Placental ultrastructural and immunohistochemical changes in preeclampsia with concomitant fetal growth restriction
Aim. To investigate the ultrastructural and immunohistochemical changes occurring in the placenta in preeclampsia (PE) with concomitant fetal growth restriction (FGR). Materials and methods. The study comprised 33 patients who underwent operative delivery. Among them, 15 and 8 women had severe and moderate PE, respectively. The control group included 10 women with an uncomplicated full-term pregnancy. Six women with severe PE had FGR. A histological examination of hematoxylin-eosin stained sections was performed along with immunohistochemical studies using primary polyclonal antibodies to TLR8 (1: 250; GenTex). Besides, 10 placental specimens were examined using a Philips CM100 transmission electron microscope (Philips/FEI Corporation, Eindhoven, Holland). Results. In patients with severe PE, the findings of electron microscopy showed damage to membranes with a reduction of microvilli on the surface of syncytiotrophoblast (SCT), cytoplasm vacuolization, damage to membranes and apical parts of cells. In patients with moderate PE, microvilli remained on the SCT surface. A characteristic change in the SCT ultrastructure in moderate PE was the appearance in the cytoplasm of numerous vacuoles of different sizes formed by swelling and dilatation of cisterns of granular endoplasmic reticulum. The findings of immunohistochemical studies demonstrated that membrane and cytoplasmic TLR8 staining of SCT and syncytial knots (SK ) was most pronounced in PE with concomitant FGR and the least in uncomplicated physiological pregnancy (p<0.01). Besides, in moderate PE, membrane staining in SCT and SK was predominant, while severe PE was associated with mainly cytoplasmic staining (p <0.01). Conclusion. Severe PE is associated with damage to SCT membranes, which disrupts the placental barrier resulting in placental insufficiency. Patients with severe PE have over-expression of TLR8 in SCT. It cannot be ruled out that FGR is caused by the activation of inflammatory cascades resulting from over-expression of TLR8 and the high pro-inflammatory response of trophoblast.Nizyaeva N.V., Amiraslanov E.Yu., Lomova N.A., Pavlovich S.V., Savel’eva N.A., Nagovitsyna M.N., Sukhacheva T.V., Serov R.A., Shchegolev A.I., Kan N.E.
Keywords
Preeclampsia (PE) accounts for 2 to 8% of hypertensive disorders in pregnancy [1]. PE is a multisystem disorder characterized by new onset of arterial hypertension (AH) after 20 weeks gestation with proteinuria (> 0.3 g/l in a 24-hour urine collection), edema, and multiple organ failure [1, 2]. The pathogenesis of PE remains elusive, and no significant progress has yet been made in prevention and treatment of this disease [3, 4], which may be attributed to the fact that PE is the final clinical manifestation of disorders of various origins. Severe PE is often co-occurs with fetal growth restriction (FGR), while moderate PE is characterized by normal fetal growth and development [5, 6]. In recent years, studies investigating pathogenesis of PE have been increasingly focused on the role of placenta and immune tolerance [5, 7–9]. One of the important links of innate immunity associated with the regulation of inflammatory reactions is Toll-like receptors (TLRs) that can bind to ligands and as a result contribute to the development pro-inflammatory reactions and inadequate immune tolerance [10, 11]. Among TLRs, the least explored to date is TLR8. Besides, TLR8 is thought to be associated with autoimmune diseases and a systemic inflammatory response [8, 12–14].
The present study aimed to investigate the ultrastructural and immunohistochemical changes occurring in the placenta in PE with concomitant FGR.
Materials and methods
The study analyzed clinical and anamnestic data of 33 women who underwent operative delivery at 26–39 weeks’ gestation, meet the inclusion criteria and signed informed consent to take part in the study. Severe and moderate PE was diagnosed in 15 and 8 pregnant women, respectively. The control group consisted of 10 women with an uncomplicated full-term pregnancy. Among patients with severe PE group, 6 women had FGR.
Criteria for inclusion in the group with moderate and severe PE were determined according to the relevant guidelines [1, 2].
The criteria for inclusion in the group with severe PE with FGR were the combination of PE with stage II and III FGR (fetal weight less than 5 percentiles) [1, 12].
The criteria for inclusion in the control group were uncomplicated full-term pregnancy, cesarean delivery for post-surgical uterine scar, abnormal fetal position, anatomically narrow pelvis, and high progressive myopia. Thus, 4 groups were formed.
Exclusion criteria from all groups were acute and chronic inflammatory diseases, severe extragenital comorbidities, multiple pregnancies, the use of assisted reproductive technologies (ART), a history of internal organ transplantation, a history of cancer, diabetes mellitus (DM), congenital fetal malformations, and spontaneous delivery.
