Preimplantation genetic testing of embryos in assisted reproductive technology programs in patients with external genital endometriosis
According to published data, external genital endometriosis (EGE) leads to decline of oocyte quality/quantity and the effectiveness of ART programs. In patients with EGE, oocytes may be chromosomally unstable. This may affect the incidence of embryonic aneuploidy. Aim: To assess the incidence of aneuploid embryos in women with EGE versus women of appropriate age without EGE, who underwent fertility treatment with ART programs. Materials and methods: The group of women with EGE included 113 women, and the control group included 211 women. The group of women with endometriosis consisted of 113 patients, the control group comprised 211 women. Each group was divided into three subgroups depending on the age (<35 years, 35–37 years, >37 years). To assess whether an embryo was aneuploid, high-resolution next-generation sequencing (NGS) was performed after trophectoderm. Results: There were no statistically significant differences in embryonic parameters, aneuploidy rates in embryos, and the outcomes of ART programs in patients with EGE versus the control when comparing the subgroups of appropriate age. Conclusion: It was shown, that there is no obvious impact of EGE on the effectiveness of ART programs after embryo transfer. However, a potential effect of endometriosis on the number of removed MII oocytes, as well as the rate of aneuploid embryos cannot be ignored. For detailed study of the effect of EGE on the incidence of aneuploidy further prospective research is necessary.Kulakova E.V., Nepsha O.S., Ekimov A.N., Drapkina Yu.S., Makarova N.P., Ibragimova L.K., Sysoeva A.P., Kalinina E.A.
Keywords
Extragenital endometriosis (EGE) is a chronic inflammatory disease, which is characterized by ectopic endometrial cells outside the uterine cavity. Among fertile women, the average incidence of this disease in 10-15 %, while among infertile women about 30–50% [1].
Despite significant advances in the field of assisted reproductive technologies (ART), the absence of pregnancy in women with endometriosis is associated with activation of various pathogenetic mechanisms underlying infertility in women with EGE. In patients with endometrial cysts, depletion of the pool of primordial follicles in the ovarian cortex, decreased ovarian response to stimulation leading to a decrease in the number and quality of mature oocytes was observed compared to the group of women without EGE of similar age. It is also known that, EGE is associated with impairment of transformation of the endometrium in luteal phase of the natural or stimulated cycle; and this can reduce the chance of embryo implantation and conception using ART programs [2].
EGE is accompanied by macrophage activation in response to local inflammatory reaction, release of cytokines and prostaglandins, release of reactive oxygen species (ROS), mobilization of antioxidant response and formation of local redox imbalance with subsequent damage to lipids, proteins and nucleic acids [3]. The impact of such "toxic environment" affects both the quality of gametes and the process of fertilization, reducing not only the chances of spontaneous conception, but also having a negative impact on the quality of embryos and their ability to develop using ART programs [4]. The role of ROS in defects of meiotic spindle formation during meiosis is still open for discussion: complete maturation of the nucleus depends on formation of normal fission yeast spindles that control chromosome segregation [5, 6]. Meiotic errors of maternal origin are the main cause of chromosomal aneuploidies. These errors occur at different stages of oogenesis, primarily when homologous chromosomes or sister chromatids do not undergo an inversion, and this leads to unequal distribution of genetic material [6]. Accordingly, oocytes obtained after ovarian stimulation in women with EGE may have chromosomal instability, this can affect the incidence of aneuploidies in embryos in this group of patients and lead to worsening outcomes of ART programs.
Assessment of embryo’s chromosomal status using preimplantation genetic testing for aneuploidy (PGT-A) is aimed at optimizing the choice of embryos for transfer in order to increase the effectiveness of ART programs [7]. Currently, one of widely used approaches for assessment of embryo’s chromosomal status is the next-generation sequencing (NGS) [8].
Currently, the data on the effect of EGE on the embryo’s genetic status are rather contradictory. However, the results of recent studies have shown that the outcomes of ART program in patients with EGE and in women without EGE in the control group are comparable. [9].
The aim of this study was to assess the incidence of aneuploid embryos in women with EGE versus women of appropriate age without EGE, who underwent infertility treatment using ART programs.
Materials and methods
Retrospective analysis of medical records was performed, the clinical data of 324 married couples were analyzed and compared. The women underwent infertility treatment in Prof. B.V. Leonov Department of Assisted Technologies in Infertility Treatment of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov from January, 2018 to December, 2020. The group of women with EGE included 113 women, and the control group included 211 women without EGE. Each group was divided into three subgroups depending on the age (<35 years, 35–37 years, >37 years) in order to minimize the impact of age factor on the research results.
