Immune status of COVID-19 patients with different disease severity
Aim. To investigate the immune status and compare immunological parameters in COVID-19 patients with different disease severity.Krechetova L.V., Inviyaeva E.V., Sadykov V.F., Vtorushina V.V., Ivanets T.Yu., Silachev D.N., Pyregov A.V., Dolgushina N.V., Sukhikh G.T.
Materials and methods. The prospective study included 62 patients with COVID-19. The patients were stratified into three groups based on the disease severity, including mild (group 1, n=29), moderate (group 2, n=17), and severe (group 3, n=16) forms of COVID-19. On days 3–7 from the onset of the disease, peripheral blood lymphocytes were phenotyped by flow cytometry. Cytokine concentrations were measured using a multiplex immunoassay-standard 48-plex Bio-Plex Pro™ Human Cytokine Screening test system (Bio-Rad, USA) on a flow-based laser immuno-analyzer Bio-Plex 200.
Results. Patients with severe COVID-19 had higher levels of leukocytes, neutrophils, CRP, and lower relative and absolute lymphocyte counts. There were low counts of CD3+, CD3+CD4+, CD3+CD8+, and T-lymphocytes expressing the activation marker HLA-DR (CD3+HLA-DR+), NK-cells, and PAN. In group 3, changes in 39 of the 48 investigated soluble factors were observed.
Conclusion. High levels of leukocytes, neutrophils, CRP, neutrophilic-leukocyte index, low levels of absolute and relative lymphocyte counts, pronounced changes in immunological parameters, a systemic inflammatory reaction associated with the release of mediators called cytokines ("cytokine storm") predispose to a severe course of COVID-19.
Keywords
The novel coronavirus infection (SARS-CoV-2) epidemic first broke out in Wuhan, China, in December 2019 and quickly spread worldwide. To date, enough data has accumulated indicating that the spectrum of COVID-19 ranges from asymptomatic to severe forms, accompanied by extensive lung damage, leading to acute respiratory distress syndrome, multiple organ failure, and sepsis [1, 2]. The mortality rate ranges from 0.5–3.5% [3].
The impaired immune response plays a significant role in the development of severe forms of the disease; therefore, early detection of immune dysregulation markers can help understand the pathogenesis of severe forms of COVID-19 and develop methods of therapy and prevention these complications.
The present study aimed to investigate the immune status and compare immunological parameters in COVID-19 patients with different disease severity.
Materials and methods
The prospective study included 62 patients with COVID-19. The inclusion criteria were confirmed diagnosis of COVID-19, age 18+ years, signed informed consent for inclusion in the study, and the blood sampling on days 3–7 from the onset of the disease. The exclusion criteria were HIV infection and other congenital and acquired immunodeficiencies, chronic infectious, oncological, autoimmune, and rheumatic diseases, pregnancy and lactation for women, taking immunomodulatory drugs for at least three months before the onset of the illness and during the disease.
The patients were stratified into three groups based on the disease severity, including mild (group 1, n=29), moderate (group 2, n=17), and severe (group 3, n=16) forms of COVID-19.
The criteria for a mild form of COVID-19 were the detection of SARS-CoV-2 RNA by reverse transcription-polymerase chain reaction (RT-PCR) in an oropharyngeal smear in combination with subfebrile fever (<38°C) and the absence of severe and moderate infection criteria.
The criteria for the moderate form of the disease were the detection of SARS-CoV-2 RNA by RT-PCR in an oropharyngeal smear in combination with any of the following clinical manifestations: body temperature above subfebrile (>38°C), respiratory rate (RR)> 22 breaths per min, shortness of breath on exertion, oxygen saturation (SpO2) <95%; the presence of pneumonia on chest computed tomography (CT) with a minimum or medium lung involvement (CT 1–2).
