Comparative analysis of the reliability of data on the molecular karyotype of the embryo obtained by whole-genome amplification methods based on NGS technology of trophectoderm of individual cells
Glotov O.S., Saifitdinova A.F., Pavlova O.A., Leonteva O.A., Poliakova I.V., Maslennikov A.B.
Aneuploidies in human embryos play a significant role in the development of implantation failures and early reproductive losses in assisted reproductive technology (ART) programs. Preimplantation genetic testing for aneuploidy (PGT-A) is the most commonly used method for assessing embryo ploidy. The introduction of PGT-A into clinical practice has greatly reduced the chances of transferring aneuploid embryos to the uterus and has increased the effectiveness of ART programs.
Different PGT-A methods and sampling techniques have advantages and disadvantages, and there are various reasons for potential false-positive and false-negative PGT-A results. Since only a few cells are used for the study, the uniformity of DNA amplification is crucial for obtaining reliable analysis results. Therefore, the most important stage of PGT-A is whole-genome amplification (WGA).
This article presents an analysis of the current literature regarding the effectiveness and reliability of methods for amplifying small quantities of DNA. The results of our study, which compares different methods of whole-genome amplification of individual trophectoderm cells, are presented in order to obtain reliable data on the molecular karyotype of embryos using NGS. The effectiveness of using domestic WGA kits has been demonstrated.
Conclusion: Comparing PGT-A results using various kits has shown that the "hybrid" protocol we developed, which allows for the use of both foreign and domestic WGA kits, is applicable. This approach has demonstrated the high reliability of WGA kits with SD polymerase for preparing materials for PGT of human embryos, and can also be applied in other areas of biomedicine and forensic research where high reliability and accuracy in amplifying extremely small quantities of DNA is required.
Authors' contributions: Leonteva O.A. – cultivation of embryos, biopsy of trophectoderm cells; Glotov O.S., Saifitdinova A.F., Pavlova O.A., Poliakova I.V. – sample preparation, whole genome amplification, NGS; Glotov O.S., Saifitdinova A.F. – data analysis; Glotov O.S., Saifitdinova A.F., Maslennikov A.B. – manuscript drafting and editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Gratitude: The authors express gratitude to Puppo (Trofimova) I.L., researcher at the V.A. Almazov National Medical Research Center, for initiating the work on collecting embryos for studies.
Ethical Approval: The collection of material for the study was performed on the basis of the donors' informed consent approved by the local ethics committee of the Almazov National Medical Research Center (No. 02-21 of February 15, 2021).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Glotov O.S., Saifitdinova A.F., Pavlova O.A., Leonteva O.A., Poliakova I.V., Maslennikov A.B. Comparative analysis of the reliability of data on the molecular karyotype of the embryo obtained by whole-genome amplification methods based on NGS technology of trophectoderm of individual cells.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (6): 66-74 (in Russian)
https://dx.doi.org/10.18565/aig.2024.78
Keywords
Aneuploidies in human embryos significantly contribute to implantation failure and early reproductive losses in assisted reproductive technology (ART) programs. To reduce the risk of transferring an embryo with a chromosomal abnormality into the uterine cavity, preimplantation genetic testing for aneuploidy (PGT-A) is performed. The implementation of PGT-A in clinical practice has improved the effectiveness of ART programs; however, current PGT-A methods have several limitations [1]. The reasons for false-positive and false-negative PGT-A results, and consequently, the incorrect assessment of an embryo’s karyotype, include errors during preliminary embryo manipulations, methods of material collection, and direct molecular genetic studies of the biopsy specimen. The most common problems are contamination of the biopsy material and uneven whole-genome DNA amplification (WGA) [2].
On average, contamination occurs in approximately 0.4% of biopsy specimens, but this rate can be significantly higher in some clinics [2]. Therefore, constant monitoring of possible contamination is crucial to avoid misdiagnoses.
Until recently, most commercially available platforms for PGT-A used whole-genome amplification followed by sequencing random DNA fragments throughout the genome using next-generation sequencing (NGS) technology. However, simple quantitative measurements of DNA fragments from each chromosome provided by these methods cannot detect whether a biopsy sample is contaminated with nonfetal DNA. Negative controls are rarely used during PGT-A, and are inadequate because they do not assess contamination in tubes containing biopsy specimens.
