Существование человека в окружающей среде, заполненной микроорганизмами, возможно только благодаря высокоэффективным механизмам защиты. Врожденная иммунная система представляет собой барьер первой линии, механизм быстрого реагирования для предотвращения микробной инвазии. Ее компоненты наследуются от родителей и направлены против молекул, экспрессируемых только микроорганизмами [1, 2]. Время размножения большинства бактерий составляет 20–30 мин, а для развития специфического адаптивного иммунного ответа с образованием антител необходимо от нескольких дней до 2–3 недель. Врожденная иммунная система защищает хозяина в период времени между инфицированием и появлением первичной иммунной реакции [2].
Механизмы, с помощью которых функционируют врожденная и адаптивная (приобретенная) иммунные системы, принципиально отличаются. Врожденная иммунная система распознает микробы через рецепторы распознавания образов (PRR), специфичных для молекулярных компонентов микроорганизмов. Гены, кодирующие экспрессию данных рецепторов, подвержены незначительной модификации от поколения к поколению [1, 3]. Адаптивные иммунные ответы постоянно корректируются на протяжении жизни человека. Каждый Т- и В-лимфоцит в процессе функционирования и контакта с возбудителями приобретает уникальный набор рецепторов, который не передается по наследству. Основными функциями врожденной иммунной системы являются обнаружение и защита от чужеродных микроорганизмов, поддержание «иммунологического гомеостаза», активация и индивидуализация адаптивных иммунных реакций и регуляция процессов воспаления [4]. Компонентами врожденной иммунной системы являются [5, 6]:
- физические барьеры – плотные соединения на коже, поверхностях эпителия и слизистой оболочки, слизь, эндотелиальные клетки сосудов;
- ферменты, вырабатываемые в эпителиальных и фагоцитарных клетках (лизоцим);
- сывороточные белки, участвующие в воспалительных реакциях (компоненты комплемента, С-реактивный белок, лектины и фиколины);
- антимикробные пептиды (дефенсины, кателицидины) на поверхности клеток и в гранулах фагоцитов;
- клеточные рецепторы, реагирующие на микроорганизмы и запускающие продукцию цитокинов и интерферона (Toll-подобные рецепторы TLR);
- клетки, выделяющие цитокины и другие медиаторы воспаления (макрофаги, тучные клетки, естественные клетки-киллеры (NK), врожденные лимфоидные клетки (ILCs));
- фагоциты (нейтрофилы, моноциты, макрофаги).
Основным внешним барьером являются эпителиальные клетки, которые функционируют как тканевые дозорные за счет экспрессии мембраносвязанных PRR. PRR позволяют идентифицировать патоген-ассоциированные молекулярные структуры (PAMP) и реагировать быстрее, чем нейтрофилы и моноциты. Эпителиальные клетки обеспечивают непрерывный физический барьер, а также механизмы очистки (мукоцилиарная система, поверхностные антимикробные пептиды) для защиты от внешней среды [7].
Секретируемые и циркулирующие молекулы распознавания образов включают в себя антимикробные пептиды (АМП; лектины и пентраксины). Они обеспечивают непосредственное уничтожение микробов, действуют как вспомогательные белки для трансмембранных рецепторов и функционируют в качестве усилителей фагоцитоза (опсонинов) для эффекторных клеток.
AMП представляют собой группу секретируемых PRR, которые важны для защиты кожи и слизистых оболочек, а также для уничтожения фагоцитированных организмов. AMП, выделяемые на эпителиальные поверхности в месте повреждения, создают микробицидный щит, который повреждает микроорганизмы до их прикрепления и вторжения. Они обладают бактерицидным действием против широкого спектра бактерий, грибков, хламидий, паразитов и вирусов [5–9]. AMП существуют во многих различных формах и структурах, но все они содержат кластеры гидрофобных, катионных аминокислот, которые связываются с отрицательно заряженными фосфолипидами во внешнем бислое бактериальных мембран. Наружные клеточные мембраны человека содержат липиды (включая холестерин), которые отличаются от таковых у микробов, и AMП на них не реагируют. Благодаря многообразию АМП, бактерии не приобретают к ним устойчивости. Вторая важная функция АМП заключается в контроле над составом комменсальных микроорганизмов, которые колонизируют тело человека [5, 6].
