Овариальная стимуляция (ОС) – один из ключевых этапов проведения программы экстракорпорального оплодотворения (ЭКО). Основной целью ОС является получение оптимального числа зрелых ооцитов, способных к оплодотворению, что особенно важно для повышения частоты наступления беременности и коэффициента живорождения при проведении программ вспомогательных репродуктивных технологий (ВРТ) у женщин с бесплодием [1]. Известно, что чем старше возраст женщины, тем большее количество ооцитов потребуется для повышения шансов на получение одного эуплоидного эмбриона [2]. Так, у женщин в возрасте до 35 лет вероятность получения эуплоидных эмбрионов составляет 60% на один цикл стимуляции яичников, в возрасте до 40–42 лет – снижается до 30% и в возрасте старше 42 лет – не превышает 5% [3].
Пациентки со сниженными показателями овариального резерва и «бедным» ответом на ОС составляют большую когорту среди всех женщин с бесплодием. Согласно статистическим данным, распространенность «бедного» ответа на ОС гонадотропинами варьирует от 9 до 24%, что является основной причиной снижения частоты наступления беременности и частоты живорождения в данной группе пациенток [1, 4, 5].
Как известно, пациентки с недостаточным ответом яичников на ОС имеют повышенный базальный уровень фолликулостимулирующего гормона (ФСГ), сниженные показатели антимюллерова гормона (АМГ) и количества антральных фолликулов (КАФ). Установлена связь сниженного овариального ответа со старшим репродуктивным возрастом женщины, наличием оперативных вмешательств на яичниках в анамнезе, а также избыточной массой тела или ожирением [6]. Однако, причина недостаточного овариального ответа на ОС у женщин с сохраненным овариальным резервом до сих пор не ясна [7], и вышеуказанные параметры не являются прогностически значимыми для оценки динамики роста фолликулов в ответ на гонадотропную стимуляцию яичников. Необходимо проведение дальнейших исследований, направленных на поиск наиболее информативных маркеров для прогнозирования «бедного» ответа яичников на ОС и достижения оптимального результата в циклах ВРТ [8].
Концепция классификации POSEIDON
«Болонские» критерии, принятые в 2011 г. группой ESHRE для определения «бедного» ответа яичников на ОС, не охватывают всю когорту пациенток с низким овариальным резервом. Согласно данным критериям, прогнозирование «бедного» ответа яичников правомочно при соблюдении двух из трех нижеперечисленных условий:
- возраст женщины ≥40 лет, а также наличие оперативных вмешательств в анамнезе;
- получение ≤3 ооцитов в предыдущем цикле овариальной стимуляции в стандартном протоколе ЭКО;
- сниженные показатели овариального резерва (АМГ≤0,5–1,1 нг/мл; КАФ≤5–7).
Однако, молодая женщина в возрасте до 35 лет с бесплодием и признаками сниженного овариального резерва, раннее не предпринимавшая попыток ЭКО, не удовлетворяет данным критериям [8, 9].
Новая классификация POSEIDON (Patient-Oriented Strategies Encompassing Individualized Oocyte Number), принятая в 2016 г., дает возможность персонализировать протоколы ОС в различных возрастных группах с разными показателями овариального резерва (КАФ и уровень АМГ) и наличием сниженного ответа яичников на ОС в предыдущем протоколе ЭКО, что более объективно характеризует пациенток с «бедным» ответом яичников и помогает стратифицировать женщин с ожидаемым и неожиданно «бедным» ответом на ОС [10, 11]. У таких пациенток снижена кумулятивная частота живорождения в связи с недостаточным количеством полученных ооцитов и эмбрионов, соответственно, а также с наличием ооцитов и эмбрионов неудовлетворительного качества в группе пациенток старшего репродуктивного возраста (>36 лет) [12, 13]. Согласно классификации POSEIDON женщины с «бедным» ответом на ОС стратифицируются на 4 группы. Необходимо отметить, что, по данным современной литературы, эти группы пациенток составляют фактически 47% от общего количества женщин, обратившихся для проведения программ ВРТ [8]. Пациентки с неожиданно «бедным» или субоптимальным ответом на проведение ОС в анамнезе, но с сохраненными клинико-лабораторными показателями овариального резерва (КАФ≥5; АМГ≥1,2 нг/мл) составляют 1 и 2 группы POSEIDON:
POSEIDON 1: Возраст<35 лет, АМГ≥1,2 нг/мл, КАФ≥5. В свою очередь, данная группа включает в себя 2 подгруппы, исходя из результатов ОС:
- подгруппа 1а (неожиданно «бедный» ответ): <4 ооцитов;
- подгруппа 1b (неожиданно субоптимальный ответ): 4–9 ооцитов;
POSEIDON 2: Возраст≥35 лет, АМГ≥1,2 нг/мл, КАФ≥5. Данная группа также включает в себя 2 подгруппы, исходя из результатов ОС:
- подгруппа 2а (неожиданно «бедный» ответ): <4 ооцитов;
- подгруппа 2b (неожиданно субоптимальный ответ): 4–9 ооцитов.
