Lysol oxidase-like (LOXL1) levels in vaginal secretions in women of reproductive age with pelvic organ prolapse and the normal position of pelvic organs
Ivanyuk I.S., Remneva O.V., Korenovsky Yu.V., Gal’chenko A.I.
Objective: To evaluate the potential of non-invasive method for determining LOXL1 levels for the diagnosis of pelvic organ prolapse (POP) in women of reproductive age.
Materials and methods: A total of 48 women with POP and 32 women who had no vaginal prolapse were examined. LOXL1 levels in vaginal secretions were determined using enzyme-linked immunosorbent assay.
Results: LOXL1 levels were significantly higher in women with vaginal prolapse and in women with hypertension (p<0.05). LOXL1 levels were lower in women with varicose veins of the lower extremities, hemorrhoids and nephroptosis.
Conclusion: The study evaluated the potential of quantitative measurement of LOXL1 in vaginal secretions in women of reproductive age, that can become an effective prospective method to predict the diagnosis of pelvic organ prolapse.
Authors' contributions: Ivanyuk I.S., Remneva O.V., Korenovsky Yu.V. – the concept and design of the study; Ivanyuk I.S., Korenovsky Yu.V. – material collection and processing, statistical data processing; Ivanyuk I.S. – manuscript writing; Gal’chenko A.I. – manuscript editing.
Conflicts of interest: The authors confirm that they have no conflict of interest to declare.
Funding: The study was conducted without any sponsorship.
Ethical Approval: The study was approved by the local Ethics Committee at Altai State Medical University, Ministry of Health of Russia (protocol No. 11 of December 24, 2021).
Patient Consent for Publication: The patients have signed informed consent for publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Ivanyuk I.S., Remneva O.V., Korenovsky Yu.V., Gal’chenko A.I. Lysol oxidase-like (LOXL1) levels in vaginal secretions in women of reproductive age with pelvic organ prolapse and the normal position of pelvic organs.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (5): 110-116 (in Russian)
https://dx.doi.org/10.18565/aig.2024.329
Keywords
Pelvic organ prolapse (POP) is a pathological condition and is defined as downward displacement of pelvic organs from their normal anatomical position. This condition can lead to impaired urination and/or defecation. Women with POP have sexual dysfunction and depressive episodes [1, 2]. Currently, there is no clear information about the causes of this pathology. The leading risk factor is considered to be pelvic floor trauma during vaginal birth [3]. At the same time, it has been shown that occurrence of pelvic organ prolapse depends not on obstetric events, but on pelvic floor health before the first birth [4, 5].
Once the process in started, POP can progress throughout a woman’s life. The pattern of occurrence and severity of anatomical changes is not clear. In some cases, the prolapse is mild, in other cases it becomes severe. Conservative treatment methods can help reduce the symptoms caused by POP and slow down the disease progression [6, 7]. The rate of complications after surgical treatment of POP remains high [8].
The position of the organs in the pelvis is determined by the state of the supporting structures of the pelvic floor: fascia, ligaments and muscles. Extracellular matrix (ECM) metabolism plays an important role in maintaining the structural integrity and properties of elastic and collagen fibers of the pelvic floor tissues. Extracellular matrix (ECM) metabolism plays an important role in maintaining the structural integrity and properties of elastic and collagen fibers of the pelvic floor tissues [9, 10]. The effect of lysyl oxidase-like 1 (LOXL1) on ECM formation and restoration has been widely explored [11]. Lysyl oxidase-like-1 (LOXL1) belongs to the lysyl oxidase (LOX) family containing five family members: LOX and LOX-like LOXL1, LOXL2, LOXL3 and LOXL4. LOXL1 is involved in elastic fiber synthesis, polymerizing tropoelastin monomers into elastin polymers [12].
Li Y. et al. (2021) significantly contributed to the exploration of POP pathogenesis. The studies in mice confirm the association between LOXL1 and occurrence of POP. In LOXL1 knockout mice, elastic fibers of the vaginal wall are characterized by thin and short rod-like structure. Thereby formation of the phenotype specific for POP occurs [13, 14].