The criteria for a severe form of the disease were the detection of SARS-CoV-2 RNA by RT-PCR in an oropharyngeal smear in combination with any of the following clinical manifestations: RR>30 breaths per min; SpO2≤93%; PaO2/FiO2≤300 mm Hg; decreased level of consciousness, agitation; unstable hemodynamics (systolic blood pressure less than 90 mm Hg or diastolic blood pressure less than 60 mm Hg, urine output less than 20 ml/hour); changes in the on chest computed tomography or X-ray, typical for a viral lesion (the volume of the lesion is significant or subtotal; CT score 3–4); qSOFA> 2 [4].
The virus was identified using the Reagent kit for detecting RNA of SARS-CoV coronaviruses and similar SARS-CoV by real-time reverse transcription and polymerase chain reaction (SARS-CoV-2/SARS-CoV) (DNA-Technology, Russia).
Three regions of the genome were selected as targets, including coronavirus ЅARЅ-CoV-2 specific regions of the N and E genes, as well as the conserved region of the E gene, typical for a group of coronaviruses similar to ЅARЅ-CoV (including ЅARЅ-CoV and ЅARЅ-CoV-2). Amplification was performed on a DT-964 device (NPO DNA-Technology LLC, Russia). The results were processed automatically using the device software.
On days 3–7 from the onset of the disease, peripheral venous blood samples were collected from the study subjects and tested for serum levels of anti-SARS-CoV-2 IgG antibodies and immune profile, including total lymphocyte count with an analysis of the composition of lymphocyte subpopulation: CD3+, CD3+CD4+, CD3+CD8+, CD19+, CD3-CD56+CD16+, CD3+CD56+CD16+, CD19+CD5+, Treg, with the calculation of the ratio of T-lymphocytes with cytotoxic and helper function (CD8+/CD4+), assessment of peripheral blood activated lymphocyte count (CD3+HLA-DR+, CD3+CD25+, CD25+), as well as the phagocytic activity of neutrophils (PAN) with the calculation of the stimulation index (SI).
Phenotyping of peripheral blood lymphocytes was performed by flow cytometry using monoclonal antibodies (mAbs) labeled with FITC or PE against antigens CD3(FITC), CD4(PE), CD5(PE), CD8(PE), CD16(PE), CD19(FITC), CD56(PE), CD25(FITC), HLA-DR(FITC (Becton Dickinson and eBioscience, USA). The distribution of the main immunocompetent T cell subpopulations (CD3+, CD4+, CD8+), B cells (CD19+), B1 cells (CD19+ CD5+), NK cells (CD56+ CD16+) was assessed. The lymphocyte gate, which allows the exclusion of other blood cells from the analysis, was detected using anti-CD45 mAbs labeled with peridinin chlorophyll protein (PerCP) (Dako, Denmark). Corresponding FITC or PE-labeled isotypic IgGs were used to assess positively stained subpopulations.
Whole blood Tregs with the intracellular expression of FOXP3 of patients with idiopathic recurrent miscarriage were defined as a subpopulation with the СD4+CD25highCD127low phenotype using a combination of mAbs to CD4 antigens labeled with PerCP (eBioscience, USA), CD25 labeled with FITC (Becton Dickinson, USA) and PE-labeled CD127 (eBioscience, USA). The proportion of Tregs among CD4+ cells was estimated. Monoclonal antibodies were added directly to whole blood and then treated with FACS Lysing Solution (Becton Dickinson, USA).
PAN was assessed using the FagoFlow method (ExBio, Czech Republic). The test is based on the assessment of the oxidative burst in granulocytes after stimulation with E. coli. The ratio of the mean fluorescence intensity (SIF) of activated granulocytes of stimulated samples and negative controls reflects the intensity of the oxidative burst of granulocytes after stimulation with E. coli; it is referred to as the stimulation index (SI).
Lymphocyte phenotyping and PAN assessment were performed on a Gallios Flow Cytometer (Beckman Coulter, USA) using the Kaluza software.