В свою очередь, группы 3 и 4 включают пациенток с ожидаемым «бедным» ответом на ОС в циклах ВРТ (КАФ<5; АМГ<1,2 нг/мл) [3]. Согласно современной тенденции к отсроченному материнству, POSEIDON 4 составляет самую обширную группу пациенток (~50%), в то время как POSEIDON 3 встречается только в 10% всех случаев [14, 15]:
POSEIDON 3: Возраст<35 лет, АМГ<1,2 нг/мл, КАФ<5.
POSEIDON 4: Возраст≥35лет, АМГ<1,2 нг/мл, КАФ<5.
Патогенез «бедного» ответа на овариальную стимуляцию
До сих пор неизвестны патогенетические механизмы, задействованные в развитии «бедного» ответа на гонадотропную стимуляцию яичников в программах ЭКО. Исключением являются пациентки с резекцией яичников в анамнезе. В настоящее время активно обсуждаются следующие вероятные механизмы развития субоптимального и «бедного» ответа на ОС: влияние вредных факторов окружающей среды, воздействие оксидативного стресса и митохондриальной дисфункции, применение субоптимальных доз гонадотропинов, асинхронный рост фолликулов в цикле стимуляции яичников, возникновение технических сложностей, ассоциированных с введением триггера овуляции и/или проведением трансвагинальной пункции яичников [7]. Более того, в научной литературе появляется все больше данных о зависимости чувствительности яичников на ОС у пациенток с сохраненным овариальным резервом от индивидуального генетического профиля каждой пациентки, что дало основание для развития фармакогеномики ОС [16, 17].
Известно, что ген рецептора ФСГ (FSHR) является наиболее изученным среди других генов, имеющих прямое отношение к гонадотропной стимуляции яичников. В настоящее время установлено, что два однонуклеотидных полиморфизма гена FSHR: rs6165 и rs6166, которые образуют сцепленные блоки и, как следствие, наследуются вместе, могут играть важную роль в исходах ОС [18, 19]. В исследовании L. Lazaros et al. (2013) показано, что аллель 680Ser полиморфизма гена FSHR 2039 A>G (Asn680Ser) ассоциирован с повышенным базальным уровнем ФСГ и обоснованностью применения увеличенных доз препаратов рекомбинантного ФСГ (рФСГ) в программах ВРТ у таких пациенток [19]. Напротив, B. Lledo et al. (2016) в ретроспективном когортном исследовании отметили, что при проведении ОС у женщин c генотипом Ser/Ser полиморфизма гена FSHR 2039 A>G (Asn680Ser) более целесообразно использование менотропинов, чем препаратов рФСГ, так как они обладают длительным периодом полувыведения и способствуют поддержанию необходимого уровня ФСГ более продолжительное время [20].
По данным нескольких исследований было продемонстрировано, что полиморфизм гена рецептора FSHR [rs1394205] также может быть ассоциирован с развитием «бедного» ответа на гонадотропную стимуляцию. Отмечено, что пациенткам, гомозиготным по аллелю АА полиморфизма гена рецептора FSHR [rs1394205] требовалась большая доза препарата рФСГ, по сравнению с носителями аллелей GG и AG полиморфизма гена рецептора FSHR [rs1394205] (р<0,001). Также показано, что у гетерозигот по аллелю AG полиморфизма гена рецептора FSHR [rs1394205] потребление препаратов рФСГ было ниже, чем у гомозигот по аллелю АА данного полиморфизма (р=0,002) [21–23].