The results of the studies devoted to determination of the levels of LOXL1 in vaginal wall tissues, ligaments and muscles of female pelvic floor have been described. The samples were taken for examination during surgical treatment [15, 16]. Wu X. et al. used similar tissue samples, but obtained conflicting results [17]. The necessity to obtain biopsy material restricts possibilities to determine the levels of LOXL1, especially in healthy women. It is relevant to explore the possibility of using non-invasive method for determining the level of LOXL1.
The aim of the study was to evaluate the potential of non-invasive method for determining the levels of LOXL1 for the diagnosis of pelvic organ prolapse (POP) in women of reproductive age.
Materials and methods
The study was carried out at the Department of Obstetrics and Gynecology, Altai State Medical University (ASMU) of the Ministry of Health of Russia.
The study was approved by the local Ethics Committee of ASMU of the Ministry of Health of Russia (protocol No. 11 of December 24, 2021).
The total of 80 women of reproductive age were examined. The main group consisted of 48 women with POP. The control group consisted of 32 women, who had no POP.
Inclusion criteria in the main group were: age of 18–45 years, POP (stages II–III). Inclusion criteria in the control group were: age of 18–45 years, absence of POP. Exclusion criteria from the study were: pregnancy at the time of the study and postpartum period less than 12 months, acute phase of chronic conditions, malignant neoplasms.
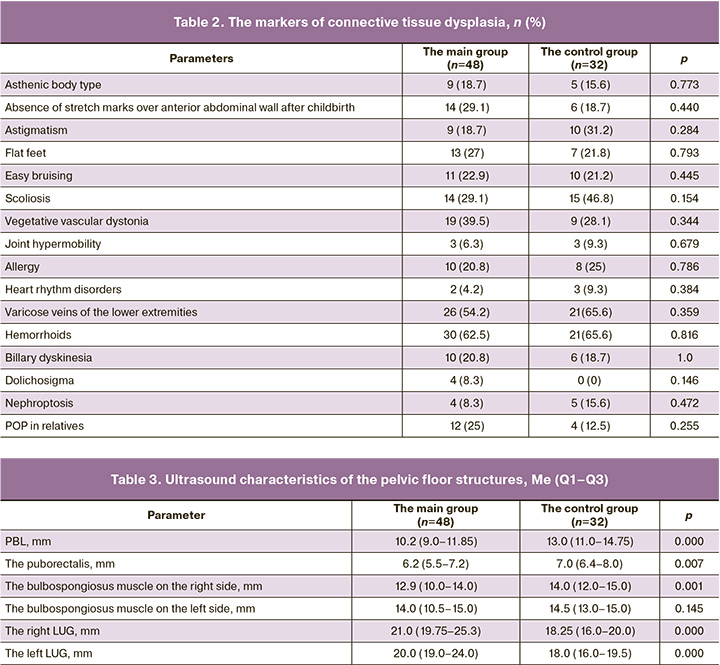
The scope of gynecological examination included visual examination of the external genitalia at rest and during functional tests, bimanual vaginal examination. The clinical and anamnestic data, including the signs of connective tissue dysplasia were assessed in each patient.
Assessment of health status of pelvic organs, the thickness and structure of pelvic floor muscles was performed by ultrasound diagnostic examination using GE Voluson E8 (GE HealthCare, Austria) with vaginal sensor RIC5-9-D.
Vaginal secretion samples were collected with Fashion cotton swabs using rubbing motions from the anterior and posterior vaginal walls and vaginal vault, spending 5 seconds for each area to obtain enough material for biochemical tests. Cotton swabs with samples were placed in microtubes, frozen and stored at -20°C before testing.
Prior to testing the samples were thawed at room temperature for 15 minutes. Then 300 µl of RIPA Lysis Buffer (strong) (Servicebio, PRC, cat. No. G2002-30ML) and 100 µl of the protease inhibitor aprotinin 10000 ATpE/mL (NovaMed Pharmaceuticals, Pakistan) were added. The samples were incubated for 15 minutes at room temperature.
Protein concentration in the samples was determined using reagent kit (Protein PGR) (“Vector Best”, Russia, cat. No. В8047) using semi-automatic biochemistry analyzer (Photometer 5010 V5+).
The levels of LOXL1 in the samples were determined using Human lysyl oxidase like protein 1 ELISA kit (ABclonal, USA, cat. No. RK01795) in accordance with the manufacturer’s instructions and using the Bio-Rad Model 680 microplate reader (USA).