A histological examination of hematoxylin-eosin stained sections was performed along with immunohistochemical studies. Immunohistochemistry was conducted using a closed type Ventana Immunostainer (Roche, UK) with a closed kit with primary polyclonal antibodies to TLR8 (1: 250; cat. number GTX2120; Gentex) [15]. The negative and positive control was done according to the manufacturer’s instructions. The reaction product was visualized as brown staining of the cell membrane and cytoplasm. For quantitative assessment, we evaluated membrane and cytoplasmic staining of syncytiotrophoblast (SCT) and syncytial knots (SK) in standard units using a Nikon Eclipse Microscope System and NIS-Elements imaging software (Czech Republic). In each sample, no less than 20 fields of view were analyzed at optical magnification of x400. For ease of presentation, all data was multiplied by 100.
Besides, 10 placental specimens were examined using an electron microscope to estimate the state of SCT and SK in placental villi at the ultrastructural level. For transmission electron microscopy, placental fragments sized 1 mm3 were taken from the thickness of the placental disc 2–5 minutes after cesarean section. The material was fixed in a solution of 2.5% glutaraldehyde and 1% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4), and fixed in a 1.5% OsO4 solution, dehydrated, and poured into Araldite. Semi-thin sections were stained by the SEC method with additional dyeing by methylene blue. Ultrathin sections were contrasted with uranyl acetate and lead citrate and examined under a Philips CM100 electron microscope (Philips / FEI Corporation, Eindhoven, Holland).
Statistical analysis was performed using the SPSS Statistics for Windows v.21. Given a small sample size, the distribution of continuous variables was tested for normality using the Kolmogorov-Smirnov test, which did not show normal distribution. Comparing numerical data between groups was performed with nonparametric tests. Quantitative variables were expressed as the median and quartiles Me (Q1; Q3). Kruskal–Wallis test was used for comparing numerical data between 4 groups.
Results
The mean age of patients with PE [33 (28;39) years] was statistically significantly higher than that in the control group [27 (25; 32) years] (p = 0.01). This observation indicates a possible association between PE and the woman’s age. No significant difference in age was found between the groups of severe and moderate PE. There was a significant difference in gestational ages at delivery between patients in the severe PE group and the control group (p < 0.02). Thus, the mean gestational ages at delivery among patients with severe PE was 35 (32; 38) weeks, which was significantly lower than in the control group [39 (36; 40) weeks] (p = 0.02). The need for an emergency preterm delivery in patients with severe PE was usually related to severe fetal distress detected by Doppler ultrasound scan and an increase in PE severity despite antihypertensive therapy.
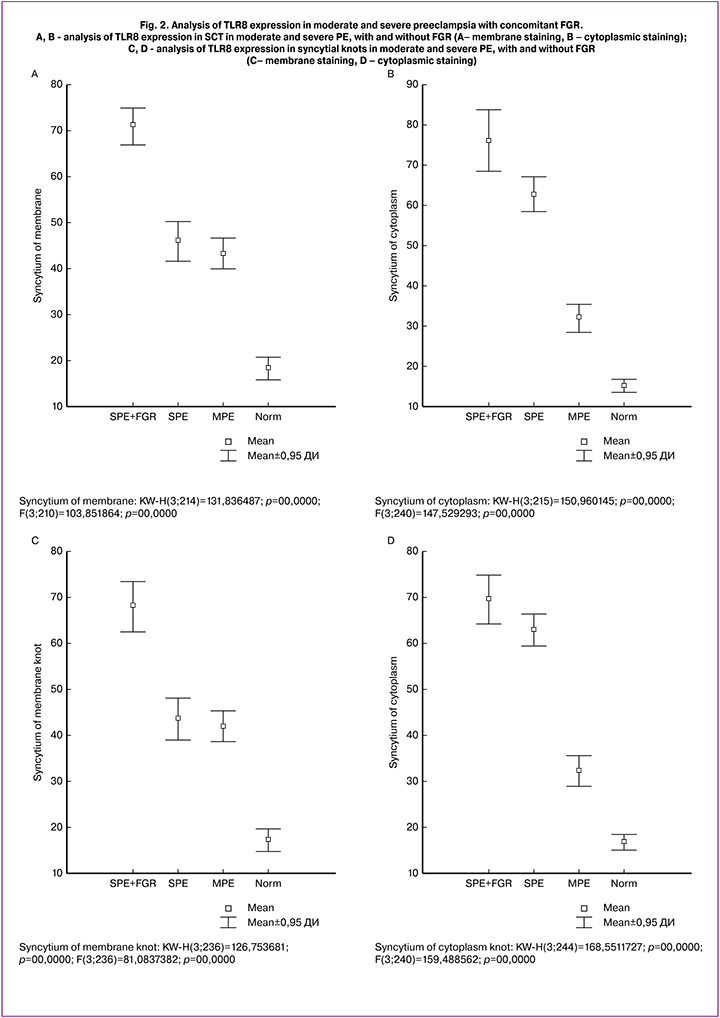
Immunohistochemical analyses revealed the presence of products of TLR8 immunohistochemical reaction in the membrane and cytoplasm of the cytotrophoblast, CCT, and SK, in decidual cells, amniotic epithelium, blood vessel endothelium, macrophages (Kashchenko-Hoffbauer cells), and in villi stroma cells. A quantitative assessment showed that the expression level of TLR8 in severe PE with concomitant FGR was 69 (55; 77) and 77 (71; 81) in SCT and 66 (59; 73) and 70 (62; 75 ) in SK (membrane and cytoplasmic staining, respectively), which was significantly higher than in all other groups (p <0.01) (Fig. 1, A – B).