В систематическом обзоре и мета-анализе 2018 г., включающем 57 крупных исследований, подтверждена клиническая значимость определения ряда генетических полиморфизмов для прогнозирования «бедного» ответа на ОС, а именно: FSHR [rs6165], FSHR [rs6166], FSHR [rs1394205], LHB [rs1800447], LHCGR [rs2293275] и LHCGR [rs13405728]. Установлено, что пациентки с генотипом GG полиморфизма гена FSHR [rs6166] обладали более выраженной резистентностью к препаратам гонадотропинов, по сравнению с носителями генотипов АА и AG полиморфизма гена FSHR [rs6166] (р<0,001), что было причиной получения меньшего количества ооцитов в результате гонадотропной стимуляции яичников [17].
Помимо полиморфизмов гена рецептора ФСГ, в научной литературе обсуждается роль полиморфных вариантов гена, кодирующего бета-цепь лютеинизирующего гормона (ЛГ) в развитии «бедного» ответа ОС. C. Alviggi et al. (2013) продемонстрировали, что наличие генетически измененного типа ЛГ (v-бета-ЛГ) может приводить к значительному увеличению суммарной дозы рФСГ, необходимой для адекватного ответа на ОС. По-видимому, полиморфизм гена ЛГ представляет собой менее активную форму ЛГ, неспособную поддерживать достаточный уровень ФСГ в протоколе ЭКО. Вероятно, для таких пациенток целесообразно рассмотреть дополнительное введение препаратов рекомбинантного ЛГ (рЛГ) вместо увеличения дозы ФСГ в ОС [24]. Необходимо проведение дальнейших исследований для оценки влияния генетических вариантов гонадотропинов и их рецепторов, как предикторов «бедного» овариального ответа на ОС.
Прогностические маркеры «бедного» ответа на овариальную стимуляцию
Несомненно, возраст женщины является основным прогностическим маркером «бедного» ответа на ОС и снижения частоты наступления беременности в связи с обратной зависимостью возраста женщины и числа полученных эуплоидных эмбрионов [25, 26].
По данным ретроспективного когортного исследования С. Esteves et al. (2021), с участием 9073 женщин в программе ЭКО/ИКСИ, кумулятивная частота живорождения у пациенток с «бедным» ответом на ОС была в среднем на 50% ниже, чем у пациенток с нормальным ответом на стимуляцию яичников, а также существенно зависела от возраста женщин. Так, у пациенток старшей возрастной группы (POSEIDON 4) кумулятивная частота родов была ниже (p<0,001), по сравнению с другими группами POSEIDON, что указывает на первостепенную роль возраста женщин с бесплодием, как прогностического маркера «бедного» ответа на гонадотропную стимуляцию [27]. В ретроспективном когортном исследовании M. Luo et al. (2021), проводилось предимплантационное генетическое исследование на анеуплоидии (ПГТ-А) 3016 бластоцист, полученных у пациенток с «бедным» ответом на ОС в соответствии с критериями POSEIDON. В результате проведенного ПГТ-А частота эуплоидии в общей популяции составила 39,1%. Согласно классификации POSEIDON, частота встречаемости эуплоидных эмбрионов составила 57,2, 34,9, 52,4 и 26,2% (p<0,001) в четырех группах POSEIDON соответственно, что, несомненно, подтверждает влияние возраста женщин на качество получаемых эмбрионов [28].
В настоящее время для прогнозирования «бедного» ответа на ОС, помимо общепринятых клинико-лабораторных маркеров (возраст, наличие оперативных вмешательств на яичниках в анамнезе, уровня АМГ, ФСГ и КАФ) обсуждается возможность введения в клиническую практику ряда новых диагностических маркеров ответа яичников, а именно: FORT (follicle output rate, скорость фолликулярного выхода), FOI (follicle-to-oocyte index, фолликулярно-ооцитарный индекс) и OSI (ovarian sensitivity index, индекс чувствительности яичников) [29].