The levels of LOXL1 were measured in ng/100 mg of protein.
Statistical analysis
Statistical data processing was performed using software program IBM SPSS Statistics 26. The Shapiro–Wilk test was used to test the normality of distribution. The Mann–Whitney test was used to test the significance of differences between the groups. The descriptive statistics are represented as median (Me) and interquartile range (Q1–Q3). Spearman’s rank correlation coefficient was used to test the relationship between the quantitative variables. Statistical significance was at p<0.05.
Results and discussion
The demographic, obstetric, gynecological, and somatic anamnestic data are represented in Tables 1 and 2.
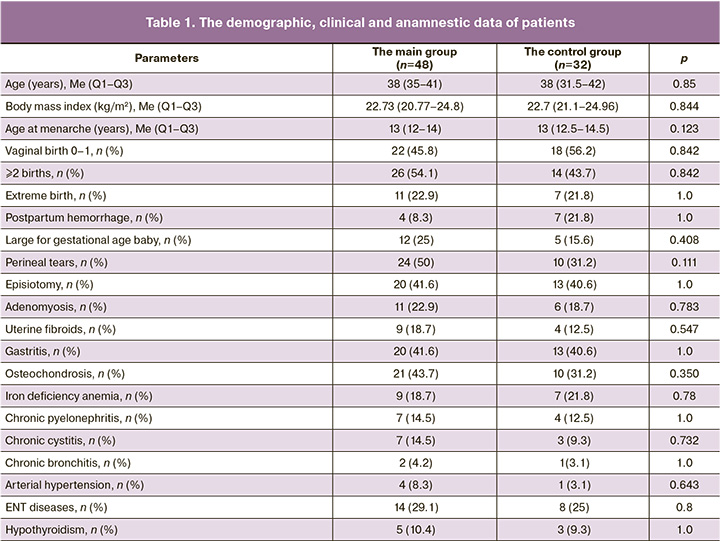
Comparison of the parameters in both groups found no statistically significant differences between women with POP and women in the control group. Ultrasound assessment of pelvic muscles showed statistically significant reduction in the perineal body length (PBL) and thickness of the puborectalis and bulbospongiosus muscles on the right side, increased right and left levator-urethra gap (LUG) in the main group (Table 3). The sonography data obtained by us are comparable with the results reported by other authors [18].
The levels of LOXL1 in biopsy samples of the pelvic floor ligaments were measured using polymerase chain reaction, and the researchers obtained mixed results. A number of authors reported that LOXL1 expression was reduced in women with POP compared with healthy patients. [19]. Other researchers reported that LOXL1 expression was increased in women with POP [20].
Garcia B. et al. demonstrated the possibility for detecting LOXL1 in the vaginal secretions in pre- and postmenopausal women [21]. They analyzed 48 samples and found this protein in 45 cases. In patients with POP, LOXL1 protein expression was higher compared with women without this pathology. These results can be explained as a compensatory protein expression increase in response to formation of altered elastic fibers [20]. In our study, LOXL1 was detected in all samples. Table 4 represents the quantitative indicators of LOXL1 in women with and without POP, taking into account the somatic pathology and the features of obstetric and gynecological anamnesis, and the presence of markers of connective tissue dysplasia.