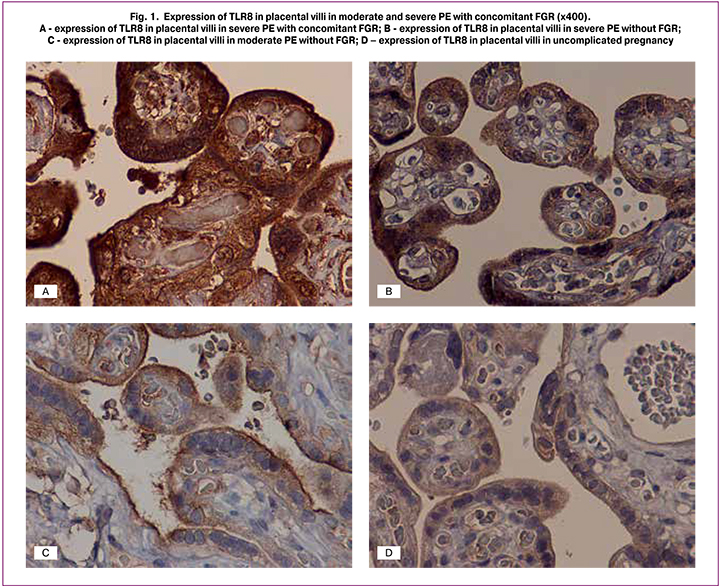
Along with this, in the control group, membrane and cytoplasmic staining was only 17 (12; 20) and 15 (11; 21) in SCT and 17 (11; 20) and 16 (11; 20) in SK, respectively; p = 0.04 (Fig. 1, C– D; Fig. 2, A – D). The same tendency was observed in moderate PE with the predominance of membrane staining in SCT and SK. The ratio of membrane to cytoplasmic staining in SCT was 0.8 (0.4; 1.3), 0.8 (0.6; 1.1), 1.4 (1.1; 1.7), and 1.3 (0.9; 1.4) in severe PE with concomitant FGR, severe PE without FGR, moderate PE, and in the control group, respectively. A similar trend was noted in the staining type in SK: 0.7 (0.5; 1.0), 1.3 (0.9; 1.5) 1, 1 (0.8; 1.4) (p = 0.04), and 1.0 (0.8; 1.4) in patients with severe PE, moderate PE and in the control group (p = 0.04), respectively, and 1.0 (0.8; 1.4) in patients with severe PE with FGR.
Therefore, moderate PE and uncomplicated full-term pregnancy are characterized by the preponderance of SCR and SK membrane staining of TLR8 over cytoplasmic, and in severe PE, there was the preponderance of cytoplasmic staining over the membrane (p < 0.03), which may be associated with a transition to a state of decompensation.