V. Genro et al. (2011) впервые был предложен прогностический маркер «бедного» ответа на ОС – скорость фолликулярного выхода (FORT). FORT представляет собой отношение преовуляторных фолликулов в день введения триггера овуляции к КАФ в день начала ОС.

Авторы отметили, что показатель FORT<50% в настоящем цикле ЭКО может быть использован, как предиктор для определения тенденции к недостаточному ответу на ОС в последующих программах ВРТ и может дополнить общепринятые клинико-лабораторные показатели (АМГ и КАФ) [30].
Другим предлагаемым прогностическим маркером является индекс чувствительности яичников (OSI) [31, 32]. Данный параметр рассчитывается, как отношение суммарной дозы гонадотропинов к числу полученных после трансвагинальной пункции ооцитов.
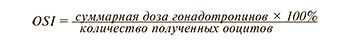
Существует мнение, что OSI является более прогностически значимым параметром «бедного» ответа на ОС, так как оценивается непосредственное число полученных ооцитов, что ассоциировано с кумулятивной частотой живорождения [32].
V. Biasoni et al. (2011) отмечают отрицательную корреляционную связь между OSI и АМГ (r=-0,67; p=0,0001), и эта корреляция является более сильной, чем между АМГ и суммарной дозой рФСГ (r=-0,49, p<0,01) или АМГ и числом полученных ооцитов (r=0,41, p<0,01) [32]. Однако необходимо проведение дальнейших исследований на большей выборке пациенток, поскольку до сих пор не установлено пороговое значение данного маркера и целесообразность его использования.
Также рассматривается еще один новый прогностический маркер «бедного» ответа на ОС – фолликулярно-ооцитарный индекс (FOI, follicle-to-oocyte index). Данный показатель представляет собой отношение числа полученных ооцитов после трансвагинальной пункции яичников к КАФ и может быть применен для выявления тенденции к неожиданно «бедному» или субоптимальному ответу на ОС у пациенток с бесплодием, а также свидетельствовать об овариальной резистентности к стандартным протоколам ЭКО [11].
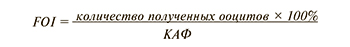
Сниженные показатели FOI (<50%) демонстрируют, что только часть антральных фолликулов из числа визуализируемых на старте ОС (2–3-й дни менструального цикла) подверглась росту под действием гонадотропинов, что поможет внести коррективы в разработку персонифицированного протокола стимуляции яичников [29].
В исследовании L. Chen et al. (2020) отмечается, что показатели FORT и FOI были выше в группе POSEIDON 3, по сравнению с остальными группами. Более того, несмотря на нормальные уровни АМГ и КАФ в группах POSEIDON 2 и 1, индексы FORT и FOI у пациенток с неожиданно «бедным» ответом на ОС были ниже, чем у пациенток в группе POSEIDON 3 [33].
В качестве дополнительных возможностей для прогнозирования «бедного» ответа на ОС обсуждается использование ВРТ калькулятора (http://www.members.groupposeidon.com/Calculator/). Калькулятор ВРТ основан на вычислении количества зрелых ооцитов (MII), полученных в результате стимуляции яичников и необходимых для получения одного эуплоидного эмбриона в программе ЭКО, пригодного для переноса в полость матки, с учетом возраста женщины и параметров спермограммы мужчины [2, 13]. В крупном многоцентровом исследовании S. Esteves et al. (2019) с помощью ВРТ-калькулятора было продемонстрировано, что необходимо минимум 10 ооцитов MII (95% CI 9–13) для 90% вероятности получения одного эуплоидного эмбриона у женщин в возрасте 35 лет [2].
Вышеперечисленные прогностические маркеры «бедного» ответа на ОС пока не внедрены в клиническую практику. Вероятно, применение комплексной оценки с учетом рутинных (возраст, АМГ, КАФ) и новых диагностических маркеров (FORT, FOI, OSI) поможет персонифицировать протоколы ЭКО для пациенток, стратифицируемых в соответствии с критериями POSEIDON. В связи с ограниченными данными прогностическая ценность этих новых маркеров требует дальнейшего изучения [34, 35].