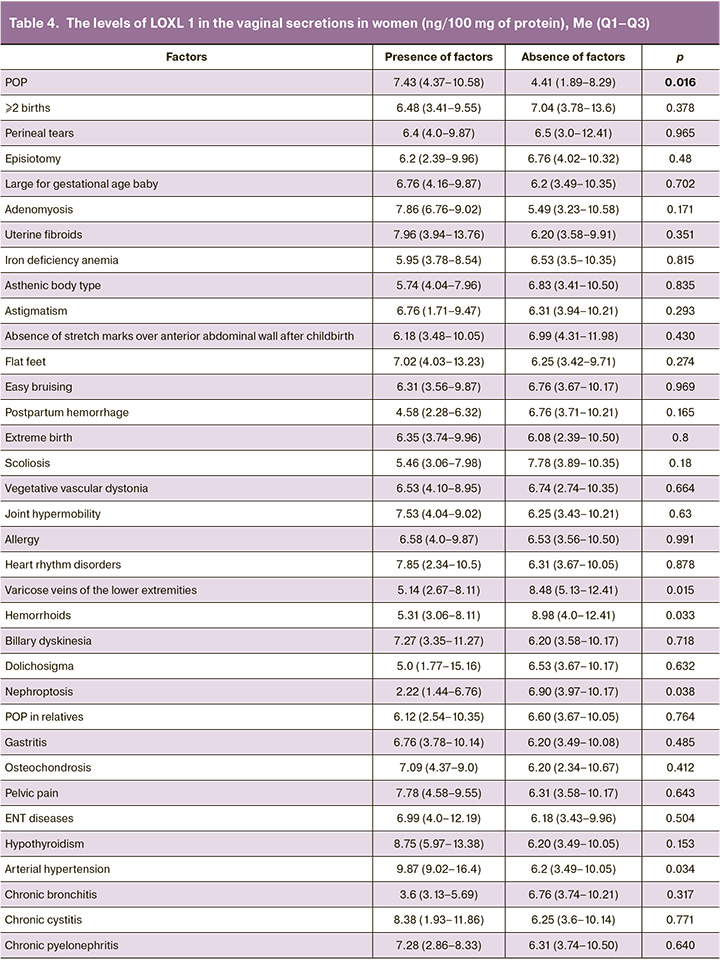
Significantly increased LOXL1 protein concentrations were found in the main group compared with the control group. There was no relationship found between the levels of LOXL1 and indicators of obstetric and gynecological anamnesis. In women with arterial hypertension, LOXL1 protein concentrations were higher compared with women with normal blood pressure (p=0.03). The levels of LOXL1 were lower in women with varicose veins of the lower extremities (p=0.01), hemorrhoids (p=0.03) and in patients with nephroptosis (p=0.03).
Given the differences between the results of ultrasound assessment of pelvic floor obtained in the groups (Table 3), we performed correlation analysis of the PBL, thickness of the bulbocavernosus and puborectalis muscles, the values of LUG and the quantitative measurement of LOXL1 levels. A positive correlation was found between the PBL (p=0.018, rs=0.417), the thickness of the puborectalis muscle (p=0.004, rs=0.491) and the level of LOXL1 in the vaginal secretions in women without POP.
Jameson S.A. et al. found that in the absence of LOXL1, non-epithelial vaginal cells from mice generate less elastin compared with vaginal cells from the LOXL1 knockout mice [22]. LOXL1 deficiency prevents the accumulation of elastic fibers during recovery of the female reproductive tract after pregnancy, childbirth and birth trauma, that can lead to POP [23].
Elastin provides elasticity and flexibility of connective tissues of the organs and systems, including the walls of blood vessels [24]. Increased expression of lysyl oxidase increases vascular wall stiffness and oxidative stress, and causes changes in elastin fibers. In contrast, inhibition of lysyl oxidase reduces vascular stiffness, changes in elastin structure, and oxidative stress associated with hypertension. Increased arterial wall stiffness is a causative factor of essential hypertension [25]. The results of our study indicate that in women with arterial hypertension, increased levels of LOXL1 are in vaginal secretions compared with the patients with normal blood pressure.
The study by Pascual G. et al. reported that LOXL1 expression is reduced in women with venous insufficiency [26]. Varicose veins are one of the clinical manifestations of venous insufficiency [27]. According to the results obtained in our study, LOXL1 concentrations are lower in women with varicose veins of the lower extremities, hemorrhoids and nephroptosis compared with patients without these pathologies (p<0.05). These conditions are related to clinical manifestations of connective tissue dysplasia [28, 29]. The obtained results confirm that dysregulated LOXL1 activity is one of possible mechanisms of pathological restructuring of the connective tissue [30].
Further research with a larger patient sample is needed, including a study on LOXL1 concentration in vaginal secretions and, in parallel, and LOXL1 expression in the ligamentous and muscular apparatus in women, to obtain more accurate results.
Conclusions
- LOXL1 concentration in vaginal secretions in women can be determined using a non-invasive method.
- In patients with POP, the levels of LOXL1 are significantly higher compared with women without POP.
- There is a direct dependence of LOXL 1 concentration on the perineal body length and thickness of the puborectalis in women without POP.