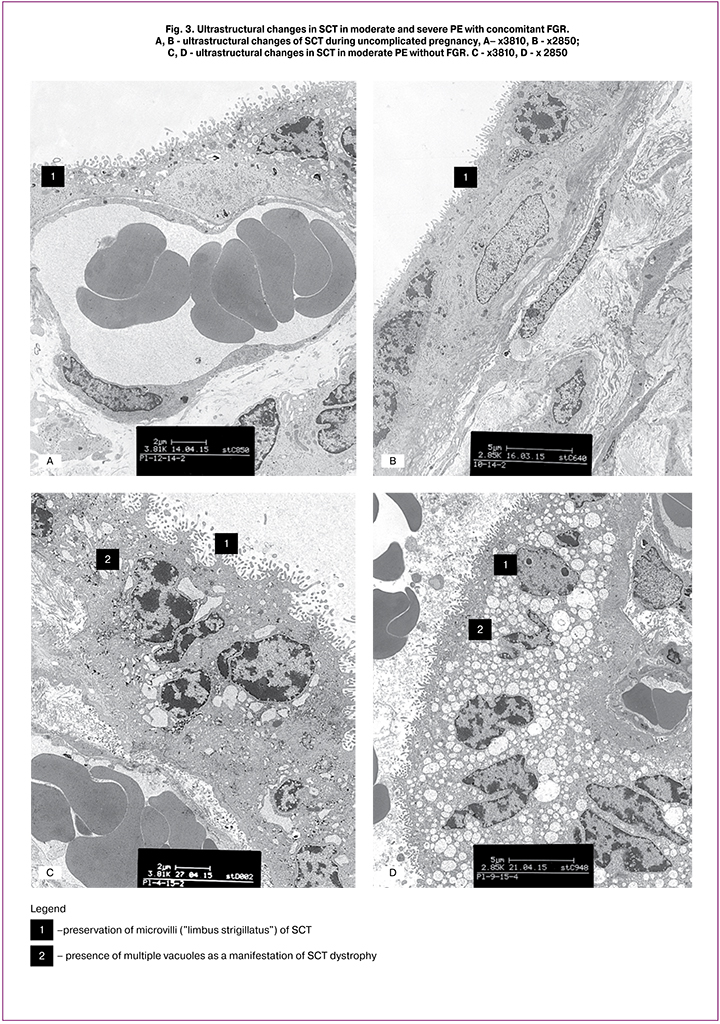
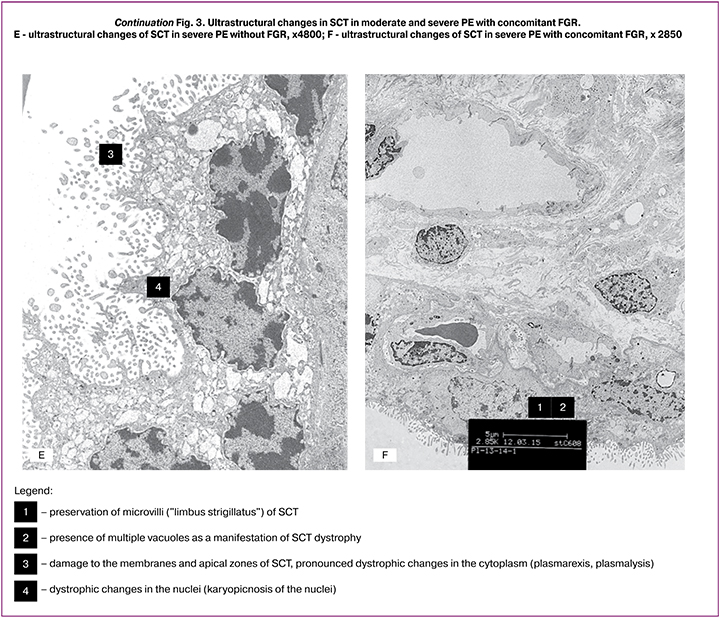
The findings of transmission electron microscopy showed that during physiological pregnancy, microvilli (like a limbus strigillatus) were observed on the surface of the SCT villi. Small vacuoles formed by cisterns of the granular endoplasmic reticulum were found in the SCT cytoplasm (Fig. 3, A – B). In moderate PE, microvilli remained on the SCT surface; in some areas, the SCT membrane protruded with microvilli. A characteristic change in the SCT ultrastructure in moderate PE was the appearance in the cytoplasm of numerous vacuoles of different sizes formed by swelling and dilatation of cisterns of granular endoplasmic reticulum. Due to these changes, the SCT height increased by 1.3 times, compared with the control (Fig. 3, C – D). In patients with severe PE, SCT ultrastructure changes consisted of significant condensation of the nuclear chromatin and reduction of the number of microvilli on the surface. The remaining microvilli were deformed and had a bizarre shape. Accumulation of vacuoles of different sizes was detected in the SCT cytoplasm, signs of dystrophic changes were revealed - myelin-like bodies, phagosomes, damage to the apical portions of SCT, as well as the presence of apoptotic bodies and focal SCT desquamation (Fig. 3, E). The decrease in TLR8 membrane staining in SCT and SK in severe PE can be explained by a reduction in the number of microvilli. At the same time, in PE with concomitant FGR, the membranes and apical sections of SCT and SK were partially preserved, although multiple vacuoles were present in the cytoplasm (Fig. 3, F).