Подходы к ведению пациенток с «бедным» ответом на овариальную стимуляцию в соответствии с критериями POSEIDON
В настоящее время с целью оптимизации программ ВРТ в группах POSEIDON активно обсуждаются различные варианты протоколов для улучшения исходов у пациенток с «бедным» ответом на ОС, которые часто используют эмпирически, а именно: повышенные дозы препаратов рФСГ, комбинированное использование менотропинов и рФСГ, применение рЛГ, различные режимы супрессии гипофиза («длинные» проколы с агонистом гонадотропин рилизинг-гормона (ГнРГ) и протоколы с антагонистом ГнРГ), применение «двойной» стимуляции в одном цикле и адъювантная терапия (препараты андрогенов, дегидроэпиандростерон (ДГЭА), ингибиторы ароматазы, а также коэнзим Q10 (CoQ10) [8, 12, 36].
Подходы к ведению пациенток POSEIDON 1 и 2
Пациентки с неожиданно «бедным» или субоптимальным ответом (1 и 2 группы POSEIDON) на ОС составляют 10–15% среди женщин с нормальными параметрами овариального резерва. В научной литературе появляется все больше примеров того, как специфические генетические характеристики гонадотропинов и их рецепторов могут влиять на реакцию яичников при проведении ОС, а также обоснованности проведения генетического скрининга для выявления значимых полиморфизмов гонадотропинов и их рецепторов, как предикторов неожиданно «бедного» ответа у женщин с нормальными параметрами овариального резерва [16, 37]. Внедрение фармакогеномного подхода уже хорошо зарекомендовало себя и явилось рентабельной стратегией в нескольких областях медицины, в т.ч. в онкологии и кардиологии [36]. Все больше данных указывает на необходимость применения фармакогеномного подхода к ОС. Так, пациентки с неожиданно «бедным» ответом на ОС и полиморфизмами гонадотропинов и их рецепторов нуждаются в увеличении дозы препаратов рФСГ и/или их комбинации с препаратами рЛГ для получения оптимального ответа на стимуляцию яичников [17, 37, 38].
В исследовании Behre et al. (2005) отмечено, что носителям аллеля 680Ser полиморфизма гена FSHR 2039 A>G (Asn680Ser) обоснованно увеличение дозы препаратов рФСГ при ОС [38]. Эти данные также демонстрируют и другие исследования в различных популяциях, что подтверждает влияние генетической изменчивости FSHR на число полученных ооцитов [8, 11, 37].
В ретроспективном исследовании Р. Drakopoulos et al. (2018) был проведен анализ пациенток с нормальными показателями овариального резерва и «бедным» ответом на ОС в предыдущем протоколе ЭКО. При увеличении дозы рФСГ отмечалось большее число ооцитов (9 против 6, р<0,001) и эмбрионов оптимального качества (4 против 3, р<0,001). Методом регрессионного анализа установлено, что повышение стартовой дозы рФСГ на 50 МЕ/сутки способствует получению большего числа ооцитов [35].
По результатам систематического обзора и метаанализа С. Alviggi et al. (2018) была рассмотрена целесообразность применения рЛГ в двух подгруппах пациентов: у пациенток с оптимальными параметрами овариального резерва и неожиданно «бедным» или субоптимальным ответом на ОС (1 и 2 POSEIDON), а также у пациенток старшего репродуктивного возраста (4 POSEIDON), в связи с активацией андрогенпродуцирующей функции тека-клеток фолликулов и оптимизацией фолликуло- и оогенеза при дополнительном назначении рЛГ пациенткам данных групп [39, 40]. По итогам исследования А. Conforti et al. (2019) показано значительное повышение числа полученных ооцитов (OR 1,98, р=0,03), частоты имплантации (OR 2,62, р=0,004) и частоты наступления беременности (OR 2,03, р=0,003) у пациенток с «бедным» ответом на ОС при добавлении рЛГ к препаратам ФСГ в протоколе ОС [41]. В ретроспективном когортом исследовании E. Papaleo et al. (2014), проведенном при участии 65 пациенток с неожиданно «бедным» ответом на ОС в анамнезе (1 и 2 POSEIDON), при добавлении 150 МЕ/сутки рЛГ в cледующем цикле ОС отмечалось получение большего количества ооцитов (р<0,001) и зрелых ооцитов (МII) (р<0,05), по сравнению с предыдущим протоколом ОС и монотерапией рФСГ [42]. Однако, по мнению S. Canosa et al. (2022), результаты гонадотропной стимуляции пациенток 1 и 2 группы POSEIDON с неожиданно «бедным» ответом на ОС, получавших комбинированную терапию препаратами рФСГ и рЛГ сопоставимы с пациентками, получавшими только препараты рФСГ (р<0,01). Скорее всего, применение препаратов рЛГ способствует улучшению качества и зрелости ооцитов, но не увеличивает их количество [43]. В связи с противоречивыми данными целесообразность применения комбинированной терапии препаратами рФСГ и рЛГ у пациенток с неожиданно «бедным» или субоптимальным ответом на ОС требует дальнейшего изучения.