References
- Anglès-Acedo S., Ros-Cerro C., Espuña-Pons M., Valero-Fernandez E.M.; en nombre del GISPEM. Sexual activity and function of women with severe pelvic organ prolapse subjected to a classical vaginal surgery. A Multicentre study. Actas. Urol. Esp. (Engl. Ed.). 2019; 43(7): 389-95. https://dx.doi.org/10.1016/j.acuro.2019.02.003
- Abebe S.A., Gashaw Z.M., Ayichew Z., Angaw D.A., Kindie E.A. Prevalence and associated factors of depression among women with advanced pelvic organ prolapse in Northwest Ethiopia: cross-sectional study. BMC Womens Health. 2024; 24(1): 313. https://dx.doi.org/10.1186/s12905-024-03162-4
- Handa V.L., Blomquist J.L., Roem J., Munoz A., Dietz H.P. Pelvic floor disorders after obstetric avulsion of the levator ani muscle. Female Pelvic Med. Reconstr. Surg. 2019; 25(1): 3-7. https://dx.doi.org/10.1097/SPV.0000000000000644
- Reimers C., Siafarikas F., Stær-Jensen J., Smastuen M.C., Bo K., Ellström Engh M. Risk factors for anatomic pelvic organ prolapse at 6 weeks postpartum: a prospective observational study. Int. Urogynecol. J. 2019; 30(3): 477-82. https://dx.doi.org/10.1007/s00192-018-3650-2
- Handa V.L., Blomquist J.L., Carroll M.K., Munoz A. Genital hiatus size and the development of prolapse among parous women. Female Pelvic. Med. Reconstr. Surg. 2021; 27(2): e448-e452. https://dx.doi.org/ 10.1097/SPV.0000000000000960
- Mendes L.C., Bezerra L.R.P.S., Bilhar A.P.M., Neto J.A.V., Vasconcelos CTM, Saboia D.M. et al. Symptomatic and anatomic improvement of pelvic organ prolapse in vaginal pessary users. Int. Urogynecol. J. 2021; 32(4): 1023-9. https://dx.doi.org/10.1007/s00192-020-04540-w
- Короткевич О.С., Эйзенах И.А., Мозес В.Г., Захаров И.С. Клиническая эффективность вагинального тренажера в лечении несостоятельности мышц тазового дна у женщин пожилого возраста. Фундаментальная и клиническая медицина. 2018; 3(4): 32-8. [Korotkevich O.S., Eizenakh I.A., Mozes V.G., Zakharov I.S. Clinical efficiency of vaginal training device in treatment of pelvic organ prolapse in elderly women. Fundamental and Clinical Medicine. 2018; 3(4): 32-8. (in Russian)]. https://dx.doi.org/10.23946/2500-0764-2018-3-4-32-38
- Vandendriessche D., Sussfeld J., Giraudet G., Lucot J.P., Behal H., Cosson M. Complications and reoperations after laparoscopic sacrocolpopexy with a mean follow-up of 4 years. Int. Urogynecol. 2017; 28(2): 231-9. https://dx.doi.org/10.1007/s00192-016-3093-6
- Черёмин М.М., Смольнова Т.Ю., Красный А.М., Чупрынин В.Д., Особенности генной экспрессии у пациенток с пролапсом гениталий. Акушерство и гинекология. 2024; 3: 50-6. [Cheremin M.M., Smolnova T.Yu., Krasnyi A.M., Chuprynin V.D. Gene expression features in patients with genital prolapse. Obstetrics and Gynecology. 2024; (3): 50-6. (in Russian)]. https://dx.doi.org/10.18565/aig.2024.17
- Li Y., Zhang Q.Y., Sun B.F., Ma Y., Zhang Y., Wang M. et al. Single-cell transcriptome profiling of the vaginal wall in women with severe anterior vaginal prolapse. Nat. Commun. 2021; 12(1): 87. https://dx.doi.org/10.1038/s41467-020-20358-y
- Martinez-Gonzalez J., Varona S., Canes L., Galan M., Briones A.M., Cachofeiro V. et al. Emerging roles of lysyl oxidases in the cardiovascular system: new concepts and therapeutic challenges. Biomolecules. 2019; 9(10): 610. https://dx.doi.org/10.3390/biom9100610