The diagnosis of EGE was based on pelvic ultrasound (PU) results or histologic confirmation of laparoscopic findings. The women with EGE stages I-II were included in the study. The control group consisted of female patients with tubo-peritoneal factor (TPF) of infertility, poor response, and poor ovarian reserve; married couples with idiopathic infertility; as well as couples with male factor (MF) infertility. Married couples with severe MF infertility (the absence of spermatozoa in the ejaculate, pronounced oligozoospermia (number of sperm <5 million/ml), testicular sperm aspiration) were excluded from the study due to possible association between severe forms of pathozoospermia and increased levels of aneuploidy in gametes and embryos obtained from ART programs [10]. ART programs using donor cells were excluded from the study.
Spermogram parameters were assessed during preliminary examination of married couples before using ART program and on the day of transvaginal ovarian drillingt (TVOD). The material was collected in a sterile container after 3–5 days of sexual abstinence. Prior to intracytoplasmic sperm injection (ICSI), sperm processing density gradient centrifugation was performed. All specimens were further washed in buffer. Identification of cumulus oocyte complexes (COCs) and assessment of the degree of oocytes maturation was carried out using stereo microscope with heating surface of sterile laminar flow cabinet. COCs were washed and released from follicular fluid and blood and placed in sterile plates (Nunc, Denmark) with Continuous Single Culture Medium (CSCM, Irvine Sc., USA) for incubation at T=+37.0°С in an atmosphere of 6% CO2 for 2–3 hours. Oocyte denudation was performed in a hyaluronidase solution for 20 sec (Irvine Sc., USA). Further COCs were washed in CSCM (Irvine Sc., USA) and placed back in the incubator. After fertilization using ICSI technique, the oocytes were transferred into CSCM (Irvine Sc., USA) for further cultivation. The stage of two pronuclei was assessed 14–16 hours after fertilization. Fertilization was considered a failure, when 2 pronuclei were absent. Cultivation at all stages was conducted in multi-gas incubators COOK (Ireland) with 25 μL drops overlaid with oil (Irvine Sc., USA). CSCM (Irvine Sc., USA) remained the same during the whole process of cultivation. Appropriate blastocysts for genetic analysis were evaluated according to the classification adopted by the Istanbul Consensus Workshop on embryo assessment and corresponded to grade 3BB and higher [11]. Biopsy of trophectoderm cells was performed on the 5th or 6th day of cultivating the embryos, followed by cryopreservation of biopsied embryos. The obtained trophectoderm cells were transferred into transferred into the Eppendorf tubes containing lysis buffer for molecular genetic diagnosis. The following steps were performed in preimplantation genetic testing for aneuploidy (PGT-A): step 1 was whole genome amplification and library preparation for sequencing. During library preparation, unique molecular barcodes were attached to DNA fragments. Further, ion semiconductor sequencing was performed, followed by bioinformatic analysis of the results and preparation of the research report. The analysis of ART programs included single euploid embryo transfer in the cryoprotocol. Clinical pregnancy was registered 21 days after embryo transfer by visualization of the gestational sac in the uterine cavity at ultrasound examination.
All patients underwent a comprehensive examination before ART programs, including assessment of follicle stimulating hormone (FSH) and anti-Müllerian hormone (AMH) levels, as well as pelvic ultrasound for antral follicle count (AFC) on cycle day 2–3. All participants of the study underwent fertility treatment using assisted reproductive technology in full conformity with commonly used techniques and the guidelines approved for ART clinical protocols [12]. All patients have signed informed voluntary consent, which was approved by the Ethical Committee, and has consented to processing their personal data. Ovarian stimulation was started on days 2–3 of the menstrual cycle according to the standard protocol using gonadotropin-releasing hormone antagonist (GnRH-ant) and recombinant follicle-stimulating hormone (FSH) or human menopausal gonadotropin (HMG). To prevent premature luteinizing hormone (LH) rise/peak when the follicle reached a diameter of 14 mm, the patients received GnRH-ant at a dose of 0.25 mg per day. The procedure of final maturation of oocytes was performed after the follicle reached a diameter of ≥17 mm; human chorionic gonadotropin (HCG) was administered at a dose of 10 000 IU for ovulation induction. In case of a risk of ovarian hyperstimulation syndrome (OHSS), triptorelin at a dose of 0.2 mg was administered to trigger final maturation of oocytes. TVOD was carried out in the operating room under intravenous anesthesia 35 h after triggering ovulation. Follicular fluid samples were collected and the quality of oocytes was assessed. The obtained embryos were vitrified for PGT-A according to culture media manufacturer’s manual for embryo vitrification and thawing (Kitazato, Japan) [13]. After getting the results of biopsy, the patients underwent endometrium preparation for cryopreserved embryos transfer with hormone replacement treatment (estradiol valerate at a dose of 8 mg per day on the 4–5th day of the menstrual cycle, micronized progesterone at a doze of 400–600 mg per day on the 15–16th day of the menstrual cycle). Embryo transfer was performed on the 20–21th day of the menstrual cycle. Embryos were thawed according to the culture media manufacturer’s manual for embryo vitrification and thawing (Kitazato, Japan) [13].