To determine the incidence of contamination, a retrospective study analyzed 49,287 trophectoderm biopsies subjected to PGT-A. Embryos that were found to have contaminated biopsies were usually rebiopsied. In such cases, the results of the two samples were compared to determine whether the original result of the contaminated sample would have been erroneous if only the relative chromosome copy number had been examined, as is typical for most NGS-based PGT-A methods. Single nucleotide polymorphism (SNP) analysis of genome-wide amplification products was used to identify the true origin of data from the samples. The genotype of each SNP and the relative number of DNA fragments containing each allele allowed for the detection of triploidy, haploidy, and contamination. Of the 49,287 biopsies analyzed, non-fetal DNA contamination was detected in 218 (0.44%). Across the 25 clinics that provided samples, contamination rates varied from 0 to 1.5%, with one clinic having a contamination rate of 7.7% (26 biopsies) [2].
The results were divided into three categories: 1) no change in interpretation between the first (contaminated) and second biopsy samples; 2) false positive–a contaminated sample would have been euploid but incorrectly interpreted as triploid, leading to its unnecessary discard and potentially affecting ART effectiveness; and 3) false negative–the contaminated sample was completely aneuploid but incorrectly classified as mosaic or euploid, making it suitable for transfer and potentially resulting in implantation failure or abnormal pregnancy. A false-negative result was obtained in 19% of the contaminated samples, while a false-positive result simulating triploidy was obtained in 24% [2].
The authors highlight the limitations and challenges of their research. It is impossible to confidently determine the origin of contaminants without DNA from the source for comparison. Additionally, it is not possible to determine whether contamination is more likely during in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) cycles, as only 3% of the samples were fertilized using IVF [2].
Thus, detecting contamination during PGT-A is crucial to prevent errors in embryo karyotyping. Missed contamination and incorrect karyotype assessment may lead to the discarding of potentially viable embryos or the transfer of aneuploid embryos misclassified as mosaic, thereby increasing the incidence of implantation failure, miscarriage, and aneuploid pregnancies.
A second equally important issue that can lead to misinterpretation of PGT-A aneuploidy results is Whole Genome Amplification (WGA). Problems leading to inaccurate reproducibility of WGA include preferential amplification and allele dropout.
Genome-wide DNA amplification methods have been developed for genomic research projects since the late 20th century. These methods were primarily aimed at obtaining reliable means of scaling small quantities of DNA without labor-intensive and expensive cloning methods, which neither guaranteed accuracy nor prevented the loss of hereditary information. However, the limited capabilities of early genetic engineering allowed the use of only the large subunit of DNA polymerase I from Escherichia coli (Klenow fragment) to amplify DNA in the presence of random hexanucleotide primers. This method can only double the amount of original DNA by replicating the second strand from the sequences of denatured double-stranded DNA3]. This was insufficient to amplify extremely small amounts of DNA from the individual cells. Real progress in scaling the original sample came with the development of a polymerase chain reaction (PCR) method based on thermostable DNA polymerase [4].
In 1992, a method for whole-genome amplification was developed using PCR with degenerate primers (Degenerate Oligonucleotide Primed, DOP-PCR). This method employed primers with internal sequences consisting of six random nucleotides: 5'-CCGACTCGAGNNNNNNATGTGG-3'[5]. The amplification protocol began with cycles of low-temperature annealing and ramping, resulting in DNA fragments flanked by an adapter sequence that could be further amplified using a standard PCR protocol. However, the authors of this method did not achieve uniform DNA amplification because of the limited understanding of metazoan genome organization and coding sequence proportions [5]. Further developments in DOP-PCR involved modifying primer sequences and optimizing low-temperature cycle conditions. These improvements have formed the basis for several commercial WGA kits, although none have achieved complete and uniform DNA amplification across different sections and entire chromosomes of individual cells [5].
