Tuberculosis of respiratory tract and chemotherapy: the influence on the state of vaginal microbiota
Aim. To assess the state of the vaginal microbiota in patients with tuberculosis (TB) of the respiratory tract during the monitoring of chemotherapy (CTH).Kayukova S.I., Ergeshov A.E., Lulueva Zh.S., Bagdasaryan T.R., Donnikov A.E., Shelykalina S.P., Karpina N.L., Evseeva N.I., Uvarova E.V.
Materials and methods. The study “case–control” was conducted in compliance with STROBE recommendations, where the «case» meant the presence of TB of the respiratory tract and CTH; “control” – the absence of TB of the respiratory tract and CTH. 108 women of reproductive age were examined. They were stratified into 2 groups: group 1 – 54 patients with TB of the respiratory tract treated with CTH; group 2 – 54 women without TB of the respiratory tract and CTH. The standard obstetrical-gynecological and phthisiological researches were used; special study of vaginal microbiota was conducted with the use of “Femoflor” test system with a real-time polymerase chain reaction and quantitative characteristics of 28 conditionally pathogenic microorganisms. Statistical data analysis was performed using scripts in the R programming language version 3.5 in the R Studio environment.
Results. With prolongation of therapy (for 60 and 150 days after CTH), a statistically significant decrease in Lactobacillus spp. – 6 [5,7; 6,6], 0 [0; 4] и 0 [0; 3] (р≤0,0001), a gradual increase in the majority of associates of conditionally pathogenic microflora (the most indicative were Gardnerella vaginalis and Candida spp.) were noted in microbiota. The microscopic characteristic of the vaginal biotope was the presence of normocenosis prior to CXT in the vast majority in group 1 – 47 (87%). 60 and 150 days after CXT, there was absence of normocenosis –
0 (0%), and statistically significant increase in the incidence of dysbiosis and an inflammatory bowel disease was 29 (54%) (р≤0,0001) and 12 (22%) (р=0,02); 25 (46%) (р≤0,0001) and 29 (54%).
Conclusion. The presence of 2 aggressive factors – TB of the respiratory tract and CXT damage the vaginal biotope resulting in formation of aerobic-anaerobic dysbiosis. No statistically significant relationship was observed between the development of vaginal dysbiosis and the clinical form of TB of the respiratory tract, the severity of the specific process and the selected chemotherapy regimen. Further studies in this direction should be continued.
Keywords
It is known that infectious diseases, long-term use of antibiotic therapy, hormonal imbalance, and stress are associated with a high probability of dysbiosis of the mucous membrane of the genital tract [1]. One of such infections is tuberculosis, and long-term chemotherapy (CT) may cause adverse effects in the female reproductive system [2–4]. In recent decades, thanks to the widespread introduction of the molecular genetic research methods: genome sequencing, polymerase chain reaction (PCR), DNA hybridization, much evidence has been obtained about the composition and functioning of the vaginal microbiota in women of reproductive age. These methods allowed overcoming the limitations of the «classical» bacteriologic, investigation which for many years was the «gold standard» in the study of human microbiota [5, 6]. No research of vaginal microbiota in women of reproductive age under the impact of two aggressive factors: active tuberculosis infection and prolonged CT has been carried out so far. Currently, the use of innovative diagnostic test systems for quantitative assessment of the vaginal microbiota in this category of patients is an urgent task of modern phthisiology and gynecology.
Materials and methods
The study «case – control» of 108 women of reproductive ages was conducted in the Central Research Institute of Tuberculosis in compliance with STROBE recommendations, where «case» meant the presence of TB of the respiratory tract and CT; «control» – the absence of TB of the respiratory tract and CT. The patients were stratified into 2 groups: group 1 – 54 patients with TB of the respiratory tract treated with CT; group 2 – 54 women without TB of the respiratory tract and CT (Fig. 1). The planned sample size was calculated using the Altman normogram.