Statistical analysis
Microsoft Excel tables and SPSS Statistics 22 (USA) were used for statistical analysis. Quantitative data analysis was performed with Kolmogorov–Smirnov test (graphical comparison of distributions between the groups). Chi-squared (χ2) test was used for statistical analysis of categorical data, and Mann–Whitney U-test was used for paired comparison, when distribution was different from normal. The distribution of variables different from normal were described as median values (Me) and quartiles (Q1 and Q3), i.e. Me (Q1; Q3). The level of significance was p< 0.05.
Results
Comparison of patients of similar age group allowed an objective assessment of the impact of EGE on the incidence of aneuploidy and on the stages of ART programs.
A comparative analysis in both groups showed that an average number of COCs obtained on the day of TVOD, mature oocytes and normally fertilized oocytes (2PN 2PB zygotes) in the group of women with EGE was significantly higher versus the same parameters in the comparison group. This was due to certain selection criteria: the comparison group included both women with tubo-peritoneal factor of infertility and poor response and a low level of AMH. It should be noted, that that most women in the comparison group were of higher reproductive age (>37 years, n=103) (Table 1).
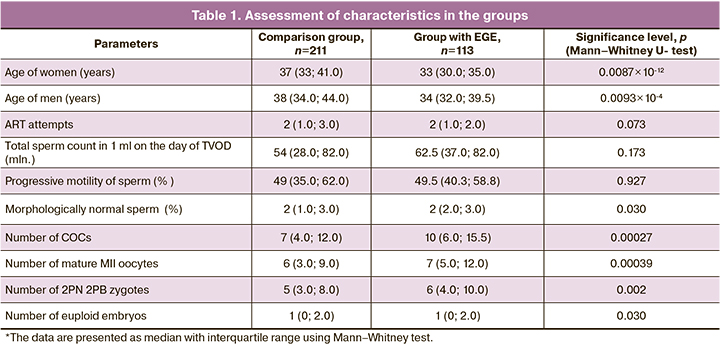
Statistically, in the subgroup of women over 37 years of age in the comparison group, there were more patients of a higher age compared to the subgroup of women with EGE of similar age. Significantly larger number of ART attempts was in patients in the group without EGE versus the group with EGE in the subgroup of patients under the age of 35 year. Semen analysis results on the day of TVOD showed that in the subgroup of patients under the age of 35 years in the comparison group, the percentage of morphologically normal sperm in men was lower (2% versus 3%) compared to the same parameter among the partners of women in the group with EGE (p=0.001). This can be explained by the fact that married couples with MF infertility were included in this group. There were no statistically significant differences regarding other semen characteristics. Comparative analysis of embryonic stage showed similar results with regard to the number of obtained COCs and mature oocytes, as well fertilization rate among the subgroups of women of appropriate age both in the group with and without EGE (Table 2).