A breakthrough occurred in the discovery of polymerases with helicase activity, which enabled the amplification of hairpin-enriched regions and the development of isothermal amplification methods. The large subunit of DNA polymerase I Bst from the thermophilic bacterium Geobacillus stearothermophilus (previously known as Bacillus stearothermophilus until 2001) has a higher optimal temperature for synthetic activity, lacks 3'-5' exonuclease activity, and can displace the second strand from the duplex and continue synthesis [6]. Its use in WGA led to the development of multiple strand displacement amplification (MDA) methods, which increased product yield in one round of synthesis owing to overlapping fragments [7]. However, this amplification method is limited by fragment length and enzyme processivity, as well as error accumulation [7].
Previous attempts at MDA-based WGA have used the Phi29 phage polymerase, which is known for its significantly higher speed and accuracy [8]. This polymerase forms the basis of commercial kits such as REPLI-g (Qiagen) and has been successfully used to amplify small amounts of DNA during comparative genomic hybridization [9]. Despite these advances, this method has not been widely adopted in clinical practice due to its inability to achieve uniform amplification. In addition, DNA fragmentation (the next step of PGT-A after WGA) is a significant obstacle for MDA-based methods, making PCR-based methods preferable [10].
The use of hexanucleotide random primers, due to the low-temperature optimum of the enzyme (30°C) isolated from the bacterium Bacillus subtilis phage, and the uncontrolled branching of the product, complicates the procedure for DNA linearization and standardization of subsequent library preparation for NGS. A significant breakthrough in WGA method development was the combination of MDA and DOP-PCR protocols, which led to the creation of the MALBAC protocol [11]. This protocol was later standardized by Rubicon Genomics (now part of Takara) and used under license as part of the SurePLEX DNA Amplification System Kit (Illumina). Commercial WGA kits for extremely small quantities of DNA from individual cells feature an invariant part of primers based on a combination of two nucleotides (G and T) and the inclusion of six variable (random) nucleotides in the sequence. The first step uses polymerase with helicase activity, whereas the second step employs PCR.
Ignatov K.B. et al. reported improvements in DNA amplification from single cells using SD DNA polymerase, a mutant of Taq DNA polymerase with strong strand displacement activity (unlike Taq polymerase) and high thermal stability. Both these properties of SD DNA polymerase, combined with high polymerase activity, provide a marked improvement in the sensitivity and efficiency of PCR (including amplification of GC-rich templates and complex secondary structures), long-range PCR (LR-PCR), loop amplification (LAMP), and polymerase chain displacement reaction (PCDR) [12]. In a more recent study, the authors described a new variant of DOP-PCR with improved WGA performance, which was achieved using SD polymerase. They also compared improved DOP-PCR (iDOP-PCR) with «classical» DOP-PCR [5] using the commercially available PicoPlex technique from Rubicon Genomics Inc. (Michigan, USA), which is currently the predominant method used for preimplantation genetic diagnosis (PGD) and other medical applications [13].
Our previous study proposed the use of two-step whole-genome amplification to increase the reliability of the molecular karyotype data of the original sample, based on the analysis of 20 pg of DNA. In the first step of flanking DNA fragments and second-strand displacement amplification, a pair of partially degenerate primers was used: 5’-TGTGTTGGGTGTGTTTGGNNNNNNGG and 5’-TGTGTGTTGGGTGTGTTTGGNNNNNNTTT. In the second stage of the polymerase chain reaction, we used a primer based on the constitutive part 5’-TGTGTTGGGTGTGTTTGG. This study provides reaction conditions and a comparison of the use of various polymerases, as well as their combinations. The feasibility of using a combination of Bst and Pfu polymerases in the presence of 10 mM magnesium ions for the first stage was demonstrated, and the potential for using Klentaq1 polymerase, which carries the D732N substitution, for the development of whole-genome amplification methods was revealed. It has been demonstrated that the proposed method allows scaling up an initial extremely small amount of DNA to obtain a sample suitable for analysis by massively parallel sequencing (next generation sequencing, NGS) using standard commercial data interpretation protocols [14].
Another approach was demonstrated by the Russian manufacturer of PGT-A kits, Biolink LLC, with its SC WGA Display kit (Biolink, Novosibirsk), which offers the use of SD polymerase, which can withstand heating up to 92 °C, making it possible to perform both stages of amplification with one enzyme in one tube, further reducing the risk of sample contamination.