В литературе обсуждается целесообразность проведения «двойной» стимуляции (DuoStim) у пациенток с неожиданным и ожидаемым «бедным» ответом [44, 45]. Так, использование DuoStim в 1 и 2 группах POSEIDON, предполагающую ее проведение в фолликулярную и в лютеиновую фазы менструального цикла, может увеличить число полученных ооцитов, поскольку известно, что за один менструальный цикл происходит рекрутирование двух или трех когорт антральных фолликулов [46]. Данный подход увеличивает шансы на получение генетически нормального эмбриона за более короткий срок времени; в связи с чем, DuoStim является оптимальной стратегией для 2 группы POSEIDON, включающей женщин старше 35 лет, поскольку с возрастом увеличивается частота анэуплоидии эмбрионов [45]. В работе M. Eftekhar et al. (2020) отмечалось получение большего числа ооцитов (р=0,004), зрелых ооцитов (р=0,016) и эмбрионов (р=0,013) в лютеиновую фазу менструального цикла, по сравнению с фолликулярной фазой при проведении «двойной» стимуляции пациенткам 1 и 2 групп POSEIDON [47].
Подходы к ведению пациенток POSEIDON 3 и 4
До сих пор нет единого мнения о преимуществах использования «длинного» протокола и протокола с антГнРГ у пациенток с ожидаемым «бедным» ответом. Известно, что использование протокола с антГнРГ сокращает продолжительность ОС (р=0,001); при этом используются меньшие дозы препаратов рФСГ (р=0,0001), а количество полученных ооцитов (р=0,02) и процент отмены циклов ЭКО сопоставимы с результатами, полученными в «длинном» протоколе [48].
У пациенток с «бедным» овариальным ответом (3, 4 POSEIDON) используют также программу ЭКО в естественном цикле и его модификации, стимуляцию яичников в лютеиновую фазу цикла, протокол Random-start (начало ОС с любого дня менструального цикла), комбинированную стимуляцию с использованием ингибиторов ароматазы [14, 15, 49, 50]. В случае отсутствия эффекта от проводимой терапии рекомендовано проведение программы ЭКО с донорскими ооцитами, что существенно повышает шансы на наступление беременности (р<0,001) [51].
У пациенток 3 и 4 групп POSEIDON предлагается использование протокола с антГнРГ и повышенных доз препаратов рФСГ (300–375 МЕ/сутки), а также комбинированное применение препаратов рФСГ и рЛГ, что способствует рекрутированию антральных фолликулов через синергическое действие с инсулиноподобным фактором роста-1 и получению большего числа ооцитов (95% СI 0,14–1,36) [15, 34, 43].
«Двойная» стимуляция также активно применяется и является методом выбора у пациенток 3 и 4 групп POSEIDON. В исследовании D. Cimadomo et al. (2018) при проведении «DuoStim» 188 пациенткам 3 и 4 POSEIDON было установлено, что качество и зрелость ооцитов, полученных в фолликулярную фазу, соответствовала качеству и зрелости ооцитов, полученных в лютеиновую фазу менструального цикла. Кроме того, количество ооцитов MII при ОС в лютеиновую фазу было выше, по сравнению с фолликулярной фазой цикла (р=0,05) [52].