Универсальной антибактериальной молекулой является дефенсин. Он представляет собой короткий пептид (30–45 аминокислот), составляет от 2 до 4% клеточного белка нейтрофилов и высвобождается в фагоцитарную вакуоль с захваченными микроорганизмами. Дефенсины напрямую убивают микроорганизмы или образуют микроскопические наносети, которые захватывают клетки в ловушку [7]. Альфа-дефенсины содержатся в нейтрофилах и синтезируются клетками Панета в кишечных криптах [6, 7]. Бета-дефенсины экспрессируются на всех эпителиальных поверхностях, включая дыхательные пути, мочеполовой и желудочно-кишечный тракты, рот, роговицу, конъюнктиву и кожу. Инфекционное или травматическое повреждение эпителия вызывает образование воспалительных цитокинов, которые стимулируют выработку бета-дефенсина [7–9].
Toll-подобные рецепторы (TLR) представляют собой трансмембранные PRR, которые обнаруживаются в моноцитах, макрофагах, эпителиальных клетках, нейтрофилах, а также в дендритных и многих других клетках [10, 11]. TLR распознают множество PAMP, включая микробные компоненты клеточной стенки, белки и нуклеиновые кислоты. Передача сигналов TLR приводит к изменениям транскрипционных факторов, которые регулируют множество генов, в том числе кодирующих важные провоспалительные цитокины. TLR транскрибируют секрецию мощных провоспалительных цитокинов, включая фактор некроза опухоли (TNF), интерлейкин-6 (IL-6) и про-IL-1-бета. TLR3, -4, -7, -8 и -9 могут запускать продукцию интерферонов 1-го типа (IFN-альфа, -бета и -лямбда). Именно через активацию сигналов через TLR на амниотические эпителиальные клетки происходят выброс цитокинов и прерывание беременности [12].
Микробиом человека – это совокупность бактерий, грибков и вирусов, которые в норме живут в теле человека и являются компонентами врожденной иммунной системы [11, 12]. Микробный состав организма напрямую влияет на созревание иммунного ответа и его эффективность, защищает от чрезмерного роста патогенных микроорганизмов и модулирует баланс между воспалением и иммунным гомеостазом [13, 14]. Изменение состава, разнообразия или метаболитов микробиома называется дисбиозом. Считается, что первоначально развивается дисбаланс факторов врожденного иммунитета, что приводит к нарушению бактериологического гомеостаза и развитию дисбиоза [15, 16]. В связи с этим без восстановления нормального состояния факторов врожденного иммунитета достичь нормализации показателей микробиоценоза может быть невозможно или эффект терапии окажется кратковременным.
Целью нашего исследования стало изучение показателей врожденного иммунитета слизистой оболочки влагалища в I триместре беременности при бактериальном вагинозе.
Было проведено проспективное исследование 50 беременных с бактериальным вагинозом. Критериями включения стали: срок гестации 12 недель, первая спонтанная одноплодная беременность, отсутствие эндокринных, инфекционных и анатомических причин невынашивания беременности, отсутствие инфекций, передающихся половым путем, аэробного вагинита и кандидозного кольпита, отсутствие воспалительной реакции во влагалище (уровень лейкоцитов ≤15), согласие на участие в исследовании, возраст от 18 и до 40 лет, диагностированный бактериальный вагиноз (по критериям Амселя). Дизайн работы представлен на рис. 1.