All approaches to whole-genome DNA amplification have their advantages and disadvantages, so the choice of a particular method depends on its application. In our case, a reliable, stable, and fail-safe method was required to perform PGT-A. Other considerations when deciding which method to use for WGA include the protocol length, cost, and automation potential.
The objective of this study was to perform a comparative analysis of methods for whole-genome DNA amplification from trophectoderm biopsy cells of embryos on the 5th day of development in order to obtain reliable data on the molecular karyotype of the embryo by NGS using the commercial reagents Sureplex (Illumina, USA) and PGT Display (Biolink, Novosibirsk) = iDOP-PCR (Bioron, Germany).
Materials and methods
The study used human embryos obtained by fertilization of oocytes from donors aged 20–32 years. Oocytes were obtained on the basis of individual voluntary consent, developed in part for research purposes, and fertilized with sperm from donors in the sperm bank of the International Center for Reproductive Medicine (ICRM). Embryos were cultured in COOK media until day 5–6 of development, according to a previously published protocol [15]. For excellent and good quality blastocysts, trophectoderm cell biopsy was performed using a laser. Aneuploid embryos (as determined by PGT-A) were thawed and a second biopsy was performed as previously described [16]. Trophectoderm cell samples obtained from the seven biopsies were used for further research. As a positive control, we used diluted male standard DNA (Agilent Technologies, USA) to obtain 20 pg of DNA in one reaction (which is the expected amount of DNA from trophectoderm biopsy cells). Whole-genome amplification and library preparation were performed using VeriSeq PGS (Illumina, USA) and PGT Display kits (Biolink, Novosibirsk). DNA concentration in the samples after WGA was measured on a Qubit 4 fluorimeter (Thermo Fisher Scientific, USA) using the intercalating dye Pico488 (Lumiprobe, Moscow). To compare the amplification results, we used commercial kits of reagents for single-cell DNA amplification SurePLEX DNA Amplification System (Illumina, USA) and SC WGA Display (Biolink, Novosibirsk) in different combinations. Libraries were prepared using VeriSeq PGS (Illumina, USA) and PGT Display (Biolink, Novosibirsk) kits, followed by sequencing (NGS) on a MiSeq system (Illumina, USA). Library size and quality were controlled using a High Sensitivity DNA D5000 ScreenType with a Type Station System (Agilent Technologies, USA). Analysis of the quality of determination of the molecular karyotype of the original sample was performed using BluFuse Multi v4.3 (Illumina, USA) and Display (Biolink, Novosibirsk) software.
Results and discussion
In this study, we conducted a comparative analysis of methods for whole-genome DNA amplification from trophectoderm biopsy cells of embryos on the 5th day of development to assess the reliability of data obtained from the molecular karyotype of the embryo by NGS using the commercial reagents Sureplex (Illumina, USA) and PGT Display (Biolink, Novosibirsk).
Because of the complexity of using many patented imported methods and kits in the Russian Federation, the main goal of this study was to conduct a comparative analysis of the kit for whole-genome amplification available in our country with the «reference» kit, as well as to analyze the possibility of using various programs for data interpretation.
To test the optimal conditions for conducting WGA, in the first stage, we analyzed the resulting WGA products using the High Sensitivity DNA D5000 ScreenType with the Type Station System (Fig. 1).