Discussion
In recent years, the concept of PE has transformed from being a pregnancy-induced disease characterized by a combination of renal pathology and hypertension to a heterogeneous multisystem disorder that affects maternal and child health during the subsequent life. Women with a history of PE are at high risk for developing coronary heart disease and other vascular disorders, including hypertension, stroke, venous thromboembolism, renal failure, type 2 diabetes, and hypothyroidism [7, 8, 11, 16, 17].
According to several studies, patients with cardiovascular disease have an elevated serum myosin level [17–21]. Besides, along with fragments of DNA molecules and small interfering RNAs (siRNAs), myosin is a key ligand for TLR8 [9, 17, 22–25]. Myocardial and blood vessel wall injury results in the release of myosin molecules into the systemic circulation, thus activating TLR8 receptors and launching inflammatory cascades on monocytes, mediating the development of a systemic inflammatory response [24–26], which leads to the formation of a vicious circle and disease progression. It has been shown that an increased maternal level of myosin (TLR8 ligand) can be a starting mechanism of cardiovascular disease [6], and likewise, severe PE can also be associated with the increased myosin concentration mediating the development of a systemic inflammatory response.
The study results suggest that precipitous development of PE is accompanied by the destruction of SCT membranes, which occurs over a short time, while in severe PE with concomitant FGR, this process is stretched in time due to activation of compensatory mechanisms. Failure of compensatory mechanisms and decompensation develop gradually, which allows some SCT and SK membranes to survive. That is, the placental barrier changes from functional compensation to decompensation and after a while, lead to FGR with complete decompensation of the maternal-fetal placental system.
It should be noted that an increase in the number of SKs is a result of compensatory changes aimed at improving the maternal-fetal exchange. SKs are the centers of SCT proliferation in the form of closely spaced nuclei surrounded by a common cytoplasm and located on the surface of chorionic villi [27]. Along with this, SKs are associated with SCT apoptosis, which is especially pronounced in obstetric pathology and has been proven by immunohistochemical studies and electron microscopy [28, 29].
Besides, SKs synthesize a wide range of biological substances, hormones, including chorionic gonadotropin and miRNAs [12, 30], which then enter the maternal bloodstream and help to maintain the physiological course of pregnancy. Dystrophic changes and defective SKs in severe PE lead to an accelerated decrease in placental compensatory capacities and placental insufficiency. It has been proven that a decrease in SKs is often associated with FGR [12]. Considering that SCT forms the placental barrier, its damage contributes to the development of placental insufficiency [31], and the damage of SK impairs trophoblast invasion ability.
In our opinion, damage to SCT membranes negatively affects uteroplacental perfusion and causes placental insufficiency that restricts fetal growth, which often leads to preterm delivery. However, it cannot be ruled out that FGR is caused by the activation of inflammatory cascades resulting from over-expression of TLR8 and the high pro-inflammatory response of trophoblast. Along with this, the preservation of membrane staining in severe PE concomitant with FGR may be attributed to a partial preservation of placental compensatory capabilities.
Taking into account that SKs produce a wide range of biologically active substances that support normal gestation, their deficiency may be an additional factor reducing the placental compensatory capabilities. New insights into the differences in the course of severe PE may help develop treatments aimed at preserving and regenerating trophoblast, thus contributing to the preservation and prolongation of pregnancy.
Conclusion
Patients with severe PE were found to have TLR8 over-expression in SCT. Severe PE is associated with mainly cytoplasmic TLR8 staining, while in moderate PE, membrane staining is predominant. Besides, severe PE is associated with damage to SCT membranes, which disrupts the placental barrier resulting in placental insufficiency.
References
- Адамян Л.В. Серов В.Н., ред. Гипертензивные состояния во время беременности, в родах послеродовом периоде. Преэклампсия. Эклампсия. Клинические рекомендации (Протокол лечения), 2016. [Adamjan L.V. Serov V.N., red. Gipertenzivnye sostojanija vo vremja beremennosti, v rodah poslerodovom periode. Prejeklampsija. Jeklampsija. Klinicheskie rekomendacii (Protokol lechenija), 2016. (in Russ.)]