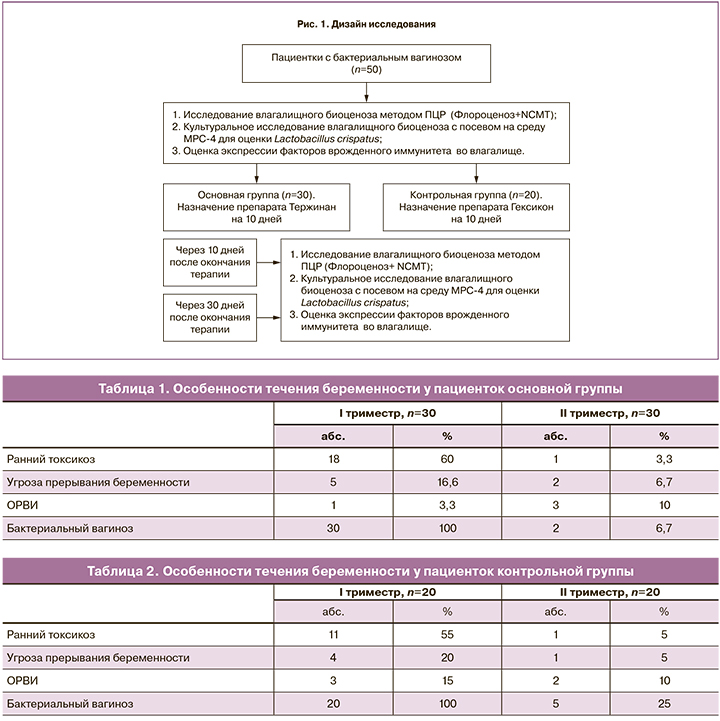
Материалы и методы
Возраст пациенток основной группы находился в пределах от 20 до 38 лет (31±3,1 года), возраст беременных контрольной группы был в пределах 18–37 лет (28±2,4 года). В структуре экстрагенитальных заболеваний патология органов желудочно-кишечного тракта (ЖКТ) встречалась с большей частотой у пациенток основной группы (43,3% против 25%), хронические инфекционные заболевания дыхательной (33,3 и 30%) и мочевыделительной (43,3 и 40%) систем по частоте встречаемости в группах отличий не имели.
Менструальный цикл у большинства пациенток сформированных групп был регулярным (93,3 и 85%). Среди гинекологических заболеваний наиболее распространенными были кандидозный кольпит (86,6 и 75%), эктопия шейки матки (50 и 70%), миома матки (6,6 и 0%).
Особенности течения данной беременности отражены в табл. 1–2.
В I триместре у всех пациенток среди осложнений преобладали ранний токсикоз (60 и 55%) и угроза прерывания беременности (16,6 и 20%). Наиболее распространенным осложнением во II триместре была угроза прерывания беременности (6,7 и 5%). На момент включения в исследование пациентки не получали терапию с интравагинальным способом введения препаратов.
Микробиологическое исследование. Качественный и количественный анализ микрофлоры влагалища проводился методом комплексного ПЦР-анализа, Флороценоз+NCMT (лаборатория CMD), и культурального исследования. Материал из влагалища брали до проведения мануального исследования, после введения зеркал. Исследование проводилось троекратно: в момент включения, через 10 и 30 дней после окончания терапии. Полученный материал помещался в специальные пробирки с транспортной средой и поглотителем кислорода Эймса (Medical Wire, Англия). Пробирки в течение 1,5–2 ч доставляли в лабораторию, где проводился посев на селективные и дифференциально-диагностические среды (среда Эндо, агар Сабуро, 5% кровяной агар, агар МРС-4). Посевы инкубировались в течение 24–48 ч, видовую идентификацию выделенных лактобактерий проводили масс-спектрометрическим методом.
Иммунологическое исследование проводили на кафедре иммунологии МБФ ФГБОУ ВО «РНИМУ им. Н.И. Пирогова» (зав. кафедрой д.м.н., профессор Ганковская Л.В.). Была определена экспрессия генов врожденного иммунитета TLR9, дефенсина HBD1 и TNF-α в эпителиальных клетках многослойного плоского эпителия влагалища. В ходе работы использовали наборы для выделения РНК и проведения реакции обратной транскрипции и ПЦР в режиме реального времени. Забор материала проводили при помощи цитощеток из заднего свода влагалища (до лечения, через 10 и 30 дней после окончания терапии), переносили в пробирки и замораживали при температуре –25о С. Реакцию обратной транскрипции проводили в объеме 25 мкл. Реакционная смесь содержала 3 мкл РНК-матрицы, 1 мкл random (Синтол, РФ) и 9 мкл ddH2O. Смесь инкубировали при температуре +75о С в течение 5 минут, далее пробирки охлаждали до +4 оС. Полученную кДНК хранили при –70о С. Реакцию ПЦР в режиме реального времени проводили в объеме 25 мкл с использованием праймеров фирмы Синтол (РФ). Подсчет уровня экспрессии целевых генов оценивали относительно гена домашнего хозяйства – β-актина.
