Генитальный герпес (ГГ) – инфекция, передаваемая половым путем (ИППП), может быть вызвана вирусами простого герпеса (ВПГ) 1-го и 2-го типов. ВПГ-2 является наиболее распространенной причиной генитальных язв во многих странах. 19,2 миллиона новых инфекций, вызванных ВПГ-2, произошло среди взрослых и подростков в возрасте 15–49 лет в мире в 2012 году, причем самые высокие показатели наблюдались среди младших возрастных групп [1]. Глобальная распространенность ГГ, вызванного ВПГ-2, составляет 11,3% (417 млн человек) [2]. ВПГ-1 обычно передается без половых контактов (оральная инфекция). Однако этот тип вируса может также передаваться половым органам через оральный секс и все чаще отмечается как причина ГГ, особенно в развитых странах [3]. 140 млн людей имели генитальную инфекцию, вызванную ВПГ-1, в 2012 году [4].
В Российской Федерации, по данным официальной статистики, показатель заболеваемости ГГ в 2014 году составил 14,2 случая на 100 000 населения, у лиц в возрасте старше 18 лет – 17,2 случая [5].
У женщин ВПГ-2 встречается чаще, чем у мужчин: по данным ВОЗ в 2012 году носителями инфекции являлись 267 млн женщин и 150 млн мужчин. Это объясняется тем, что половым путем ВПГ передается легче от мужчины к женщине, чем от женщины к мужчине [6].
ВПГ-инфекция вызывает особую озабоченность в связи с ее эпидемиологической синергией с ВИЧ-инфекцией [7]. Люди, инфицированные ВПГ-2, примерно в 3–5 раз чаще инфицированы ВИЧ, и генитальный герпес встречается у 80% лиц с ВИЧ-инфекцией [8]. Поэтому лица с ВПГ, должны быть тестированы на ВИЧ. Ко-инфекция ВПГ-2 с ВИЧ часто протекает в более тяжелой форме и с более частыми рецидивами [9].
ГГ является пожизненным состоянием, при котором вирус персистирует в нервных ганглиях и характеризуется периодическими симптоматическими рецидивами после первого эпизода, иногда – частыми. Первичная инфекция в большинстве случаев является бессимптомной или нетипичной, поэтому не диагностируется. Классическая клиническая картина при первом эпизоде заболевания встречается только в 10–25% случаев [10].
Инфекция сопровождается периодическим выделением вируса со слизистой оболочки половых органов, даже при отсутствии симптомов. В результате ВПГ-2 часто передается во время полового контакта партнерам от пациентов, которые не знают о ее наличии [9].
Принципы лечения генитального герпеса
Тактика лечения ГГ должна рассматриваться для следующих клинических условий: первый клинический эпизод ГГ; рецидивирующий ГГ (эпизодическая терапия); рецидивирующий ГГ с частыми обострениями и/или тяжелым течением или сопровождающийся осложнениями (супрессивная терапия) [9].
Противовирусная химиотерапия ациклическими нуклеозидами, блокирующими репликацию вируса (ацикловир, валацикловир и фамцикловир) является основным методом лечения пациентов с клиническими проявлениями [9–11]. Указанные средства помогают снизить тяжесть, продолжительность и частоту симптомов, однако не уничтожают вирус, не влияют на риск, частоту или тяжесть последующих рецидивов после прекращения приема препарата.
Частота и выраженность клинических проявлений заболевания связана с состоянием иммунной системы организма [12, 13]. При этом клеточные интерферон-γ-зависимые механизмы играют доминирующую роль в контроле распространения инфекции [13].
Предупреждение инфицирования ВПГ среди беременных женщин особенно важно на поздних сроках беременности, поскольку в этот период риск развития неонатального герпеса является максимальным [11].
Современные рекомендации для лечения генитального герпеса
На протяжении последних 3 лет ведущими медицинскими сообществами были пересмотрены рекомендации по лечению многих ИППП, в том числе и ГГ. Это связано с достижениями медицины в области профилактики, диагностики и лечения ИППП. Так, выявлены новые данные в эпидемиологии ИППП (вирусные патогены приобрели бóльшую распространенность, чем бактериальные) и определены изменения устойчивости к противомикробным препаратам. В связи с этим возникла необходимость в увеличении продолжительности лечения для первого эпизода ВПГ-инфекции и в проведении супрессивной терапии среди пациентов с ВПГ-инфекцией в районах с высоким уровнем ВИЧ [9].
Целью лечения клинических эпизодов ГГ является уменьшение продолжительности и тяжести генитальных поражений.