- Dong X., Gou W., Li C., Wu M., Han Z., Li X., Chen Q. Proteinuria in preeclampsia: Not essential to diagnosis but related to disease severity and fetal outcomes. Pregnancy Hypertens. 2017; 8: 60-64. https://doi.org/ 10.1016/j.preghy.2017.03.005
- de Haas S., Ghossein-Doha C., Geerts L., van Kuijk S.M., van Drongelen J., Spaanderman M.E. Cardiac remodelling during normotensive and hypertensive complicated pregnancies: a systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2017; 50(6): 683-696. https://doi.org/: 10.1002/uog.17410
- Roberts J.M., Bell M.J. If we know so much about preeclampsia. why haven’t we cured the disease? J Reprod Immunol. 2013; 99(1-2): 1-9.https://doi.org/10.1016/j.jri.2013.05.003
- Ломова Н.А., Кан Н.Е., Ванько Л.В., Донников А.Е., Матвеева Н.К., Беляева А.С., Тютюнник Н.В., Сухих Г.Т. Диагностическая значимость факторов врожденного иммунитета при плацентарной недостаточности. Акушерство и гинекология, 2014; 1: 29-35. [Lomova N.A., Kan N.E., Vanko L.V., Donnikov A.E., Matveeva N.K., Belyaeva A.S., Tyutyunnik V.L., Sukhikh G.T. Diagnosis value of innate immunity in placental insufficiency. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2014; (2): 29-35 (in Russian)].
- Баев O.P., Карапетян А.О., Низяева Н.В., Садекова А.А., Красный А.М. Содержание внеклеточной ДНК плода в материнской крови и экспрессия ДНК-распознающих ZBP-1 рецепторов в структурах плаценты при преэклампсии и преждевременных родах. Клеточные технологии в биологии и медицине. 2019; 3:179-185. [Baev O.P., Karapetjan A.O., Nizjaeva N.V., Sadekova A.A., Krasnyj A.M. Soderzhanie vnekletochnoj DNK ploda v materinskoj krovi i jekspressija DNK-raspoznajushhih ZBP-1 receptorov v strukturah placenty pri prejeklampsii i prezhdevremennyh rodah. Kletochnye tehnologii v biologii i medicine. 2019; 3:179-185. (in Russ.)]
- Cухих Г.Т., Ванько Л.В. Иммунные факторы в этиологии и патогенезе осложнений беременности. Акушерство и гинекология. 2012; 1: 128-136.[Sukhikh G.T., Vanko L.V. Immune factors in the etiology and pathogenesis of pregnancy complications. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2012; 1: 128–136. (in Russian)].
- Behrens I., Basit S., Lykke J.A., Ranthe M.F., Wohlfahrt J., Bundgaard H., Melbye M., Boyd H.A. Hypertensive disorders of pregnancy and peripartum cardiomyopathy: A nationwide cohort study. PLoS One. 2019; 14(2): e0211857. https://doi.org/10.1371/journal.pone.0211857.
- Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006; 124 (4): 783-801. DOI: 10.1016/j.cell.2006.02.015
- Krieg A.M., Vollmer J. Toll-like receptors 7, 8, and 9: linking innate immunity to autoimmunity. Immunol Rev. 2007; 220:251-69. DOI: 10.1111/j.1600-065X.2007.00572.x
- Mihu D., Razvan C., Malutan A., Mihaela C. Evaluation of maternal systemic inflammatory response in preeclampsia. Taiwan J Obstet Gynecol. 2015; 54(2): 160–6. https://doi.org/: 10.1016/j.tjog.2014.03.006
- Benirschke K., Burton G.J. Baergen R.N. Pathology of the human placenta, Sixth ed., NewYork: Springer; 2012. 939 р.
- Guiducci C., Gong M., Cepika A.M., Xu Z., Tripodo C., Bennett L., Crain C., Quartier P., Cush J.J., Pascual., Coffman R.L., Barrat F.J. RNA recognition by human TLR8 can lead to autoimmune inflammation. J Exp Med. 2013;210(13): 2903–19. https://doi.org/10.1084/jem.20131044
- Thwaites R., Chamberlain G., Sacre S. Emerging role of endosomal toll-like receptors in rheumatoid arthritis. Front Immunol. 2014; 5: e. 1. https://doi.org/10.3389/fimmu.2014.00001
- Низяева Н.В., Волкова Ю.С., Муллабаева С.М., Щеголев А.И. Методические основы изучения ткани плаценты и оптимизация режимов преподготовки материала. Акушерство и гинекология. 2014; 8: 10-18. [Nizyaeva N.V., Volkova Yu.S., Mullabaeva S.M., Shchegolev A.I. The methodical bases for placental tissue examination and the optimization of material pre-preparation regimens. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2014; 8: 10-18. (in Russian)].