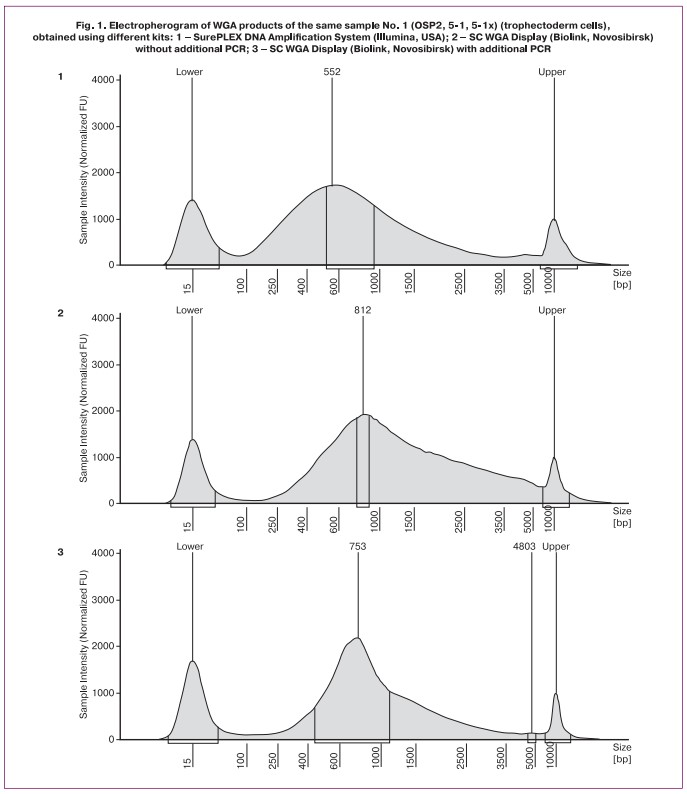
As shown in Figure 1, the WGA size is slightly larger for the SC WGA Display than for the SurePLEX DNA Amplification System. The additional PCR step slightly reduced the size of the WGA product, but the reduction was not significant. Thus, the obtained results of «similar length» fragments allowed for the continued use of various combinations of sample preparation protocols.
In the second phase of the study, we examined seven embryos using different «combinations» of kits and programs for Sureplex (Illumina, USA) and PGT Display (Biolink, Novosibirsk) (Fig. 2). We used Nextera XT (Illumina, USA) as the main protocol for library preparation. The libraries and further software analyses are shown in Figure 2.
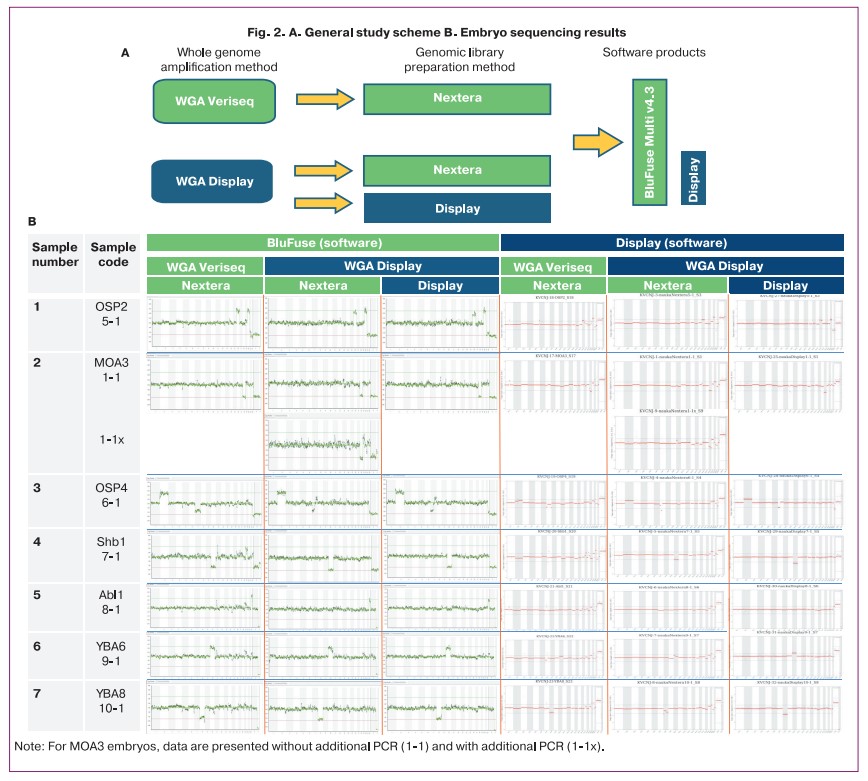
All genomic libraries were sequenced in one run using the MiSeq System (Illumina, USA) to eliminate the influence of different conditions and sequencing reagents.
As shown in Figure 2, similar results were obtained for most of the samples. Thus, the karyotypes coincided for all samples, except for sample No. 3. In this sample, a rearrangement was detected – monosomy 7p22.3-p11.2 using the Illumina protocol. However, when using the combination WGA Display + Nextera (Display), partial trisomy 7q31.1-q36.3 was detected, which is more clearly visible when processing sequencing data using BluFuse Multi v4.3 (Illumina, USA). Perhaps, these features are associated with the nuances of the WGA process itself or the presence of mosaicism and mitotic error. A similar mechanism has been described in [17]. However, due to the lack of rebiopsy material, it was not possible to verify this using FISH.