Лечение первого эпизода генитальной инфекции проводится по стандартным схемам: ацикловир 200 мг 5 раз в день или 400 мг 3 раза или валацикловир 500 мг 2 раза или фамвир 250 мг 3 раза (уровень доказательности – А). Продолжительность терапии клиническими рекомендациями 2014 года (RCOG) [14] определена в течение 5 дней, 2015 года (CDC [11], РОДВиК [15]) – 7–10 дней, однако уже в 2016 году рекомендуемая экспертами ВОЗ продолжительность лечения первого эпизода составляет 10 дней [9].
Данная рекомендация применима к взрослым, подросткам, а также к людям, живущим с ВИЧ, с ослабленным иммунитетом, с тяжелым эпизодом и беременным женщинам [9].
Отмечается, что все из перечисленных выше препаратов имеют одинаковую эффективность (А). При этом среднее время до заживления герпетических поражений сопоставимо (5,1, 4,25, 4,08 дня соответственно) [16], а продолжительность выделения вируса снижается на 9 и более дней (-9,2 дн.; 95% ДИ: 11,1–7,29) по сравнению с плацебо [17].
Однако, принимая во внимание современные требования к лекарственным средствам в отношении комплаентности, следует отметить, что преимуществом валацикловира является удобство менее частого режима дозирования. Объясняется это тем, что ацикловир плохо абсорбируется в желудочно-кишечном тракте – его пероральная биодоступность составляет 10–30% [18].
Валацикловир по своей структуре отличается от ацикловира наличием аминокислотного валина, присоединенного к 5-гидроксильной группе нуклеозида, и его биодоступность превосходит ацикловир более чем в 10 раз, составляя 55% (по данным F.G. Hayden – до 70%), а концентрация в крови сопоставима с парентеральным введением ацикловира [19, 20].
Фамцикловир после приема внутрь быстро превращается в активный пенцикловир, биодоступность которого составляет 77%, однако время достижения максимальной концентрации в крови аналогично валацикловиру (2 часа), а меньшая разовая доза требует более частого суточного приема, что может снизить комплаентность [21]. Кроме того, сравнение эффективности супрессивной терапии валацикловиром и фамцикловиром в двух рандомизированных клинических исследованиях, выполненное A. Wald с соавт., показало, что время до первого вирусологически подтвержденного рецидива было короче среди получателей фамцикловира (относительный риск ОР=2,15; 95% ДИ: 1,00–4,60 и 2,33; 95% ДИ: 1,18–4,89) [22].
Дополнительное преимущество было недавно описано для валацикловира – прием препарата по 500 мг два раза в день ассоциировалось с 44% вероятностью прекращения распространения поражений. Прерывание клинической манифестации генитального герпеса наблюдается почти в два раза чаще, когда лечение начинается в течение 6 часов после начала симптомов по сравнению со сроком через 6 часов. Это преимущество не было описано для ацикловира или фамцикловира [23].
Местные химиотерапевтические средства менее эффективны, чем пероральные, а сочетание орального и местного лечения не является более эффективным по сравнению с пероральным лечением [14].
По окончании острой фазы инфекции свободный ВПГ-2 более не выделяется, что обусловливает наступление латентной фазы заболевания.
Эпизодическая терапия проводится при рецидивирующем ГГ. Продолжительность ее составляет 2–5 дней в зависимости от выбранного препарата (5 дней: ацикловир 400 мг 3 раза в сутки или 800 мг 2 раза или фамцикловир 250 мг 2 раза; 3 дня: валацикловир 500 мг 2 раза; 2 дня: ацикловир 800 мг 3 раза в сутки) [9]. Лечение необходимо начинать во время продромальной фазы или в первые 24 часа после появления симптомов. Указанные дозы препаратов подходят для взрослых, подростков и беременных женщин [9]. Сокращение сроков лечения повторных эпизодов ГГ связано с тем, что рецидивы, как правило, отличаются меньшей тяжестью проявлений и более быстрым их разрешением. Для лиц, живущих с ВИЧ, терапия должна продолжаться не менее 5 дней (ацикловир 400 мг 3 раза, валацикловир или фамвир по 500 мг 2 раза в сутки) [9].
Отмечается, что кратковременные курсы эпизодической терапии являются более удобными и экономически эффективными стратегиями и должны рассматриваться как опции первой линии [14].
Оценка частоты и тяжести эпизодов проводится в первые 3–5 месяцев. Супрессивная терапия необходима при повторных клинических эпизодах, которые являются частыми (4 и более в год), тяжелыми или сопровождаются стрессом (ацикловир 400 мг 2 раза в сутки, валацикловир 500 мг или 1 г 1 раз или фамцикловир 250 мг 2 раза). Валацикловир 500 мг один раз в день может быть менее эффективным, чем доза 1 г для лиц, у которых наблюдаются очень частые рецидивы (более 10 эпизодов в год) [9].