Статистическую обработку результатов проводили с использованием программного обеспечения Microsoft Excel 2016 и Statistica 6.1. Полученные данные проходили проверку на нормальность распределения с учетом малой выборки, по результатам которой установлено, что полученные выборки имеют отклонение от нормальности, вследствие чего целесообразно применять непараметрические критерии для сравнения групп. Сравнение показателей до и после лечения проводили с использованием критерия Вилкоксона. Результаты считались достоверными при уровне вероятности ошибки p<0,01.
Результаты
Критерием включения в исследование было наличие бактериального вагиноза, установленного согласно наличию 3 из 4 критериев Амселя (обильные выделения из влагалища, рН≥4,5, положительный аминный тест и наличие ключевых клеток в мазке с окрашиванием по Граму). После постановки диагноза 30 беременных получали терапию комбинированным препаратом Тержинан (тернидазол 200 мг, неомицина сульфат 100 мг, нистатин 100 000 ЕД и преднизолон 3 мг), а 20 пациенток – препаратом хлоргексидина биглюконатом. Переносимость терапии была хорошей, все пациентки проявили высокую комплаентность.

В табл. 3 представлены результаты микробиологического исследования, выполненного методом ПЦР (Флороценоз+NCМT).
Согласно микробиологическому заключению, диагноз «бактериальный вагиноз» был подтвержден в 100% случаев, через 10 дней терапии с использованием препарата Тержинан сохранялся у 9 (30%) пациенток, через 30 дней — у 3 (10%). Это свидетельствует о том, что об окончательной эффективности терапии необходимо судить не ранее чем через 4 недели после ее окончания. Это позволит снизить частоту ложноположительных результатов и преждевременного назначения повторных курсов лечения. В случае использования препарата Тержинан эффективность лечения бактериального вагиноза составила 90%.
Среди пациенток основной группы Ureaplasma parvum выявлялась у 43,3%, Mycoplasma hominis – у 33,3%. Их титр значительно снизился к 30-му дню после окончания лечения, хотя препарат Тержинан не оказывает прямого эффекта на данных возбудителей.
Обращает на себя внимание общая высокая обсемененность бактериями, присутствующая до лечения и сохраняющаяся через 30 дней после окончания терапии. Однако меняется видовой состав представленных микроорганизмов. По данным ПЦР-диагностики, у всех беременных с бактериальным вагинозом исходно титр лактобактерий превышал 7 lg ГЭ/мл, однако при культуральном исследовании их рост отсутствовал у 30% (n=10) (рис. 2), а в остальных случаях не превышал 4 lg КОЕ/мл.
Определение видового состава лактобактерий показало, что до начала лечения L. crispatus были идентифицированы только у 10%, среди выявленных типов преобладали L. rhamnosus (у 73,3%) и L. jensenii (у 40%).
Через 30 дней после окончания терапии методом ПЦР выявлено достоверное увеличение титра лактобактерий, в 10 раз превышающее исходные показатели, по данным культурального исследования рост колоний превышал 6 lg КОЕ/мл, а у 60% пациенток отмечены L. crispatus.
У беременных контрольной группы Ureaplasma parvum выявлялась у 25%, Ureaplasma urealyticum у 15%. На фоне и после проведения терапии данные микроорганизмы сохранялись в исходном титре (табл. 4).