Адъювантная терапия
В литературе обсуждается также возможность использования адъювантной терапии в качестве подготовки к протоколу ЭКО или непосредственно в протоколе стимуляции яичников у женщин с субоптимальным или «бедным» ответом для повышения эффективности программ ВРТ.
Одним из наиболее распространенных методов в качестве прайминга перед протоколом ЭКО у пациенток с «бедным» или субоптимальным ответом на ОС является использование комбинированных оральных контрацептивов (КОК) для синхронизации роста когорты фолликулов [53]. В рандомизированном контрольном исследовании C. Farquhar et al. (2017) продемонстрировано, что применение КОК перед стимуляцией яичников в протоколе с антГнРГ было ассоциировано с более низкими показателями прогрессирующих беременностей и частотой родов, по сравнению с группой пациенток, не использовавших КОК перед ОС (ОR 0,74, 95% CI 0,58–0,95) [53]. В метаанализе J. Li et al. (2021) показано, что назначение КОК в качестве подготовительной терапии к программе ЭКО не влияло на продолжительность ОС (95% CI -0,20–0,28), дозу препаратов рФСГ (95% CI -0,11–0,30), толщину эндометрия в день трансвагинальной пункции яичников (95% CI -1,28–0,24), количество полученных ооцитов (95% CI -0,08–0,48), а также на частоту наступления беременности (95% CI 0,68–1,01) по сравнению с контрольной группой, что ставит под сомнение целесообразность использования КОК в качестве подготовки к ОС у пациенток с «бедным» ответом и требует проведения дальнейших исследований [54].
Использование препаратов эстрогенов в качестве подготовки к протоколу ОС способствует гомогенизации пула антральных фолликулов за счет снижения роста ФСГ с конца лютеиновой фазы цикла, что может приводить к получению большего числа зрелых ооцитов [55]. В метаанализе K. Reynolds et al. (2013) было продемонстрировано, что у пациенток с «бедным» ответом в анамнезе, получавших терапию препаратами эстрогенов перед следующим протоколом ОС, отмечалась меньшая частота отмены цикла стимуляции яичников (95% CI 0,45–0,78) и более высокий процент наступления беременности, по сравнению с контрольной группой (95% CI 1,02–1,72); однако не было отмечено статистически значимой разницы в количестве полученных ооцитов [56].
Известно, что андрогены и их рецепторы играют важную роль в фолликулогенезе, рекрутинге фолликулов, ароматазной активности и продукции эстрогенов. ДГЭА-предшественник тестостерона, который является промотором роста фолликулов. В метаанализе X. Lin et al. (2019) показано, что использование прайминга препаратами ДГЭА у пациенток с «бедным» ответом на ОС способствовало получению большего количества ооцитов (р=0,009) и увеличению частоты наступления беременности (р=0,04), по сравнению с контрольной группой [57].
Большой интерес в качестве адъювантной терапии также представляет использование трансдермальной формы тестостерона для повышения эффективности программ ВРТ у пациенток c «бедным» ответом на ОС. A. Neves et al. (2022) продемонстрировали большее количество полученных ооцитов (95% CI 0,46–1,42), увеличение процента наступления беременности (95% CI 1,33–3,20) и коэффициента рождаемости (95% CI 1,11–3,95) при использовании трансдермальной формы тестостерона перед протоколом ОС у пациенток с «бедным» ответом, по сравнению с контрольной группой [58]. В исследовании M. Noventa et al. (2019) также показано существенное увеличение количества полученных ооцитов (р<0,00001), количества зрелых ооцитов (р<0,006) и частоты наступления беременности (р=0,0003) при использовании препаратов тестостерона перед протоколом ОС у пациенток с «бедным» ответом [59]. Однако J. Subirá et al. (2021) демонстрируют отсутствие статистически значимой разницы в числе полученных ооцитов в группах пациенток с «бедным» ответом на ОС, получавших подготовительную терапию трансдермальной формой тестостерона, и в контрольной группе (р=0,719) [60]. В связи с противоречивыми данными, целесообразность использования препаратов тестостерона в качестве прайминга перед протоколом ЭКО требует проведения дальнейших исследований [58–61].