In general, the data for all samples showed that the additional PCR step (reamplification) recommended by Biolink in some cases (little DNA) produces more «noise» (total noise 0.35 versus 0.47 for sample 6); therefore, in our opinion, it can only be used with an initially low concentration of WGA product. The data for the remaining samples were similar (not shown in the article).
It should be noted that the BluFuse Multi v4.3 program «reads» data obtained using different sequencing protocols quite well. The display program is more compatible with «native’ reagents. The domestic protocol turned out to be longer than the foreign analog (by two working hours), which, however, is not critical. The results of NGS (different sets) on embryo biopsy samples for which aneuploidies have already been identified showed the possibility of interchangeability/compatibility of protocols, which is an important and positive point. At the same time, the «combined» protocols were practically not inferior to those recommended by the manufacturers.
The use of «combined» protocols in the study made it possible to select conditions for obtaining an amplifier, the sequencing results of which can be analyzed using common commercial algorithms and software to identify numerical chromosomal abnormalities. These methods can be implemented in the preparation of materials for the analysis of the molecular karyotype of individual cells, including the PGT of human embryos and the study of tumor cells, and may also be useful in the fields of biomedicine [18, 19] and forensic research, where uniform amplification of extremely small amounts of DNA is required.
Conclusion
We demonstrated the effectiveness of a combined DNA amplification method using partially degenerate primers, isothermal amplification with second-strand displacement, and a two-step PCR protocol. This method, using the WGA display reagent kit from Biolink LLC, Novosibirsk, has shown potential for further sequencing using NGS. Both the proprietary reagents of the manufacturer’s SurePLEX DNA Amplification System (Illumina) and the reagents and SC WGA Display (Biolink) can be used.
In addition, our «hybrid» protocol, which allows the use of both foreign and domestic whole-genome amplification kits, yielded comparable results in PGT-A. This approach has demonstrated the reliability of WGA kits with SD polymerase in preparing materials for the PGT of human embryos. In addition, it has potential applications in other areas of biomedical and forensic research, where highly reliable and accurate amplification of minute amounts of DNA is required.
References
- Малышева О.В., Бичевая Н.К., Гзгзян А.М., Глотов О.С., Кинунен А.А., Лобенская А.Ю., Мекина И.Д., Полякова И.В., Пуппо И.Л., Сайфитдинова А.Ф., Щербак С.Г., Коган И.Ю. Технологические платформы преимплантационного генетического тестирования на анеуплоидии: сравнительная эффективность диагностики хромосомной патологии. Акушерство и гинекология. 2020; 4: 65-71. [Маlysheva О.V., Bichevaya N.K., Gzgzyan А.М., Glotov О.S., Kinunen А.А., Lobenskaya А.Yu., Меkina I.D., Polyakova I.V., Puppo I.L., Saifitdinova А.F., Shcherbak S.G., Kоgan I.Yu. Technological platforms for preimplantation genetic testing for aneuplody: comparative effectiveness of diagnosing chromosomal abnormalities. Obstetrics and Gynecology. 2020; (4): 65-71. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.4.65-71.
- Clark G., Babariya D., Del A., Cano C., Fernández Marcos E., Parnell L. et al. P-727. New methods reveal the true incidence of DNA contamination in PGT-A samples for the first time and avoid errors that could result in serious misdiagnoses. Hum. Reprod. 2023; 38(Suppl.1): i171. https://dx.doi.org/10.1093/humrep/dead093.337.
- Klenow H., Henningsen I. Selective elimination of the exonuclease activity of the deoxyribonucleic acid polymerase from Escherichia coli B by limited proteolysis. Proc. Natl. Acad. Sci. USA. 1970; 65(1): 168-75. https://dx.doi.org/10.1073/pnas.65.1.168.
- Saiki R.K., Gelfand D.H., Stoffel S., Scharf S.J., Higuchi R., Horn G.T. et al. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988; 239(4839): 487-91. https://dx.doi.org/10.1126/science.2448875.