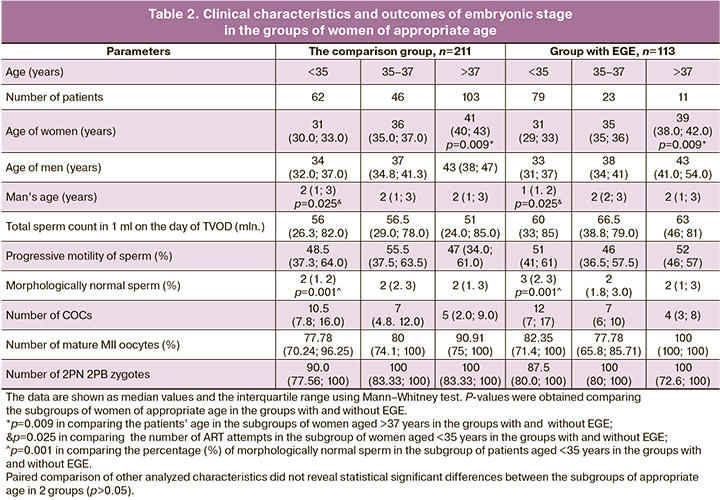
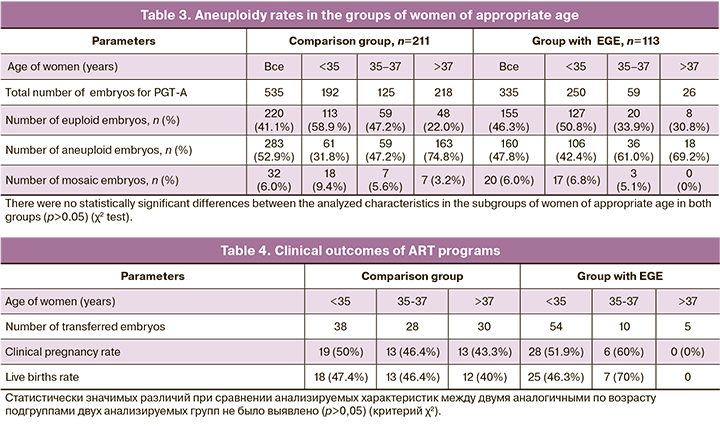
PGT-A results showed, that in the group of women without EGE, the rate of aneuploidy was 52.9% (283/535) versus 47.8% (160/335) in the group with EGE, and the differences were not statistically significant (Table 3). The similar results were obtained in comparison of the subgroup of patients of similar age. However, it should be noted that the percentage of aneuploidy among the patients with EFE in the subgroups under the age of 35 and 35–37 years was higher compared to the subgroups of women of similar age in the comparison group 42.4 and 61% versus 31.8 and 47.2%, respectively, but the differences were not statistically significant. The implantation rate in the group with EGE was 49.3% (34/69), and it was comparable with the similar value in the comparison group – 46.9% (45/96). There were 2 cases of missed miscarriage and 1 case of ectopic pregnancy in the group with EGE, and 2 cases of abortion in the comparison group. Live births rate was 46.4% in the group of women with EGE, including 1 case of twin birth, and 44.8% in the comparison group. Also, the difference was not statistically significant (Table 4).
Discussion
The effectiveness of ART programs in women with EGE is lower versus the patients without EGE. Extragenital endometriosis in medical history has a negative impact on the quality and/or number of oocytes and leads to impaired endometrial decidualization [1]. There is a large amount of inconsistent data on the mechanisms of the pathogenic effect of endometriotic foci on the endometrium and the quality/number of oocytes in ART programs. The issue of chromosomal instability of oocytes under the influence of ROS in the presence of EGE is being actively discussed. This is associated with impaired spindle assembly, unequal distribution of chromosomes, and formation of aneuploid gametes. The results of experimental research using animal models showed that in the presence of endometriosis, peritoneal and follicular fluid affects the oocytes leading to instability of microtubules and spindle, division and a high incidence of meiotic abnormalities in oocytes. [14]. However, Barcelos et al. did not find spindle abnormalities in the process of in vitro maturation of immature oocytes obtained from patients with EGE and from healthy women [15]. In this study, the comparable results with regard to the aneuploidy rates were obtained among the subgroups of appropriate age (<35 years, 35–37 years, >38 years) in the comparison group, and in the group with EGE (Table 3). However the percentage of aneuploidy in the group of patients with EGE (the subgroup of women aged <35 and 35–37 years) was higher versus the women in the subgroups of the appropriate age in the comparison group. The differences were not statistically significant, probably due to insufficient sampling of patients.
PGT-A results of 1880 embryos obtained from women with EGE and 23054 embryos from women in the subgroups of appropriate age in the control group (<35 years, 35–37 years, 38–40 years, 41–42 years, >42 years) showed that the rates of aneuploidy were comparable [16]. Similar results were described by Vaiarelli et al., who also did not find significant differences in the rates of aneuploidy, the number of obtained MII oocytes, fertilization rates and the percentage of cryopreserved blastocysts [17]. The researchers do not deny a potential effect of EGE on the number of MII oocytes obtained in ART programs, and this is confirmed by other authors who described a negative effect of IGE on the number of mature oocytes. [18].
An important issue is that EGE can affect endometrial receptivity [1]. Analysis of pregnancy outcomes and live birth rates after euploid embryo transfer in women with EGE (250 embryos) was performed in the two control groups: in patients, who underwent preimplantation genetic diagnosis of embryos for genetic and chromosomal pathologies (225 embryos), and married couples with MF infertility (1332 embryos), and no differences were found in patients with EGE versus patients in the control groups [19]. Our study also did not reveal significant differences neither in the incidence of clinical pregnancy, nor in frequency of live births, and this was consistent with the literature data. [16, 20]. Comparison of ART programs outcomes in the studies that used donor oocytes in women with EGE did not find reduced endometrial receptivity versus the control group. Transcriptome analysis of endometrial expression of 238 genes in women with EGE during implantation did not reveal any differences between the women with EGE and the control group. [21].