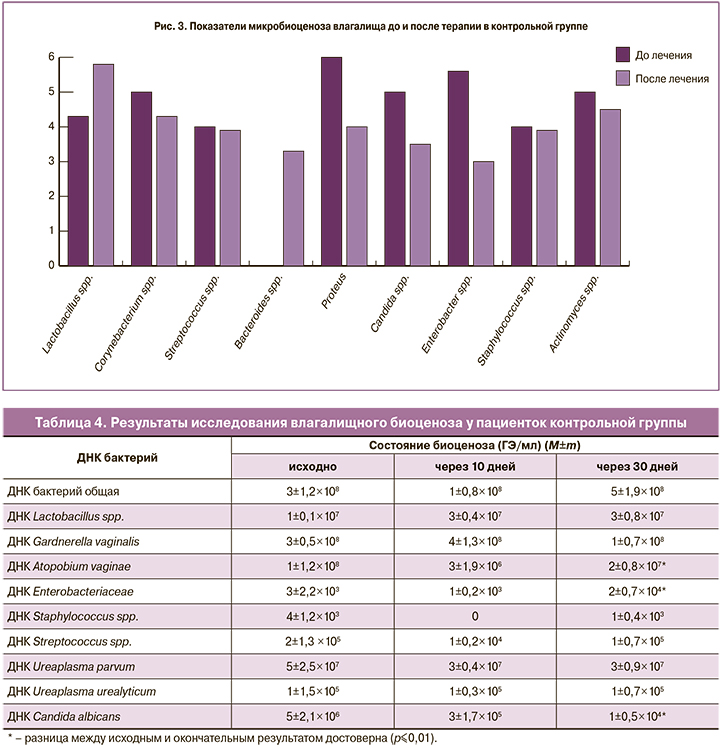
По данным ПЦР-диагностики в 100% случаев исходный титр лактобактерий был в пределах 7 lg ГЭ/мл, при культуральном исследовании рост лактобактерий отсутствовал у 35% (n=6), а в остальных случаях их титр составлял 4–5 lg КОЕ/мл. По видовому составу исходно выявлены L. rhamnosus (у 75%), L. jensenii (у 25%), L. crispatus (у 15%) и L. fermentun (у 10%).
Через 30 дней после окончания терапии методом ПЦР выявлено увеличение титра лактобактерий до 7 lg ГЭ/мл, по данным культурального исследования в среднем рост бактерий достигал 6,5 lg КОЕ/мл. Среди излеченных пациенток лактобактерии L. crispatus выявлялись в 55% случаев. Однако среди женщин с отсутствием эффекта от проведенного лечения (55%) суммарный титр лактобактерий не превышал 4 lg КОЕ/мл (рис. 3).
Эффективность терапии бактериального вагиноза в контрольной группе составила 55%, через 10 дней лечения препаратом Гексикон в 25% (n=5) сохранялись микробиологические признаки заболевания, а через 30 дней частота его возросла до 45% (n=9).
Сравнение представленных вариантов терапии изначально было неравнозначным, так как поликомпонентный препарат Тержинан очевидно более эффективен, нежели антисептическое средство. Но нас интересовала не столько эффективность лечения, сколько влияние выбранных препаратов на состояние врожденного иммунитета во влагалище.
Из показателей врожденного иммунитета мы изучали экспрессию генов TLR9, дефенсина HBD1 и TNFα. TLR9 – это рецептор, который локализован в эндосоме эпителиальных клеток. Через его активацию происходят распознавание чужеродных микроорганизмов и запуск синтеза провоспалительных цитокинов. Дефенсин является универсальной антибактериальной молекулой, реагирующей на грамположительные и грамотрицательные микроорганизмы и грибы рода Candida, а фактор некроза опухоли (ФНО) относится к провоспалительным цитокинам, участвующим в отграничении очага воспаления и активации клеток врожденного иммунитета.
Мы полагаем, что высокая частота рецидивов бактериального вагиноза, даже с учетом патогенетически обоснованного лечения, во многом обусловлена неполноценностью факторов врожденного иммунитета.
В табл. 5–6 представлены показатели экспрессии факторов врожденного иммунитета у пациенток обеих групп.