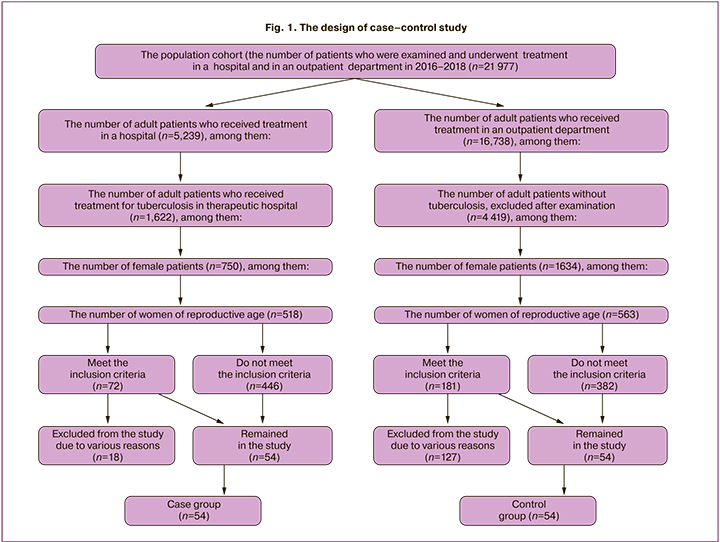
The inclusion criteria in the main group were: reproductive age, primarily detected TB of respiratory tract, application of intensive CT in the hospital; absence of severe somatic diseases; absence of sexually transmitted diseases (STDs) and pathological conditions of the uterine cervix, the patients’ informed consent to participate in the study. The inclusion criteria in the control group were: reproductive age, absence of TB of the respiratory tract and severe somatic diseases; absence of STDs and pathological conditions of the cervix, the patients’ voluntary informed consent to participate in the study. The study was approved by the Ethic Committee of the Central Research Institute of Tuberculosis (protocol № 11 of 21.05.2017). The patients underwent standard phthisiatric and gynecologic examination; the specialized study of vaginal microbiota was carried out using the «Femoflor» test system with PCR – the detection of the results in real time and quantitative characteristics of 28 conditionally pathogenic microorganisms. The vaginal microbiota in the main group was studied at time points – before CT, and after 60 and 150 days of CT; in the control group – only once. Vaginal microbiocenosis was assessed using the classification of Voroshilina E.S. et al. (2018) [1, 5]. The microscopic characteristics of the vaginal biotope were assessed using E.F. Kira's classification, 2019 [7].
Statistical analysis
The statistical data analysis was performed using the scripts in the R programming language version 3.5 in the R Studio environment. The descriptive statistics for qualitative features were shown in the form of absolute and relative frequencies, for the quantitative features – in the form of the median and upper and lower quartiles. Comparison of the quantitative parameters in independent samples was performed using Mann– Whitney U test; the qualitative features – using Fisher’s exact test. The critical level of significance was p = 0.05. To compare the values of the parameters in related samples (before and 60, 150 days after CT), the Friedman test with a critical significance level 0.05 was used; when analyzing paired differences, the Wilcoxon test was used with a critical significance level 0.025, taking into account the Bonferroni correction.
Results and discussion
In both study groups, the women of active reproductive age prevailed – 44 (81%) and 42 (78%); with a median age – 29.5 [26.3; 36.8] and 32.0 [26.5; 33.0] years respectively. The evaluation of the gynecological history in both groups showed the onset of menarche at 13 [12; 14] and 13 [12; 13] years; the average length of menstrual cycle was 28 [22.3; 28] and 29 [28; 30] days; the duration of menstrual bleeding – 5 [5; 5.8] and 5 [5; 6] days; regular menses were noted in 46 (85%) and 48 (89%) women respectively. The age of sexual debut among examined patients was 18 [16.3; 19.3] and 19 [18; 20] years. 31 (57%) women of group 1 and 48 (89%) of group 2 had pregnancies in the past (р≤0,001). The pregnancies ended with births in 27 (87%) and 47 (98%) women, with medical abortions – in 10 (32%) and 9 (19%), with miscarriage in 8 (26%) and 13 (27%) women respectively. 30 (56%) and 17 (31%) patients of groups 1 and 2, respectively (р = 0.019), had a past history of some gynecologic disease: chronic salpingitis in 15 (50%) and 7 (41%) , uterine myoma – in 6 (20%) and 4 (26%), ovarian cyst – in 6 (20%) and 6 (35%), endometriosis – in 4 (13%) and 6 (35%) women.
Among the patients with TB of the respiratory tract, poor social status (lack of permanent work, financial instability and inability to afford housing) was noted in 39 (72%) and 25 (46%) women (р = 0.011). A severe somatic disease was recorded in 28 (52%) patients in group 1 and 15 (28%) patients in group 2 (p = 0.018).
The severity of TB of the respiratory tract was defined using the important indicators: destruction of lung tissue in 23 (43%) and bacterial discharge in 36 (67%) patients (p≤0.01). Among the latter, the resistance of bacteria to anti-tuberculosis drugs was determined in 25 (69%) women (p≤0.02); multidrug resistance – in 17 (47%); broad drug resistance in 8 (22%). Various chemotherapy regimens were used in patients with TB of the respiratory tract in group 1: regimen 1 – to 14 (26%) patients, regimen II – 1 (2%), regimen III – 0 (0%), regimen IV – 18 (33%), regimen V – 7 (13%) and individual regimen – 14 (26%).