- Blanton R.M., Alcaide P. Cardiac Myosin Protein C: New Roles, New Questions, Potential Opportunities JACC Basic Transl Sci. 2017; 2(12) Is2:132-134. https://doi.org/10.1016/j.jacbts.2017.03.007.
- Dalpke A., Helm M. RNA mediated Toll-like receptor stimulation in health and disease. RNA Biol. 2012; 6: 828-42. https://doi.org/10.4161/rna.20206.
- Gladstone R.A., Pudwell J., Nerenberg K.A., Grover S.A., Smith G.N. Cardiovascular Risk аssessment and Follow-Up of Women After Hypertensive Disorders of Pregnancy: A Prospective Cohort Study. J Obstet Gynaecol Can. 2019. pii: S1701-2163(18)30836-3. https://doi.org/10.1016/j.jogc.2018.10.024
- Yu L., Wang L., Chen S. Endogenous toll-like receptor ligands and their biological significance. J Cell Mol Med. 2010; 11: 2592-603. https://doi.org/10.1111/j.1582-4934.2010.01127
- Zhang P., Cox C.J., Alvarez K.M., Cunningham M.W. Cutting edge: cardiac myosin activates innate immune responses through TLRs. J Immunol. 2009; 183(1): 27-31. https://doi.org/: 10.4049/jimmunol.0800861
- Chi H., Flavell R.A. Innate recognition of non-self nucleic acids. Genome Biol. 2008; 9 (3). e211. doi: 10.1186/gb-2008-9-3-211
- Zhang S., Hu Z., Tanji H., Jiang S., Das N., Li J., Sakaniwa K., Jin J., Bian Y., Ohto U., Shimizu T., Yin H. Small-molecule inhibition of TLR8 through stabilization of its resting state. Nat Chem Biol. 2018. 14(1): 58-64. https://doi.org/10.1038/nchembio.2518
- De Lorenzo G., Ferrari S., Cervone F., Okun E. Extracellular DAMPs in Plants and Mammals: Immunity, Tissue Damage and Repair. Trends Immunol. 2018; 39(11): 937-950. https://doi.org/10.1016/j.it.2018.09.006
- Govindan S., Kuster D.W., Lin B., Kahn D.J., Jeske W.P., Walenga J.M., Leya F., Hoppensteadt D., Fareed J., Sadayappan S. Increase in cardiac myosin binding protein-C plasma levels is a sensitive and cardiac-specific biomarker of myocardial infarction. Am J Cardiovasc Dis. 2013; 3(2): 60-70. PMCID: PMC3683403
- Marjot J., Liebetrau C., Goodson R.J., Kaier T., Weber E., Heseltine P., Marber M.S. The development and application of a high-sensitivity immunoassay for cardiac myosin-binding protein. Transl Res. 2016; 170: 17-25 e5. https://doi.org/10.1016/j.trsl.2015.11.008
- Tong C.W., Dusio G.F., Govindan S., Johnson D.W., Kidwell D.T., De La Rosa L.M., Rosas P.C., Liu Y., Ebert E., Newell-Rogers M.K., Michel J.B., Trzeciakowski J.P., Sadayappan S. Usefulness of Released Cardiac Myosin Binding Protein-C as a Predictor of Cardiovascular Events. Am J Cardiol. 2017; 120(9): 1501-7. https://doi.org/10.1016/j.amjcard.2017.07.042
- Pfeffer P.L., Pearton D.J. Trophoblast development. Reproduction. 2012; 143: 231-46. doi: 10.1530/REP-11-0374
- Ляпин В.М., Туманова У.Н., Щеголев А.И. Cинцитиальные узелки в ворсинах плаценты при преэклампсии. Современные проблемы науки и образования. 2015; 4. [Lyapin V.M., Tumanova U.N., Schegolev A.I. Syncytial nodules in the villi of the placenta with preeclampsia. Sovremennye problemy obrazovaniya/Modern problems of science and education. 2015; 4.(in Russian)]. URL: http://www.science-education.ru/ru/article/view?id=21421
- Pantham P., Askelund K.J., Chamley L.W. Trophoblast deportation part II: a review of the maternal consequences of trophoblast deportation. Placenta. 2011; 32(10): 724-31. https://doi.org/10.1016/j.placenta.2011.06.019
- Nizyaeva N.V., Kulikova G.V., Nagovitsyna M.N.,Kan, N.E., Prozorovskaya K.N., Shchegolev A.I., Sukhikh G.T. Expression of MicroRNA-146a and MicroRNA-155 in Placental Villi in Early- and Late-Onset Preeclampsia. Bull Exp Biol Med. 2017; 16: 394. https://doi.org/10.1007/s10517-017-3812-0