Сравнение показателей до и после лечения проводилось с использование критерия Вилкоксона и с учетом требований для малых выборок.
Мы обнаружили, что у пациенток с бактериальным вагинозом после 10- и 30-дневного курсов применения как препарата Тержинан, так и хлоргексидина биглюконата не отмечалось достоверных изменений в экспрессии гена ФНО и TLR9 (р≤0,01). Различия были выявлены в продукции дефенсина. В группе беременных, которым проведена терапия препаратом Тержинан, через 10 дней уровень HBD1 снизился в 1,7 раза и сохранялся к 30-му дню. В контрольной группе снижение уровня дефенсина отмечено через 10 дней терапии в 1,4 раза, однако к 30-му дню экспрессия фактора вернулась практически к исходным до терапии показателям.
Обсуждение
Барьером первой линии защиты от агрессивной окружающей среды является поверхности кожи и слизистых, покрытая индигенными микроорганизмами. Параллельно с ней функционирует врожденная иммунная система, позволяющая своевременно распознать чужеродные бактерии и ограничить их активное деление и распространение до момента выработки факторов адаптивного иммунитета.
Бактериальный вагиноз относится к невоспалительному полимикробному патологическому процессу, в основе которого лежит чрезмерное разрастание условно-патогенной микрофлоры со снижением количества индигенных лакто- и бифидобактерий. Распространенность бактериального вагиноза в популяции крайне высока. Несмотря на наличие высокоэффективных антибактериальных средств, порядка 15% случаев остаются неизлеченными, а в 20–60% развивается рецидив [14, 16].
Наличие бактериального вагиноза во время беременности ассоциировано с повышенным риском преждевременных родов и послеродовых инфекционных осложнений. В связи с этим поиск и разработка эффективных методов терапии данного состояния остаются актуальными.
В проведенном исследовании мы изучили состояние микробиоценоза влагалища с точки зрения общей бактериальной обсемененности, количества и видового состава лактобактерий и влияния типа проведенного лечения на показатели врожденного иммунитета. Мы обнаружили, что у пациенток с бактериальным вагинозом имеется крайне высокая общая бактериальная обсемененность влагалища, при этом в достаточном количестве присутствует ДНК лактобактерий. Однако при культуральном исследовании было выявлено, что присутствующие лактобактерии не способны к росту на питательных средах в достаточном титре, а среди всех выявленных видов лактобактерий L. crispatus обнаруживается только у 10% пациенток.
Массивная бактериальная обсемененность влагалища не сопровождается активацией в нем распознающих генов врожденного иммунитета (TLR9) и эффекторных генов (ФНО). Возможно, это объясняется тем, что бактериальный вагиноз является состоянием, связанным с чрезмерным разрастанием условно-патогенных микроорганизмов, являющихся комменсалами для организма человека. В противовес этому, отмечено значительное увеличение генов, отвечающих за продукцию универсальной антибактериальной молекулы дефенсина HBD1. Его экспрессия была высокой у всех беременных с бактериальным вагинозом, значительно снижалась после 10 дней терапии (в основной и контрольной группах). Стойкий эффект был отмечен только в основной группе, где уровень дефенсина сохранялся в пределах нормы и через 30 дней после окончания лечения.
После проведенного лечения стойкая эффективность и меньшее число рецидивов были отмечены в группе терапии комбинированным препаратом Тержинан. Через 30 дней после окончания лечения частота рецидива составила 10% (3 пациентки), а рост L. crispatus выявлен у 60% женщин. В контрольной группе эффективность лечения была 55%, но через 30 дней после ее окончания в 45% развивался рецидив.
Заключение
Проведенное исследование показало высокую эффективность применения препаратов Тержинан и Гексикон в терапии бактериального вагиноза у беременных. Однако стойкие клинический и микробиологический эффекты были отмечены для комбинированного препарата Тержинан. На фоне данного курса лечения зафиксированы низкая частота рецидивов, нормализация микробиоценоза влагалища с ростом протекторных видов лактобактерий и нормализация баланса в экспрессии факторов врожденного иммунитета.