Продолжительность супрессивной терапии определяется индивидуально (обычно 1 год). При стойком улучшении она может быть завершена [9]. Эффективность ее оценивают как минимум по двум рецидивам в год либо по одному – за 6 месяцев. Если течение заболевания ухудшается, то в дальнейшем супрессивная терапия может быть продолжена (С) или выбрана терапия короткими курсами по 5 дней (С) [14].
Отметим, что ранее решение о назначении супрессивной терапии рекомендовалось принимать при частоте повторных эпизодов 6 и более раз в год [14]. В настоящее время эту стратегию следует рассматривать при их частоте 4 и более в год [9].
Супрессивная терапия снижает частоту эпизодов ГГ на 70–80% у пациентов с частыми рецидивами, а их продолжительность составляет всего 7–10 часов [24]. 85% лиц, получающих такую терапию, не испытывают симптомов обострения заболевания (субклиническое течение) [25]. Лечение также эффективно у пациентов с менее частыми рецидивами. Безопасность и эффективность были подтверждены среди пациентов, получающих ежедневную терапию всеми препаратами в течение 1 года и даже периодом наблюдения при непрерывном приеме в течение 20 лет (для ацикловира и валацикловира – В) [14]. Качество жизни улучшается у многих пациентов с часто повторяющимися рецидивами, получающих супрессивную терапию, по сравнению с эпизодическим лечением [25].
Оценка эффективности подавления рецидивирующего ГГ была проведена в сравнительном рандомизированном двойном слепом исследовании среди 1479 иммунокомпетентных пациентов. Пациенты были рандомизированы для приема валацикловира (250 мг, 500 мг или 1 г один раз в день или 250 мг два раза в день), ацикловира (400 мг два раза в день) или плацебо в течение 1 года. Все дозы валацикловира были значительно более эффективными, чем плацебо, для предотвращения или задержки рецидивов (р<0,0001). Соотношение доза-реакция (р<0,0001) наблюдалось по схеме валацикловира один раз в день. Курсы лечения валацикловиром и ацикловиром дважды в день были одинаковы по эффективности. Пациенты, у которых наблюдалось менее 10 рецидивов в год, эффективно лечились валацикловиром дозой 500 мг один раз в день. Один грамм валацикловира один раз в день, 250 мг валацикловира два раза в день или 400 мг ацикловира два раза в день были эффективны у пациентов с 10 и более рецидивами в год. Профили безопасности всех видов лечения были сопоставимы [26].
Супрессивная терапия при ГГ оказывает положительное психосоциальное действие, а также уменьшает степень риска его передачи. Так, лечение валацикловиром 500 мг в день снижает вероятность передачи ВПГ-2 у дискордантных гетеросексуальных пар, в которых партнер-источник имеет генитальную ВПГ-2 инфекцию [14, 27]. Таким парам рекомендуется супрессивная антивирусная терапия как часть стратегии предотвращения передачи инфекции в дополнение к постоянному использованию презервативов и избеганию сексуальной активности во время рецидивов. Супрессивная терапия также может снизить передачу инфекции, когда используется лицами, имеющими несколько партнеров, и теми, кто является серопозитивными без клинических проявлений ГГ.
Выбор лекарственных средств зависит также от комплаентности пациента и стоимости лечения [14].
Учитывая клинические преимущества валацикловира перед ацикловиром и фамцикловиром, варианты выбора по второму показателю осуществляются между оригинальным препаратом (валтрекс) и дженериками (валвир и др.).
Качество дженерических препаратов на российском фармацевтическом рынке продолжает активно обсуждаться. При этом все авторы отмечают, что соответствие стандартам GMP (качественная производственная практика) является гарантией качества продукта (сертификаты GMP имеют все лекарства европейского производства, в отличие от российских). Помимо этого Всемирная организация здравоохранения предъявляет особые требования к эквивалентности дженериков оригинальному препарату – требуется определять биоэквивалентность (сравнительные исследования фармакокинетики), фармацевтическую эквивалентность (полное совпадение всех наполнителей и красителей в оригинальном и дженериковом препаратах) и терапевтическую эквивалентность (доказательство аналогичного клинического действия) [28]. Согласно требованиям в РФ, определяется только биоэквивалентность, причем с очень значительным разбросом данных по сравнению с оригиналом, что может привести к снижению показателей клинической эффективности.
Сравнительная стоимость препаратов валацикловира европейского производства, зарегистрированных в РФ, приводится в таблице.
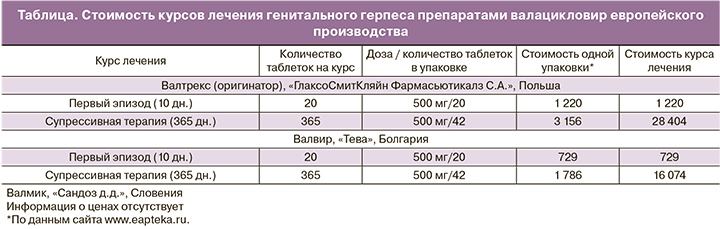
Эксперты CDC также отмечают, что стоимость супрессивной противовирусной терапии ГГ не должна рассматриваться как барьер. В США анализ стоимости ацикловира, валацикловира и фамцикловира, назначенных на один год, четко указывает на экономическое преимущество валацикловира в режиме 500 мг один раз в день [23].