- Telenius H., Carter N.P., Bebb C.E., Nordenskjold M., Ponder B.A., Tunnacliffe A. Degenerate oligonucleotide-primed PCR: general amplification of target DNA by a single degenerate primer. Genomics. 1992; 13(3): 718-25. https://dx.doi.org/10.1016/0888-7543(92)90147-k.
- Aliotta J.M., Pelletier J.J., Ware J.L., Moran L.S., Benner J.S., Kong H. Thermostable Bst DNA polymerase I lacks a 3'-->5' proofreading exonuclease activity. Genet. Anal. 1996; 12(5-6): 185-95.
- Aviel-Ronen S., Qi Zhu C., Coe B.P., Liu N., Watson S.K., Lam W.L. et al. Large fragment Bst DNA polymerase for whole genome amplification of DNA from formalin-fixed paraffin-embedded tissues. BMC Genomics. 2006; 7: 312. https://dx.doi.org/10.1186/1471-2164-7-312.
- Blanco L., Bernad A., Lázaro J.M., Martín G., Garmendia C., Salas M. Highly efficient DNA synthesis by the phage phi 29 DNA polymerase. Symmetrical mode of DNA replication. J. Biol. Chem. 1989; 264(15):8935-40.
- Linck L., Resch-Genger U. Identification of efficient fluorophores for the direct labeling of DNA via rolling circle amplification (RCA) polymerase φ29. Eur. J. Med. Chem. 2010; 45(12): 5561-6. https://dx.doi.org/10.1016/j.ejmech.2010.09.005.
- Твеленёва А.А., Мусатова Е.В., Шилова Н.В. Методы полногеномной амплификации генетического материала едничных клеток. Медицинская генетика. 2017; 16(11): 3-6. [Tveleneva A.A., Musatova E.V., Shilova N.V. Whole genome amplification as a method for analysis of single cells. Medical Genetics. 2017; 16(11): 3-6. (in Russian)].
- Zong C., Lu S., Chapman A.R., Xie X.S. Genome-wide detection of single-nucleotide and copy-number variations of a single human cell. Science. 2012; 338(6114): 1622-6. https://dx.doi.org/10.1126/science.1229164.
- Ignatov K.B., Barsova E.V., Fradkov A.F., Blagodatskikh K.A., Kramarova T.V., Kramarov V.M. A strong strand displacement activity of thermostable DNA polymerase markedly improves the results of DNA amplification. Biotechniques. 2014; 57(2): 81-7. https://dx.doi.org/10.2144/000114198.
- Blagodatskikh K.A., Kramarov V.M., Barsova E.V., Garkovenko A.V., Shcherbo D.S., Shelenkov A.A. et al. Improved DOP-PCR (iDOP-PCR): a robust and simple WGA method for efficient amplification of low copy number genomic DNA. PLoS One. 2017; 12(9): e0184507. https://dx.doi.org/10.1371/journal.pone.0184507.
- Сайфитдинова А.Ф., Павлова О.А., Зелинский А.А., Рябинина М.В., Глотов О.С., Богомаз Д.И., Рубель А.А. Полногеномная амплификация малых количеств ДНК для определения молекулярного кариотипа клеток. Интегративная физиология. 2023; 4(3): 324-34. [Saifitdinova A.F., Pavlova O.A., Zelinskii A.A., Ryabinina M.V., Glotov O.S., Bogomaz D.I., Rubel’ A.A. Whole genome amplification of small amounts of DNA to determine the molecular karyotype of cells. Integrative Physiology. 2023; 4(3): 324-34. (in Russian)]. https://dx.doi.org/10.33910/2687-1270-2023-4-3-324-334.
- Корсак В.С., Балахонов А.В., Бичевая Н.К., Корнеев И.А., Кузнецова Р.А., Леонтьева О.А., Панина А.Н., Решетников И.В., Сайфитдинова А.Ф., Трофимова И.Л. Руководство по клинической эмбриологии. 3-е изд. М.: Медиа Сфера; 2022. 250с. [Korsak V.S., Balakhonov A.V., Bichevaya N.K., Korneev I.A., Kuznetsova R.A., Leont’eva O.A., Panina A.N., Reshetnikov I.V., Saifitdinova A.F., Trofimova I.L. Clinical embryology guidelines. 3rd ed. Moscow: Media Sphere; 2022. 250p. (in Russian)].