The ambiguity of the data obtained in the above studies may be due to different inclusion/exclusion criteria and inadequate examination of the patient cohort.
ART programs failure in the group of women with EGE may be due to the morphological and functional characteristics of the obtained oocytes, affected by severity of endometriosis (stages III–IV) [22, 23]; for example, cytoplasmic defects that impair the speed of fertilization [24]. It has been shown, that morphologic abnormalities of oocytes most often were among the patients with EGE versus the women with MF infertility in the control group [25]. Various factors have impact on the quality of oocytes: the patients with IGE has increased apoptosis levels in cumulus and granulosa cells. This leads to the loss of major support that cumulus cells provide to oocytes (hormones, a number of growth factors). Comparison of spectral data using infrared and Raman spectroscopy showed a profound change in the structure of proteins, activation of mechanisms of oxidative stress, carbohydrate metabolism dysregulation, and modification of DNA methylation in granulosa cells in women with EGE [26]. In women with EGE in history, there were decreased levels of antioxidant molecules and increased levels of biomarkers of oxidative stress in follicular fluid, leading to premature “aging” of granulosa cells accompanied by mitochondrial dysfunction and increased endoplasmic reticulum stress. Transcriptomic analysis of gene expression profile of oocytes in patients with ovarian EGE versus donor oocytes showed changes in expression of different sets of genes participating in regulation of methylation processes, mitochondrial function, cell activity and growth, steroid metabolism, response to oxidative stress [27].
The results of the mentioned studies allow to make a conclusion that the quality of oocytes in women with EGE is low. However, based on the results of other studies, these abnormalities do not have apparent impact on clinical outcomes of ART programs. Analysis of large registries of the outcomes of ART programs, including about 350 000 cycles, showed that the presence of EGE in women was associated with poor ovarian response to stimulation, however the frequency of implantation was comparable with the groups of patients without EGE [28]. The results of this and other studies demonstrated that aneuploidy rates in ART programs among the women with EGE were not different compared to patients without EGE in all age- subgroups. In cases of moderate/severe stages and aggressive forms of EGE, PGT-A is recommended to raise the effectiveness of ART program [29]. The ART program, which includes PGT-A allows not only to optimize the selection of the most promising embryo for transfer, but also to minimize the financial, economic and time costs of married couples, that may be required to obtain an euploid embryo in the ART program without PGT-A.
Conclusion
This study showed that EGE did not significantly affect the incidence of aneuploidy of embryos produced by in vitro fertilization. However, the percentage of aneuploid embryos in the group of patients with EGE was higher versus the comparison group. Implantation and live birth rates were also comparable with the control group. Age of a woman is one of major predictors of the quality of oocytes obtained by TVOD. It is most probably that ART programs failure in patients with EGE may be due to insufficient number of obtained oocytes, and probably due to their morphological and functional characteristics. Poor ovarian response to stimulation and poor ovarian reserve in women with EGE in a number of cases may be due to surgical treatment. Perhaps the patients with EGE should be advised to vitrify the oocytes prior to surgery aimed at fertility preservation and reproductive function realization. Nevertheless, it should be emphasized that PGT-A is recommended for selection of an euploid embryo in case of aggressive course of EGE (stages III–IV).
References
- Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil. Steril. 2012; 98(3): 591-8. https://dx.doi.org/10.1016/j.fertnstert.2012.05.031.
- de Ziegler D., Pirtea P., Carbonnel M., Poulain M., Cicinelli E., Bulletti C. et al. Assisted reproduction in endometriosis. Best Pract. Res. Clin. Endocrinol. Metab. 2019; 33(1): 47-59. https://dx.doi.org/10.1016/j.beem.2018.10.001.
- Адамян Л.В., Сонова М.М., Арсланян К.Н., Логинова О.Н., Харченко Э.И. Окислительный стресс и эндометриоз: обзор литературы. Лечащий врач. 2019; 12: 20-5. [Adamyan L.V., Sonova M.M., Arslanyan K.N., Loginova O.N., Kharchenko E.I. Oxidative stress and endometriosis: a literature review. Medical Journal Lechaschi Vrach. 2019; 12: 20-5. (in Russian)].