The analysis of the vaginal microbiota was performed (Table).
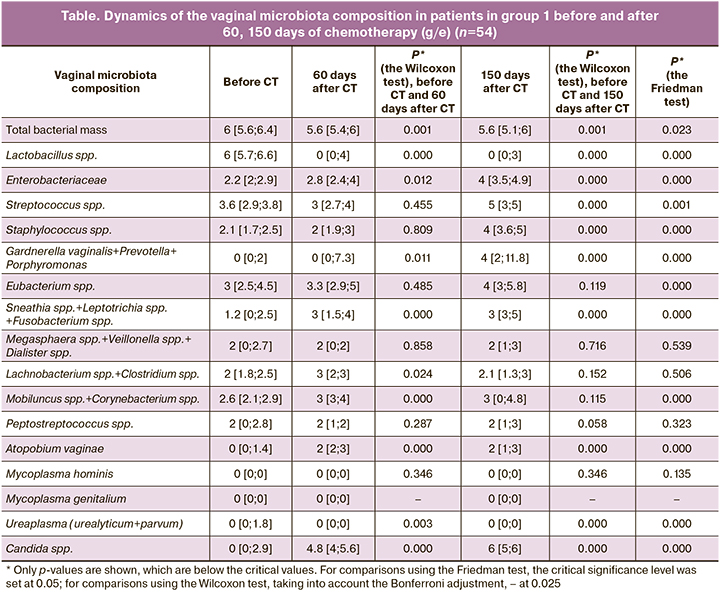
As can be seen from the Table, in patients with TB of the respiratory tract before chemotherapy, vaginal microbiocenosis was characterized by the prevalence of Lactobacillus spp. – 6 [5.7; 6.6]. Among the microorganisms that form conditionally pathogenic microflora, low values of Enterobacteriaceae – 2.2 [2; 2.9], Staphylococcus spp. – 2,1 [1,7; 2,5], Sneathia spp.+Leptotrichia spp. +Fusobacterium spp. – 1,2 [0; 2.5], Megasphaera spp.+Veillonella spp.+Dialister spp. – 2 [0; 2.7], Lachnobacterium spp.+Clostridium spp. – 2 [1.8; 2.5], Mobiluncus spp.+Corynebacterium spp. – 2.6 [2.1; 2.9], Peptostreptococcus spp. – 2 [0; 2.8] were noted, as well as the absence of Gardnerella vaginalis – 0 [0; 2], Atopobium vaginae – 0 [0; 1.4], Mycорlasma hominis – 0 [0; 0]; Mycорlasma genitalium – 0 [0; 0]; Ureaplasma (urealyticum+parvum) – 0 [0; 1.8] and Candida spp. – 0 [0; 2.9]. With the increase of the treatment duration (60 and 150 days of CT), a statistically significant decrease in Lactobacillus spp. – 0 [0; 4] and 0 [0; 3] (р≤0,0001), and a gradual increase in the majority of conditionally pathogenic microflora associates were noted. According to the used criteria of vaginal microbiocenosis classification [1, 5], the state of the microbiota among the patients in group 1 was categorized before chemotherapy as absolute normocenosis with subsequent transition to moderate dysbiosis after 60 and 150 days of anti-tuberculosis therapy. The study of the vaginal microbiota in all women in the control group showed absolute normocenosis in 54 (100%) patients, the content of Lactobacillus spp. was 6 [5,2; 6,9].
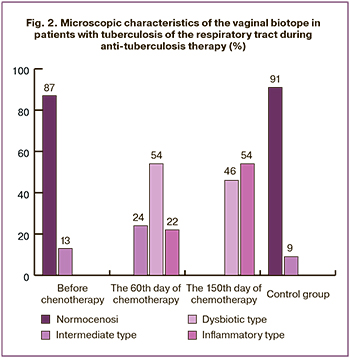 Examination of the microscopic characteristics of the vaginal biotope before chemotherapy in group 1 detected normocenosis and the intermediate type of smear in 47 (87%) and in 7 (13%) patients. After 60 days of CT normocenosis was no more present in any patient (р≤0,0001); the intermediate type was found in 13 (24%) patients (р=0,02), dysbiotic type – in 29 (54%) (р≤0,0001), inflammatory type – in 12 (22%) (р=0,02). 150 days after CT normocenosis remained undetected – 0 (0%) (р≤0,0001), the intermediate type was absent – 0 (0%) (р≤0,0001), while dysbiotic and inflammatory types were documented in 25 (46%) (р≤0,0001) and 29 (54%) patients. The study of the results of vaginal smear microscopy in the control group showed the presence of normocenosis and the intermediate type in 60 (91%) and 6 (9%) women respectively, the absence of dysbiotic and inflammatory types in no patient (0%) (Fig. 2).