- Greene A.G., Eivers S.B., Dervan E.W.J., O'Brien C.J., Wallace D.M. Lysyl oxidase like 1: biological roles and regulation. Exp. Eye Res. 2020; 193: 107975. https://dx.doi.org/10.1016/j.exer.2020.107975
- Li Y., Nie N., Gong L., Bao F., An C., Cai H. et al. Structural, functional and molecular pathogenesis of pelvic organ prolapse in patient and Loxl1 deficient mice. Aging (Albany NY). 2021; 13(24): 25886-902. https://dx.doi.org/10.18632/aging.203777
- Li Y., Wu B., An C., Jiang D., Gong L., Liu Y. et al. Mass cytometry and transcriptomic profiling reveal body-wide pathology induced by Loxl1 deficiency. Cell. Prolif. 2021; 54(7): e13077. https://dx.doi.org/10.1111/cpr.13077
- Камоева С.В., Савченко Т.Н., Абаева Х.А., Демура Т.А., Иванова А.В. Роль матриксных белков Fbln-5 и LOXL-1 в патогенезе пролапса тазовых органов. Российский вестник акушера-гинеколога. 2013; 13(3): 33‑7. [Kamoeva S.V., Savchenko T.N., Abaeva Kh.A., Demura T.A., Ivanova A.V. Role of the matrix proteins Fbln-5 and LOXL-1 in the pathogenesis of pelvic organ prolapse. Russian Bulletin of Obstetrician-Gynecologist. 2013; 13(3): 33‑7. (in Russian)].
- Гречканев Г.О., Аветисян Е.А., Старкина О.В., Бабин Ю.Ю., Никишов Н.Н., Аветисян С.М., Сучилин Д.Н., Белоглазов Д.К. Генетические предикторы пролапса тазовых органов у женщин: версии и контраверсии. Российский вестник акушера-гинеколога. 2022; 22(4): 55‑66. [Grechkanev G.O., Avetisyan E.A., Starkina O.V., Babin Yu.Yu., Nikishov N.N., Avetisyan S.M., Suchilin D.N., Beloglazov V.K. Genetic predictors of pelvic organs prolapse in women: versions and contraversions. Russian Bulletin of Obstetrician-Gynecologist. 2022; 22(4): 55‑66. (in Russian)]. https://dx.doi.org/10.17116/rosakush20222204155
- Wu X., Liu X., Li T. Potential molecular targets for intervention in pelvic organ prolapse. Front. Med. (Lausanne). 2023; 10: 1158907. https://dx.doi.org/10.3389/fmed.2023.1158907
- Токтар Л.Р., Оразов М.Р., Геворгян Д.А., Арютин Д.Г., Маркина Я. В. Достиева Ш.М., Маслюков И.А. Трансперинеальное ультразвуковое исследование в диагностике несостоятельности тазового дна. Акушерство и гинекология: новости, мнения, обучение. 2020; 8(3) Приложение: 75-9. [Toktar L.R., Orazov M.R., Gevorgyan D.A., Aryutin D.G., Markina Ya.V. Dostieva Sh.M., Maslyukov I.A. The role of transperineal ultrasound assessment in the diagnosis of pelvic floor disorder. Оbstetrics and Gynecology: News, Opinions, Training. 2020; 8(3) Suppl.: 75-9. (in Russian)]. https://dx.doi.org/10.24411/2303-9698-2020-13912
- Klutke J., Ji Q., Campeau J., Starcher B., Felix J.C., Stanczyk F.Z. et al. Decreased endopelvic fascia elastin content in uterine prolapse. Acta Obstet. Gynecol. Scand. 2008; 87(1): 111-5. https://dx.doi.org/10.1080/00016340701819247
- Jung H.J., Jeon M.J., Yim G.W., Kim S.K., Choi J.R., Bai S.W. Changes in expression of fibulin-5 and lysyl oxidase-like 1 associated with pelvic organ prolapse. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009; 145(1): 117-22. https://dx.doi.org/10.1016/j.ejogrb.2009.03.026
- Garcia B., Arthur A., Patel B., Chang J., Chen D., Lane F. A non-invasive determination of LOXL1 and fibulin-5 levels in the vaginal secretions of women with and without pelvic organ prolapse. J. Med. Res Surg. 2021; 2(2): 10.52916/jmrs214042. https://dx.doi.org/10.52916/jmrs214042