- Hutabarat M., Wibowo N., Huppertz B. The trophoblast survival capacity in preeclampsia. PLoS One. 2017. 12 (11): e0186909. https://doi.org/10.1371/journal.pone.0186909
Received 25.06.2019
Accepted 04.10.2019
About the Authors
Natalia V. Nizyaeva, PhD., MD, Senior Researcher, Pathology department, National Research Medical Center for Obstetrics, Gynecology, and Perinatology, Ministry of Healthcare of Russia; 4, Ac. Oparin street, Moscow, Russian Federation, 117997; tel.: +7 (926) 248-28-93. E-mail: niziaeva@gmail.com; orcid.org/0000-0001-5592-5690Natalia A. Lomova, PhD, Researcher, Obstetrics Department; National Research Medical Center for Obstetrics, Gynecology, and Perinatology, Ministry of Healthcare
of Russia; 4, Ac. Oparin street, Moscow, Russian Federation, 117997; orcid.org/ 0000-0002-6090-586Х natasha-lomova@yandex.ru
Elrad Yu. Amiraslanov, PhD, MD, Head, Obstetrics Department; National Research Medical Center for Obstetrics, Gynecology, and Perinatology, Ministry of Healthcare
of Russia; 4, Ac. Oparin street, Moscow, Russian Federation, 117997; E-mail: eldis@mail.ru; orcid.org/ 0000-0001-5601-1241
Nagovitsyna Мarina N., Junior Researcher, Pathology department, National Research Medical Center for Obstetrics, Gynecology, and Perinatology, Ministry of Healthcare
of Russia; 4, Ac. Oparin street, Moscow, Russian Federation, 117997; E-mail: moremore84@mail.ru; orcid.org/0000-0001-8039-6217
Natalia V. Savelyaeva, resident, National Research Medical Center for Obstetrics, Gynecology, and Perinatology, Ministry of Healthcare of Russia; 4, Ac. Oparin street, Moscow, Russian Federation, 117997
Roman A. Serov, M.D., Ph.D., Professor, Head, Department of Pathology; A.N. Bakoulev Scientific Center for Cardiovascular Surgery, Ministry of Healthcare of Russia; 135, Roublyevskoe Shosse, Moscow, Russian Federation, 121552; E-mail: seroroman@yandex.ru; orcid.org/0000-0002-7962-7273.
Tatyana V. Sukhacheva, Ph.D., senior researcher, Department of Pathology; A.N. Bakoulev Scientific Center for Cardiovascular Surgery, Ministry of Healthcare of Russia; 135, Roublyevskoe Shosse, Moscow, Russian Federation, 121552, tel.: +7 (903)152-53-23 ORCID: orcid.org/0000-0001-6127-8688
Stanislav V. Pavlovich, Ph.D., MD, Academic Secretary; National Research Medical Center for Obstetrics, Gynecology, and Perinatology, Ministry of Healthcare of Russia; 4, Ac. Oparin street, Moscow, Russian Federation, 117997; E-mail: s_pavlovich@oparina4.ru, tel.: +7-495-438-52-25. orcid.org/0000-0002-1313-7079.
Aleksandr I. Shchegolev, PhD, MD, professor, Head; Pathology department, National Research Medical Center for Obstetrics, Gynecology, and Perinatology, Ministry of Healthcare of Russia; 4, Ac. Oparin street, Moscow, Russian Federation, 117997; E-mail: ashegolev@oparina4.ru; tel.: +7 (495)438-28-92. orcid.org/0000-0002-2111-1530
Natalia E. Kan, Ph.D., M.D., professor, Obstetrics, Gynecology, and Perinatology Department of Professional Training; National Research Medical Center for Obstetrics, Gynecology, and Perinatology, Ministry of Healthcare of Russia; 4, Ac. Oparin street, Moscow, Russian Federation,
117997; E-mail: n_kan@oparina4.ru, tel.: +7 (495)438-20-88. orcid.org/0000-0001-5087-5946.
For citation: Nizyaeva N.V., Amiraslanov E.Yu., Lomova N.A., Pavlovich S.V., Savel’eva N.A., Nagovitsyna M.N., Sukhacheva T.V., Serov R.A., A.I. Shchegolev, Kan N.E. Placental ultrastructural and immunohistochemical changes in preeclampsia with concomitant fetal growth restriction.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2019; 11: 97-106. (In Russian).
https://dx.doi.org/10.18565/aig.2019.11.97-106