В научной литературе встречается все больше данных о целесообразности использования гормона роста (ГР) у женщин с «бедным» ответом на ОС [62–64]. ГР оказывает позитивное воздействие на пролиферацию гранулезных клеток за счет увеличения продукции инсулиноподобного фактора роста-1 в фолликулярной жидкости. В работе Х. Liu et al. (2021) показано значительное увеличение количества полученных ооцитов в группах пациенток с неожиданно «бедным» ответом при использовании препарата ГР перед протоколом ОС (1 и 2 группы POSEIDON) (95% Cl 1,36–1,59, р<0,001; 95% Cl 1,15–1,49, р<0,001; в 1 и 2 группах POSEIDON, соответственно). Авторы отмечают улучшение качества эмбрионов на 3-и сутки развития, по сравнению с предыдущим протоколом ЭКО у пациенток 1, 2 и 3 групп POSEIDON (95% CI 1,78–2,56, р<0,001; 95% CI 1,26–1,89, р<0,001; 95% CI 1,10–1,98, р=0,010, соответственно) [63]. Данные об успешности использования препарата ГР в качестве адъювантной терапии также подтверждаются в нескольких исследованиях [62, 64].
Снижение концентрации коэнзима Q10 (СоQ10) в плазме крови ассоциировано с митохондриальной дисфункцией, изменением уровня стероидных гормонов, гипогонадизмом у пациенток с бесплодием и увеличением частоты анеуплоидии эмбрионов. По данным Y. Xu et al. (2018), при использовании препарата СоQ10 в качестве адъювантной терапии при подготовке к протоколу ОС у пациенток с «бедным» ответом, отмечается большее количество полученных ооцитов (р=0,002) и более низкий коэффициент отмены цикла гонадотропной стимуляции, по сравнению с контрольной группой (р<0,05) [65]. В систематическом обзоре Y. Zhang et al. (2020) продемонстрировано положительное влияние сочетанной терапии CoQ10 и ДГЭА на частоту наступления беременности у женщин с «бедным» ответом на ОС, по сравнению с контрольной группой (OR 2,46, 95% CI 1,16–5,23; OR 2,22, 95% CI 1,08–4,58, соответственно). Однако эти результаты нуждаются в проведении дальнейших исследований [36].
В настоящее время аутологичная плазма, обогащенная тромбоцитами (Platelet Rich Plasma, PRP) нашла широкое применение в репродуктивной медицине. Известно, что PRP содержит множество факторов роста и цитокинов, участвующих в эффективной регенерации тканей, индукции хемотаксиса, миграции и дифференцировки клеток, а также устраняет гипоперфузию яичников, улучшая транспорт кислорода к клеткам. Данные эффекты, вероятно, способствуют улучшению качества и зрелости ооцитов, что может повысить эффективность программы ЭКО у разнородной группы пациенток с «бедным» ответом на ОС [66]. Так, в исследовании M. Farimani et al. (2021) у пациенток четырех групп POSEIDON отмечалось статистически значимое увеличение количества полученных ооцитов после проведения внутрияичниковой PRP-терапии в протоколе ЭКО (р<0,001) [67]. Более того, количество ооцитов MII было значимо выше в группах 1, 3 и 4 POSEIDON (р<0,005), по сравнению с предыдущими протоколами ОС. Стоит отметить, что наибольшая частота наступления беременности (71,42%) была установлена в 1 группе POSEIDON. В связи с недостаточностью данных, необходимо проведение дальнейших исследований о влиянии PRP-терапии в качестве адъювантной терапии в протоколах ЭКО.
Заключение
Результаты многочисленных исследований демонстрируют преимущества стратификации обширной гетерогенной группы пациенток с «бедным» ответом на ОС по критериям POSEIDON. Использование в клинической практике критериев POSEIDON, уточнение прогностической ценности новых диагностических критериев «бедного» овариального ответа на гонадотропную стимуляцию (FORT, FOI, OSI), а также оценка целесообразности тестирования на полиморфизмы генов гонадотропинов и их рецепторов пациенток с непрогнозируемым «бедным» ответом на ОС могут помочь лучше оценить большую разнородную группу пациенток с «бедным» ответом на ОС и разработать персонифицированные подходы к ОС в программе ЭКО, что требует проведения дальнейших исследований.