- Адамян Л.В., Манукян Л.М., Логинова О.Н., Арсланян К.Н., Зайратьянц В.О. Роль матриксных металлопротеиназ в патогенезе эндометриоза (обзор литературы). Проблемы репродукции. 2020; 26(2): 95-103. [Adamyan L.V., Manukyan L.M., Loginova O.N., Arslanyan K.N., Zayratyants V.O. The role of matrix metalloproteinases in the pathogenesis of endometriosis (literature review). Problems of Reproduction. 2020; 26(2): 95-103. (in Russian)]. https://dx.doi.org/10.17116/repro20202602195.
- Mandelbaum J., Anastasiou O., Lévy R., Guérin J.F., de Larouzière V., Antoine J.M. Effects of cryopreservation on the meiotic spindle of human oocytes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004; 113(Suppl. 1): S17-23. https://dx.doi.org/10.1016/j.ejogrb.2003.11.005.
- De Santis L., Cino I., Rabellotti E., Calzi F., Persico P., Borini A. et al. Polar body morphology and spindle imaging as predictors of oocyte quality. Reprod. Biomed. Online. 2005; 11(1): 36-42. https://dx.doi.org/10.1016/s1472-6483(10)61296-5.
- Александрова Н.В., Шубина Е.С., Екимов А.Н., Кодылева Т.А., Мукосей И.С., Макарова Н.П., Кулакова Е.В., Левков Л.А., Барков И.Ю., Трофимов Д.Ю., Сухих Г.Т. Сравнение результатов преимплантационного генетического скрининга, проведенного методами CGH и NGS. Молекулярная биология. 2017; 51(2): 308-13. [Aleksandrova N.V., Shubina E.S., Ekimov A.N., Kodyleva T.A., Mukosey I.S., Makarova N.P., Kulakova E.V., Levkov L.A., Barkov I.Y., Trofimov D.Y., Sukhikh G.T. Comparative results of preimplantation genetic screening by array comparative genomic hybridization and new-generation sequencing. Molecular Biology. 2017; 51(2): 269-73. (in Russian)]. https://dx.doi.org/10.7868/S0026898417010025.
- Rubio C., Rodrigo L., Garcia-Pascual C., Peinado V., Campos-Galindo I., Garcia-Herrero S. et al. Clinical application of embryo aneuploidy testing by next-generation sequencing. Biol. Reprod. 2019; 101(6): 1083-90. https://dx.doi.org/10.1093/biolre/ioz019.
- Benaglia L., Candotti G., Papaleo E., Pagliardini L., Leonardi M., Reschini M. et al. Pregnancy outcome in women with endometriosis achieving pregnancy with IVF. Hum. Reprod. 2016; 31(12): 2730-6. https://dx.doi.org/10.1093/humrep/dew210.
- Magli M.C., Gianaroli L., Ferraretti A.P., Gordts S., Fredericks V., Crippa A. Paternal contribution to aneuploidy in preimplantation embryos. Reprod. Biomed. Online 2009; 18(4): 536-42. https://dx.doi.org/10.1016/s1472-6483(10)60131-9.
- Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum. Reprod. 2011; 26(6): 1270-83. https://dx.doi.org/10.1093/humrep/der037.
- Письмо Министерства здравоохранения РФ от 15 февраля 2019 г. № 15-4/И/2-1218 О направлении клинических рекомендаций (протокола лечения) «Женское бесплодие (современные подходы к диагностике и лечению)». 4 апреля 2019 г. [Letter of the Ministry of Health of the Russian Federation dated February 15, 2019 No. 15-4/I/2-1218 On the direction of clinical recommendations (treatment protocol) "Female infertility (modern approaches to diagnosis and treatment)". April 4, 2019. (in Russian)].
- Parmegiani L., Beilby K.H., Arnone A., Bernardi S., Maccarini A.M., Nardi E. et al. Testing the efficacy and efficiency of a single "universal warming protocol" for vitrified human embryos: prospective randomized controlled trial and retrospective longitudinal cohort study. J. Assist. Reprod Genet. 2018; 35(10): 1887-95. https://dx.doi.org/10.1007/s10815-018-1276-4.
- Mansour G., Sharma R.K., Agarwal A., Falcone T. Endometriosis-induced alterations in mouse metaphase II oocyte microtubules and chromosomal alignment: a possible cause of infertility. Fertil. Steril. 2010; 94(5): 1894-9. https://dx.doi.org/10.1016/j.fertnstert.2009.09.043.