Рецидивирующие ВПГ-инфекции различной локализации следует рассматривать как клиническое проявление вторичного иммунодефицита, что является показанием для включения в схему лечения иммуномодуляторов неспецифического действия, которые способствуют активации клеток врожденного иммунитета и продукции эндогенных цитокинов, включая ИФН I и II типов, влияющие на разные этапы жизненного цикла практически всех вирусов [12, 13]. В российских клинических рекомендациях в таких случаях указывается на необходимость применения интерферона системного действия — интерферон-γ 500 000 МЕ 1 раз в сутки подкожно через день, на курс 5 инъекций (В) [15].
Кроме того, консервативная терапия препаратами из группы интерферонов или синтетических индукторов интерферона рекомендована пациентам, инфицированным ВПГ-1 и ВПГ-2, независимо от локализации простого герпеса и степени тяжести заболевания (А) [29].
Препараты с бифункциональной активностью, оказывающие одновременно противовирусное и иммуномодулирующее действие, могут иметь преимущество, в связи с отсутствием чрезмерной активации Т-клеток и/или восстановлением баланса цитокинов с преобладанием Т1, которые ассоциированы с эффективным противовирусным ответом [30].
Современные рекомендации для лечения генитального герпеса у беременных женщин
Беременным в I или во II триместре с первичным клиническим эпизодом ГГ и тем, у кого имеется высокая частота рецидивов (более 6 раз в год), рекомендован прием противовирусных препаратов для их лечения в дозах, описанных выше, а также в последние 4 недели беременности. Такая тактика снижает риск рецидива заболевания [31]. При возникновении первичного эпизода болезни в III триместре лечение рекомендуется продолжать ежедневно по схеме супрессивной терапии до родов [32].
Согласно данным, представленным в систематическом обзоре, беременные женщины, получавшие противовирусную терапию в III триместре, имели значительно меньшую вероятность рецидива ГГ при родах (относительный риск ОР=0,28; 95% доверительный интервал ДИ: 0,18–0,43) и вероятность выделения вируса во время родов (ОР=0,14; 95% ДИ: 0,05–0,39), а также значительно реже подвергались кесаревому сечению (ОР=0,30; 95% ДИ: 0,20–0,45) [33].
Ацикловир и валацикловир не лицензируются для использования во время беременности, но считаются безопасными и не связаны с увеличением числа случаев врожденных дефектов [34]. Имеются ограниченные данные для фамцикловира, и поэтому он не может считаться средством выбора для лечения герпеса во время беременности [35].
Если первичный эпизод ГГ возник после 34-й недели беременности или при наступлении родов, то существует значительный риск вирусовыделения во время родов. В этом случае профилактика неонатального герпеса может быть достигнута путем планирования кесарева сечения. При неизбежности родоразрешения через естественные родовые пути, необходимо проводить лечение не только матери, но и ребенка [36].
Риск заражения новорожденного при рецидивирующем герпесе низкий (0–3%), и роды могут быть завершены естественным путем [37].
Имеются ограниченные доказательства для выбора оптимальной акушерской тактики, когда преждевременный разрыв плодных оболочек (ПРПО) осложняется первичной герпетической инфекцией. Если решение принято в пользу немедленного родоразрешения, то ожидаемые преимущества кесарева сечения сохраняются. Если есть возможность консервативного ведения, матери следует рекомендовать принимать внутривенно ацикловир 5 мг/кг каждые 8 часов [37].
Если роды планируется завершить в течение 6 недель после первичной инфекции ВПГ, кесарево сечение может по-прежнему быть актуальным, несмотря на длительный промежуток после ПРПО. В случае ПРПО до 34 недель имеются данные, свидетельствующие о целесообразности использования выжидательной терапии на фоне перорального приема антивирусных препаратов у женщин с рецидивирующим герпесом [36].
Заключение
Обновление рекомендаций по противовирусной терапии генитального герпеса направлено на повышение комплаентности и клинической эффективности лечения.
Ограничения. Имелось мало доступных данных для прямого сравнения противовирусных средств, в частности с фамцикловиром, а также различных доз препаратов. Отмечено недостаточно сведений для ключевых групп населения, таких как лица, живущие с ВИЧ, пациенты с иммунодефицитом и беременные женщины. Не освещены вопросы резистентности к рекомендуемым средствам терапии генитального герпеса.