- Jameson S.A., Swaminathan G., Dahal S., Couri B., Kuang M., Rietsch A. et al. Elastin homeostasis is altered with pelvic organ prolapse in cultures of vaginal cells from a lysyl oxidase-like 1 knockout mouse model. Physiol. Rep. 2020; 8(11): e14436. https://dx.doi.org/10.14814/phy2.14436
- Borazjani A., Couri B.M., Kuang M., Balog B.M., Damaser M.S. Role of lysyl oxidase like 1 in regulation of postpartum connective tissue metabolism in the mouse vagina. Biol. Reprod. 2019; 101(5): 916-27. https://dx.doi.org/10.1093/biolre/ioz148
- Schlötzer-Schrehardt U., Zenkel M. The role of lysyl oxidase-like 1 (LOXL1) in exfoliation syndrome and glaucoma. Exp. Eye Res. 2019; 189: 107818. https://dx.doi.org/10.1016/j.exer.2019.107818
- Martinez-Revelles S., Garcia-Redondo A.B., Avendano M.S., Varona S., Palao T., Orriols M. et al. Lysyl oxidase induces vascular oxidative stress and contributes to arterial stiffness and abnormal elastin structure in hypertension: role of p38MAPK. Antioxid. Redox. Signal. 2017; 27(7): 379-97. https://dx.doi.org/10.1089/ars.2016.6642
- Pascual G., Mendieta C., Mecham R.P., Sommer P., Bellon J.M., Bujan J. Down-regulation of lysyl oxydase-like in aging and venous insufficiency. Histol. Histopathol. 2008; 23(2): 179-86. https://dx.doi.org/10.14670/HH-23.179
- Youn Y.J., Lee J. Chronic venous insufficiency and varicose veins of the lower extremities. Korean. J. Intern. Med. 2019; 34(2): 269-83. https://dx.doi.org/10.3904/kjim.2018.230
- Nikolenko V.N., Oganesyan M.V., Vovkogon A.D., Cao Y., Churganova A.A., Zolotareva M.A. et al. Morphological signs of connective tissue dysplasia as predictors of frequent post-exercise musculoskeletal disorders. BMC Musculoskelet. Disord. 2020; 21(1): 660. https://dx.doi.org/10.1186/s12891-020-03698-0
- Морозова Т.И., Перегудова И.Г. Проявление мезенхимальной дисплазии соединительной ткани у женщин с менструальной дисфункцией. Бюллетень медицинской науки. 2019; 4(16): 26-8. [Morozova T.I., Peregudova I.G. Manifestation of mesenchymal connective tissue dysplasia in women with menstrual dysfunction. Bulletin of Medical Science. 2019; 4(16): 26-8. (in Russian)].
- Beyens A., Van Meensel K., Pottie L., De Rycke R., De Bruyne M., Baeke F. et al. Defining the clinical, molecular and ultrastructural characteristics in occipital horn syndrome: two new cases and review of the literature. Genes (Basel). 2019; 10(7): 528. https://dx.doi.org/10.3390/genes10070528
Received 23.12.2024
Accepted 21.04.2025
About the Authors
Irina S. Ivanyuk, PhD Student, Department of Obstetrics and Gynecology, Altai State Medical University, Ministry of Health of Russia, 40, Lenin Ave., Barnaul,656038, Russia, Ivanukirina@yandex.ru, https://orcid.org/0000-0002-6895-7103
Olga V. Remneva, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology, Altai State Medical University, Ministry of Health of Russia,
40, Lenin Ave., Barnaul, 656038, Russia, rolmed@yandex.ru, https://orcid.org/0000-0002-5984-1109
Yuri V. Korenovsky, PhD, Associate Professor, Barnaul City Polyclinic No. 14, 46 Lazurnaya str., Barnaul, 656067, Russia, timidin@gmail.com,
https://orcid.org/ 0000-0002-4434-5217
Anzhelika I. Gal’chenko, PhD, Associate Professor, Department of Obstetrics and Gynecology, Altai State Medical University, Ministry of Health of Russia,
40, Lenin Ave., Barnaul, 656038, Russia, https://orcid.org/0000-0003-3013-7764