- Barcelos I.D., Vieira R.C., Ferreira E.M., Martins W.P., Ferriani R.A., Navarro P.A. Comparative analysis of the spindle and chromosome configurations of in vitro-matured oocytes from patients with endometriosis and from control subjects: a pilot study. Fertil. Steril. 2009; 92(5): 1749-52. https://dx.doi.org/10.1016/j.fertnstert.2009.05.006.
- Juneau C., Kraus E., Werner M., Franasiak J., Morin S., Patounakis G. et al. Patients with endometriosis have aneuploidy rates equivalent to their age-matched peers in the in vitro fertilization population. Fertil. Steril. 2017; 108(2): 284-8. https://dx.doi.org/10.1016/j.fertnstert.2017.05.038.
- Vaiarelli A., Venturella R., Cimadomo D., Conforti A., Pedri S., Bitonti G. et al. Endometriosis shows no impact on the euploid blastocyst rate per cohort of inseminated metaphase-II oocytes: a case-control study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021; 256: 205-10. https://dx.doi.org/10.1016/j.ejogrb.2020.11.024.
- Rossi A.C., Prefumo F. The effects of surgery for endometriosis on pregnancy outcomes following in vitro fertilization and embryo transfer: a systematic review and meta-analysis. Arch. Gynecol. Obstet. 2016; 294(3): 647-55. https://dx.doi.org/10.1007/s00404-016-4136-4.
- Bishop L.A., Gunn J., Jahandideh S., Devine K., Decherney A.H., Hill M.J. Endometriosis does not impact live-birth rates in frozen embryo transfers of euploid blastocysts. Fertil. Steril. 2021; 115(2): 416-22. https://dx.doi.org/10.1016/j.fertnstert.2020.07.050.
- Maggiore U.L.R., Gupta J.K., Ferrero S. Treatment of endometrioma for improving fertility. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017; 209: 81-5. https://dx.doi.org/10.1016/j.ejogrb.2016.02.035.
- Garcia-Velasco J.A., Fassbender A., Ruiz-Alonso M., Blesa D., D'Hooghe T., Simon C. Is endometrial receptivity transcriptomics affected in women with endometriosis? A pilot study. Reprod. Biomed. Online. 2015; 31(5): 647-54. https://dx.doi.org/10.1016/j.rbmo.2015.07.014.
- Díaz I., Navarro J., Blasco L., Simón C., Pellicer A., Remohí J. Impact of stage III-IV endometriosis on recipients of sibling oocytes: matched case-control study. Fertil. Steril. 2000; 74(1): 31-4. https://dx.doi.org/10.1016/s0015-0282(00)00570-7.
- Shebl O., Sifferlinger I., Habelsberger A., Oppelt P., Mayer R.B., Petek E. et al. Oocyte competence in in vitro fertilization and intracytoplasmic sperm injection patients suffering from endometriosis and its possible association with subsequent treatment outcome: a matched case-control study. Acta Obstet. Gynecol. Scand. 2017; 96(6): 736-44. https://dx.doi.org/10.1111/aogs.12941.
- Stilley J.A., Birt J.A., Sharpe-Timms K.L. Cellular and molecular basis for endometriosis-associated infertility. Cell Tissue Res. 2012; 349(3): 849-62. https://dx.doi.org/10.1007/s00441-011-1309-0.
- Kasapoglu I., Kuspinar G., Saribal S., Turk P., Avci B,. Uncu G. Detrimental effects of endometriosis on oocyte morphology in intracytoplasmic sperm injection cycles: a retrospective cohort study. Gynecol. Endocrinol. 2018; 34(3): 206-11. https://dx.doi.org/10.1080/09513590.2017.1391203.
- Notarstefano V., Gioacchini G., Byrne H.J., Zacà C., Sereni E., Vaccari L. et al. Vibrational characterization of granulosa cells from patients affected by unilateral ovarian endometriosis: new insights from infrared and Raman microspectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019; 212: 206-14. https://dx.doi.org/10.1016/j.saa.2018.12.054.
- Senapati S., Sammel M.D., Morse C., Barnhart K.T. Impact of endometriosis on in vitro fertilization outcomes: an evaluation of the Society for Assisted Reproductive Technologies Database. Fertil. Steril. 2016; 106(1): 164-171.e1.: https://dx.doi.org/10.1016/j.fertnstert.2016.03.037.
- Dviri M., Madjunkova S., Koziarz A., Antes R., Abramov R., Mashiach J. et al. Is there a correlation between paternal age and aneuploidy rate? An analysis of 3,118 embryos derived from young egg donors. Fertil. Steril. 2020; 114(2):293-300. https://dx.doi.org/10.1016/j.fertnstert.2020.03.034.