Examination of the microscopic characteristics of the vaginal biotope before chemotherapy in group 1 detected normocenosis and the intermediate type of smear in 47 (87%) and in 7 (13%) patients. After 60 days of CT normocenosis was no more present in any patient (р≤0,0001); the intermediate type was found in 13 (24%) patients (р=0,02), dysbiotic type – in 29 (54%) (р≤0,0001), inflammatory type – in 12 (22%) (р=0,02). 150 days after CT normocenosis remained undetected – 0 (0%) (р≤0,0001), the intermediate type was absent – 0 (0%) (р≤0,0001), while dysbiotic and inflammatory types were documented in 25 (46%) (р≤0,0001) and 29 (54%) patients. The study of the results of vaginal smear microscopy in the control group showed the presence of normocenosis and the intermediate type in 60 (91%) and 6 (9%) women respectively, the absence of dysbiotic and inflammatory types in no patient (0%) (Fig. 2).
It is known, that anti-tuberculosis drugs suppressing the vital activity of tuberculosis mycobacteria, cause adverse effects in a human body and to the specific microecosystems. Adverse reactions to the use of anti-tuberculosis drugs most commonly depends on a number of factors: type of a drug, administration way, the treatment regimen, combination with other drugs, the functional state of organs and systems, the nature of individual reactivity, and the allergy history [8].
Conclusion
The study showed that in the presence of two active aggressive factors – TB of the respiratory tract and chemotherapy in women of reproductive age, the state of the vaginal biotope is disturbed: the proportion of Lactobacillus spp. gradually decrease and the number of the majority of pathogenic microflora associates increase, resulting in formation of aerobic-anaerobic dysbiosis. No statistically significant relationship was found between the development of vaginal dysbiosis and the clinical form of TB of the respiratory tract, the severity of the specific process and the selected hemotherapy regimen. It seems that the vaginal microbiota, like any other biotope in the human body, is regulated by individual genetic and immunological defense mechanisms. Therefore, further studies in this direction should be conducted.
References
- Ворошилина Е.С., Зорников Д.Л., Плотко Е.Э. Нормальное состояние микробиоценоза влагалища: оценка с субъективной, экспертной и лабораторной точек зрения. Вестник Российского государственного медицинского университета. 2017; 2: 42-6. [Voroshilina E.S., Zornikov D.L., Plotko E.E. Normal condition of vaginal microbiocenosis: assessment from a subjective, expert and laboratory point of view. Bulletin of the Russian State Medical University. 2017; 2: 42-6. (in Russian)].
- Efared B., Sidibé I.S., Erregad F., Hammas N., Chbani L., Fatemi H. E. Female genital tuberculosis: a clinicopathological report of 13 cases. J. Surg. Case Rep. 2019; 2019(3): rjz083.10.1093/jscr/rjz083.
- Sharma B.J., Sneha J., Singh U.B., Kumar S., Kumar R. K, Singh N. et al. Effect of antitubercular therapy on endometrial function in infertile women with female genital tuberculosis. Infect. Disord. Drug Targets. 2016; 16(2): 101-8.
- Yang T.W., Park H.O., Jang H.N., Yang J.H., Kim S.H., Moon S.H., et al. Side effects associated with the treatment of multidrug-resistant tuberculosis at a tuberculosis referral hospital in South Korea. Medicine(Baltimore). 2017; 96(28): e7482. 10.1097/MD.0000000000007482.
- Ворошилина Е.С., Плотко Е.Э., Исламиди Д.К., Лаврентьева И.В., Зорников Д.Л. Микробиоценоз влагалища с точки зрения ПЦР в реальном времени. Возможности коррекции дисбиотических нарушений влагалища. Учебное пособие. Екатеринбург; 2018. 71 с. [Voroshilina E.S., Plotko E.E. Islamidi D.K., Lavrentiev I.V., Zornikov D.L. Vaginal microbiocnosis from the point of view of real-time PCR. Possibilities for correcting dysbiotic disorders of the vagina. Tutorial. Yekaterinburg. 2018. 71 p. (in Russian)].