- Saifitdinova A.F., Glotov O.S., Poliakova I.V., Bichevaya N.K., Loginova J.A., Kuznetsova R.A. et al. Mosaicism in preimplantation human embryos. Integrative Physiology. 2020; 1(3): 225-30. https://dx.doi.org/10.33910/2687-1270-2020-1-3-225-230.
- Tšuiko O., Fernandez Gallardo E., Voet T., Vermeesch J.R. Preimplantation genetic testing: single-cell technologies at the forefront of PGT and embryo research. Reproduction. 2020; 160(5): A19-A31. https://dx.doi.org/10.1530/REP-20-0102.
- Глотов О.С., Чернов А.Н., Глотов А.С., Баранов В.С. Перспективы применения экзомного секвенирования для решения проблем в репродукции человека (часть 1). Акушерство и гинекология. 2022; 12: 34-9. [Glotov O.S., Chernov A.N., Glotov A.S., Baranov V.S. Prospects for using exome sequencing to solve problems in human reproduction (Part I). Obstetrics and Gynecology. 2022; (12): 34-9. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.221.
- Глотов О.С., Чернов А.Н., Глотов А.С., Баранов В.С. Перспективы применения экзомного секвенирования для решения проблем в репродукции человека (часть 2). Акушерство и гинекология. 2022; 12: 40-5. [Glotov O.S., Chernov A.N., Glotov A.S., Baranov V.S. Prospects for using exome sequencing to solve problems in human reproduction (Part II). Obstetrics and Gynecology. 2022; (12): 40-5. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.220.
Received 02.04.2024
Accepted 30.05.2024
About the Authors
Oleg S. Glotov, Dr. Bio. Sci., Head of the Department of Experimental Medical Virology, Molecular Genetics and Biobanking, Pediatric Research and Clinical Centerfor Infectious Diseases, 197022, Russia, St. Petersburg, Professor Popov str., 9; Senior Researcher at the Department of Genomic Medicine, D.O. Ott Research Institute
of Obstetrics, Gynecology, and Reproductology, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3, olglotov@mail.ru, https://orcid.org/0000-0002-0091-2224
Alsu F. Saifitdinova, Dr. Bio. Sci., Professor at the Department of Human and Animal Anatomy and Physiology, Herzen State Pedagogical University of Russia,
191186, Russia, St. Petersburg, Moyka Embankment, 48; Deputy Head of the Laboratory of Assisted Reproductive Technologies, International Centre for Reproductive Medicine, 197350, Russia, St. Petersburg, Komendantskij Ave., 53/1, +7(812)327-19-50, saifitdinova@mail.ru, https://orcid.org0000-0002-1221-479X
Olga A. Pavlova, PhD, Biologist, Laboratory of Assisted Reproductive Technologies, International Centre for Reproductive Medicine,
197350, Russia, St. Petersburg, Komendantskij Ave., 53/1; Leading Specialist, Beagle Ltd., 192289, Russia, St. Petersburg, Bukharestskaya str., 152-1, 77,
pavlova@biobeagle.com, https://orcid.org0000-0001-9488-6903
Olga A. Leonteva, Embryologist, Laboratory of Assisted Reproductive Technologies, International Centre for Reproductive Medicine,
197350, Russia, St. Petersburg, Komendantskij Ave., 53/1, +7(812)327-19-50, olga_leont@mail.ru, https://orcid.org0000-0002-3667-0511
Irena V. Poliakova, Biologist, Laboratory «Cerbalab», 199106, Russia, St. Petersburg, Bolshoy Ave. of Vasilievsky Island, 90-2-3, irena.88@inbox.ru,
https://orcid.org/0000-0002-5738-8443
Arkadiy B. Maslennikov, PhD (Med), Head of the Novosibirsk Regional DNA-Diagnostics Laboratory, Novosibirsk City Clinical Hospital No. 1,
6300476, Russia, Novosibirsk, Zalessky str., 6-7, +7(383)226-93-35, mab2000@mail.ru, https://orcid.org/0009-0002-8046-3816
Corresponding author: Oleg S. Glotov, olglotov@mail.ru