- Shafrir A.L., Farland L.V., Shah D.K., Harris H.R., Kvaskoff M., Zondervan K. et al. Risk for and consequences of endometriosis: a critical epidemiologic review. Best Pract. Res. Clin. Obstet. Gynaecol. 2018; 51: 1-15. https://dx.doi.org/10.1016/j.bpobgyn.2018.06.001.
Received 01.04.2021
Accepted 17.05.2021
About the Authors
Elena V. Kulakova, PhD, Senior Researcher, Professor B.V. Leonov Department of IVF, Academician V.I. Kulakov National Medical Research Center for Obstetrics,Gynecology and Perinatology, Ministry of Health of the Russian Federation, e_kulakova@oparina4.ru, https://orcid.org/0000-0002-4433-4163,
117997, Russia, Moscow, Academician Oparin str., 4.
Oksana S. Nepsha, PhD (Bio), Researcher, Professor B.V. Leonov Department of IVF, Academician V.I. Kulakov National Medical Research Center for Obstetrics,
Gynecology and Perinatology, Ministry of Health of the Russian Federation, o_nepsha@oparina4.ru, https://orcid.org/0000-0002-9988-2810,
117997, Russia, Moscow, Academician Oparin str., 4.
Alexey N. Ekimov, genetic scientist, Laboratory of Molecular Genetic Methods, Academician V.I. Kulakov National Medical Research Center for Obstetrics,
Gynecology and Perinatology, Ministry of Health of the Russian Federation, a_ekimov@oparina4.ru, https://orcid.org/0000-0001-5029-0462,
117997, Russia, Moscow, Academician Oparin str., 4.
Yulia S. Drapkina, PhD, researcher, Professor B.V. Leonov Department of IVF, Academician V.I. Kulakov National Medical Research Center for Obstetrics,
Gynecology and Perinatology, Ministry of Health of the Russian Federation, yu_drapkina@oparina4.ru, https://orcid.org/0000-0002-0545-1607,
117997, Russia, Moscow, Academician Oparin str., 4.
Natalya P. Makarova, Dr. Bio. Sci., Leading Researcher, Professor B.V. Leonov Department of IVF, Academician V.I. Kulakov National Medical Research Center
for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, np_makarova@oparina4.ru, https://orcid.org/0000-0003-8922-2878,
117997, Russia, Moscow, Academician Oparin str., 4.
Luiza K. Ibragimova, postgraduate student, Professor B.V. Leonov Department of IVF, Academician V.I. Kulakov National Medical Research Center for Obstetrics,
Gynecology and Perinatology, Ministry of Health of the Russian Federation, ibragimova_luisa0693@mail.ru, https://orcid.org/0000-0002-3090-5922,
117997, Russia, Moscow, Academician Oparin str., 4.
Anastasia P. Sysoeva, embryologist, Professor B.V. Leonov Department of IVF, Academician V.I. Kulakov National Medical Research Center for Obstetrics,
Gynecology and Perinatology, Ministry of Health of the Russian Federation, sysoeva.a.p@gmail.com, https://orcid.org/0000-0002-6502-4498,
117997, Russia, Moscow, Academician Oparin str., 4.
Elena A. Kalinina, Dr. Med. Sci., Professor, Head of Professor B.V. Leonov Department of IVF, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, e_kalinina@oparina4.ru, https://orcid.org/0000-0002-8922-2878,
117997, Russia, Moscow, Academician Oparin str., 4.
Authors’ contributions: Kulakova E.V. – editing the text of the article; Nepsha O.S., Drapkina Yu.S., Sysoeva A.P., Ibragimova L.K. – collection and analysis of published data, writing the text of the article; Ekimov A.N. – preimplantation genetic testing for aneuploidy with next generation sequencing (NGS); Makarova N.P. – embryo culture, trophectoderm biopsy, embryo cryopreservation, editing and approval of the article for publication; Kalinina E.A. – editing and approval of the article for publication.
Conflicts of interest: The authors declare that they have no conflicts of interest.
Funding: The study was carried out without attraction of a third-party funding.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Kulakova E.V., Nepsha O.S., Ekimov A.N., Drapkina Yu.S., Makarova N.P., Ibragimova L.K., Sysoeva A.P., Kalinina E.A. Preimplantation genetic testing of embryos in assisted reproductive technology programs in patients with external genital endometriosis
Akusherstvo i Gynecologia/ Obstetrics and Gynecology. 2021; 11: 104-112 (in Russian)
https://dx.doi.org/10.18565/aig.2021.11.104-112