- Cartwright C.P., Pherson A.J., Harris A.B., Clancey M.S., Nye M.B. Multicenter study establishing the clinical validity of a nucleic-acid amplification-based assay for the diagnosis of bacterial vaginosis. Diagn. Microbiol. Infect. Dis. 2018; 92(3): 173-8. 10.1016/j.diagmicrobio.2018.05.022.
- Клинические рекомендации по диагностике и лечению заболеваний, сопровождающихся патологическими выделениями из половых путей женщин. М.: Российское общество акушеров-гинекологов; 2019. 57 с. [Clinical recommendations on diagnosis and treatment of diseases accompanied by pathological vaginal discharge in women. Russian Society of Obstetricians and Gynecologists. Moscow. 2019. 57p. (in Russian)].
- Вольф С.Б. Нежелательные побочные реакции на химиотерапию туберкулеза. Журнал Гродненского государственного медицинского университета. 2016; 3: 141-6. [Wolf S.B. Undesirable adverse reactions to chemotherapy of tuberculosis. Journal of Grodno State Medical University. 2016; 3: 141-6. (in Russian)].
Received 06.05.2020
Accepted 03.07.2020
About the Authors
Svetlana I. Kayukova, PhD in Medical Sciences, Senior Researcher, Central Research Institute of Tuberculosis.Tel.: +7(915)396-85-34. E-mail: kajukovalnp@gmail.com. ORCID: 0000-0002-5233-3515. 107564, Russia, Moscow, Yauzskaya alley, 2.
Atadjan E. Ergeshov, M.D., Professor, Director of Central Research Institute of Tuberculosis.
Tel.: +7(909)628-45-30. E-mail: v.kirilina@ctri.ru. ORCID: 0000-0002-2494-9275. Russia, Moscow, Yauzskaya alley, 2.
Zhanna S. Lulueva, graduate student, Central Research Institute of Tuberculosis. Tel.: +7(916)663-66-81. E-mail: zhanna_Lulueva@mail.ru.
ORCID: 0000-0002-5549-2466. 107564, Russia, Moscow, Yauzskaya alley, 2.
Tatevik R. Bagdasaryan, PhD in Medical Sciences, Head of the 1st Therapeutic department, Central Research Institute of Tuberculosis. Tel.: +7(967)182-90-04.
E-mail: tatev0812@mail.ru. ORCID: 0000-0001-9910-1570. 107564, Russia, Moscow, Yauzskaya alley, 2.
Andrey E. Donnikov, PhD in Medical Sciences, Head of the Laboratory of Molecular Genetic Methods, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Health of Russia.
Tel.: +7(903)684-52-47. E-mail: donnikov@dna-technology.ru. ORCID: 0000-0003-3504-2406. 117997, Russia, Moscow, Academician Oparin str., 4.
Svetlana P. Shelikalina, PhD in Medical Sciences, Associate Professor of the Department of Medical Cybernetics and Informatics, Russian National Research Medical University named after N.I. Pirogov of the Ministry of Health of Russia. Tel.: +7(905)771-46-48. E-mail: svetlanath@gmail.com. ORCID: 0000-0003-3292-8949.
117997, Russia, Moscow, Ostrovityanova str., 1.
Natalya L. Karpina, M.D., Professor, Head of the Center for Diagnosis and Rehabilitation of Respiratory Diseases, Central Research Institute of Tuberculosis.
Tel.: +7(916)097-36-96. E-mail: natalya-karpina@rambler.ru. ORCID: 0000-0001-7800-8158. 107564, Russia, Moscow, Yauzskaya alley, 2.
Natalya I. Evseeva, junior researcher, Clinical and Diagnostic Department, Central Research Institute of Tuberculosis.
Tel.: +7(926)324-44-10. E-mail: leopardic@inbox.ru. ORCID: 0000-0003-4055-4588. 107564, Russia, Moscow, Yauzskaya alley, 2.
Elena V. Uvarova, M.D., Professor, Corresponding Member of the RAS, Head of the Department of Pediatric and Adolescent Gynecology (2nd Gynecological Department), National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Health of Russia.
Tel.: +7(916)148-04-76. E-mail: elena-uvarova@yandex.ru. ORCID: 0000-0001-9369-0837. 117997, Russia, Moscow, Academician Oparin str., 4.
For citation: Kayukova S.I., Ergeshov A.E., Lulueva Zh.S., Bagdasaryan T.R., Donnikov A.E., Shelykalina S.P., Karpina N.L., Evseeva N.I., Uvarova E.V. Tuberculosis of respiratory tract and chemotherapy: the influence on the state of vaginal microbiota.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 8: 120-125 (in Russian).
https://dx.doi.org/10.18565/aig.2020.8.120-125



