1-й линии иммунной защиты от любой вирусной инфекции. Они представляют собой первичную быструю врожденную иммунную атаку на инфицированные вирусом клетки.
Сегодня в лечении ВПЧ-ассоциированных заболеваний доминирующее место занимают методы хирургического удаления пораженного участка ткани для предотвращения развития ее злокачественной трансформации. Однако при таком подходе мы сталкиваемся практически в 30% случаев с рецидивами заболеваний. Удаление измененной ткани не всегда способствует полной элиминации вируса, и возможно снижение локального иммунитета, что провоцирует дальнейшую персистенцию ВПЧ.
Эффективность лечения остроконечных кондилом с применением инозина пранобекса уже практически не вызывает сомнений, поскольку эти схемы апробированы во многих странах мира [34]. Во многих руководствах по лечению кондилом инозин пранобекс включен в основные схемы лечения.
Инозин пранобекс (Гроприносин) можно назначать как монотерапию для лечения кондилом, особенно когда видны нежные образования, без ороговения. Мы наблюдаем их исчезновение даже с поверхности шейки матки без каких-либо последствий для здоровья женщины. Обширные, длительно существующие кондиломы, безусловно, требуют хирургического удаления (радиоволна, лазер, криодеструкция) и параллельно назначения терапии инозина пранобексом (Гроприносином). Три 14-дневных курса с интервалом 1 месяц дают хороший результат. У таких пациентов мы практически не наблюдаем рецидива в отличие от хирургического лечения без иммуномодулирующей терапии.
Исходя из имеющихся статистических данных о благоприятном исходе ВПЧ-ассоциированных заболеваний преимущественно у молодых женщин, мы можем их наблюдать достаточно длительный период времени. Но если пациентка более старшего возраста (30 лет и старше), то, вероятно, есть необходимость использовать терапию и на раннем этапе. Это позволит предотвратить дальнейшую патологическую трансформацию эпителиального покрова и, возможно, обойтись без травмирующего ткань шейки хирургического воздействия. Таким образом, сохраняется в полном объеме репродуктивная функция многих женщин.
В нашей стране нередко доминирует агрессивная тактика ведения пациенток при практически незначительных изменениях, а зачастую даже без наличия патологии шейки матки. За рубежом принята максимально щадящая тактика, к хирургии прибегают только на уровне поражения H-SIL [35].
Пересматривается отношение к L-SIL, поскольку зачастую такое изменение клеток возможно преимущественно при хронических воспалительных процессах. Адекватная нормализация биоценоза влагалища с учетом результатов его исследования приводит к улучшению состояния шейки матки. Кроме этого, L-SIL может наблюдаться на фоне атрофических процессов различной этиологии (лактационная аменорея, перименопаузальный период, раннее истощение яичников, хирургическая аменорея и т.д.).
Чаще всего в этой ситуации и врача, и пациентку волнует вопрос: не будет ли потеряно время? Нет ли опасности резкого ухудшения состояния?
Никакой опасности нет, молниеносного развития рака под действием ВПЧ не описано с учетом особенностей развития вируса и течения самого процесса.
Применение иммуномодулирующих препаратов, в том числе инозина пранобекса (Гроприносина) дает возможность активировать локальный иммунитет, а также создает более благоприятные условия для хирургического лечения и предотвращения рецидивов [36, 37].
Надеяться на полноценный результат лечения H-SIL, H-SIL in situ только применяя инозин пранобекс, конечно, не приходится. Но комбинированное лечение с применением иммуномодулирующих препаратов однозначно позволяет избежать рецидивов и прогнозировать быструю эпителизацию в постэксцизионном периоде.
Несмотря на существование рекомендаций по ведению некоторых ВПЧ-ассоциированных заболеваний, личный опыт врача и наличие инструментария играют важную роль в выборе того или иного метода лечения [38, 39]. Инозин пранобекс – один из инструментов, который доступен врачу для использования как в виде монотерапии, так и в комбинации с другими методами. Врач при этом может быть уверен в безопасности и высокой эффективности препарата в процессе лечения ВПЧ-ассоциированных заболеваний, опираясь на данные исследований в 70 странах, демонстрирующих положительные результаты лечения ВПЧ-ассоциированных заболеваний препаратом инозин пранобекс (Гроприносин).
Инозин пранобекс (Гроприносин) не является строго специфичным против конкретного вируса. Его механизм действия универсален против различных возбудителей. Он проявляет свою активность в отношении вируса простого герпеса, цитомегаловируса, вируса кор, вируса Т-клеточной лимфомы человека, поливирусов, вирусов гриппа А и В, энтероцитопатогенного вируса, вирусов энцефаломиокардита и конского энцефалита.
Появляются сообщения о возможности влияния инозина пранобекса на вирус COVID-19 c положительными результатами. С учетом распространенности этих вирусов, дальнейшие исследования в этом направлении чрезвычайно важны.