Blood sampling to determine the concentration of cytokines was performed on days 3–7 from the onset of the disease. Fibroblast growth factor (FGF basic), Eotaxin, granulocyte colony stimulating factor (G-CSF), granulocyte macrophage colony stimulating factor (GM-CSF), interferon gamma (IFN-γ), interleukins (IL) -1β, IL-1ra, IL -1α, IL-2Rα, IL-3, IL-12 (p40), IL-16, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, GRO-α, hepatocyte growth factor (HGF), IFN-α2, leukemia inhibition factor (LIF), monocytic chemotactic protein (MCP-3), IL-10, IL-12 (p70), IL-13, IL-15, IL-17A, IP-10, MCP-1, interferon gamma-induced monokine (MIG), nerve growth factor (β-NGF), stem cell factor (SCF), stem cell growth factor (SCGF-β), stromal factor cells (SDF-1α), macrophage inflammatory protein (MIP-1α), MIP-1β, platelet growth factor (PDGF-BB), regulated upon activation normal T cell expressed and secreted (RANTES), tumor necrosis factor (TNF)-α, vascular endothelial growth factor (VEGF), cutaneous T-cell attracting chemokine (CTACK), macrophage inhibition factor (MIF), tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), IL-18, macrophage colony stimulating factor (M-CSF), TNF-β were determined in peripheral blood plasma by multiplex method using standard 48-plex assay Bio-Plex Pro Human Cytokine Screening (Bio-Rad, USA) on a flow-based laser Bio-Plex 200 system (Bio-Rad, USA) with subsequent processing of the results obtained using the Bio-Plex Manager 6.0 Properties application (Bio- Rad, USA).
According to the manufacturer's instructions of the Bio-Rad test system, for the preparation of EDTA plasma samples, blood samples were centrifuged at 4°C in two stages (at 1000g for 15 min and then at 10000g for 10 min) to remove platelets and sediments completely. Plasma samples were frozen and stored at -80°C until the analysis.
Statistical analysis
Statistical analysis was performed using Microsoft Office Excel 2007 and the MedCalc v16.8 software. The normality of the distribution was tested by the Shapiro–Wilk test. Quantitative variables showing normal distribution were expressed as means and standard deviation M (SD); otherwise, the median and the quartiles Q1 and Q3 in the Me (Q1; Q3) format were reported. Normally distributed continuous variables were compared with a Student’s t-test for independent samples with unequal variances. For non-normally-distributed parameters, the Mann–Whitney U-test was utilized for comparisons. Differences were considered statistically significant at p <0.017, taking into account the Bonferroni correction for pairwise comparisons of three study groups. Other uses of data presentation and statistical criteria used are indicated in the text.
The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P (protocol No. 4 dated 04/23/2020).
Results
There were statistically significant differences in sex, age, and body mass index (BMI). Males, older persons, and patients with higher BMI were more likely to have severe COVID-19 (Table 1).
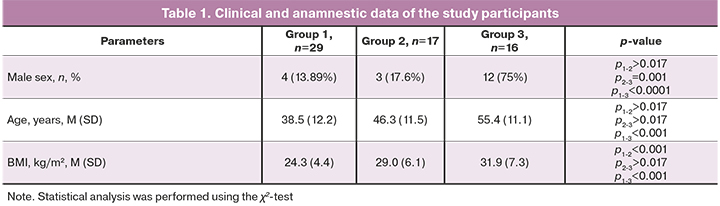
All patients groups 1 and 2 with clinical manifestations of COVID-19 were outpatients receiving one or another type of therapy. Thirty-two out of 46 people (69.5%) were administered broad-spectrum antibiotics, and 17 out of 46 people (36.9%) received low molecular weight heparins (LMWH) in prophylactic doses. Pneumonia, according to CT (CT-1 or CT-2), was diagnosed only in 12 (70.6%) patients in group 2.
Group 3 included patients with a severe course of the disease. It is important to note that this group of patients was characterized by a high heterogeneity of COVID-19 severity throughout their stay in the intensive care unit. This variability was manifested both in laboratory tests findings and, especially pronounced, in the clinical picture. Within one day and even hours, the clinical picture in these patients could change significantly. Of the 16 patients in this group, 12 (75%) were males. The mean age of the patients was 55.4±11.1 years, and the BMI was 31.9±7.3 kg/m2. The intensive care unit stay length varied significantly, ranging from 4 to 40 days (mean 15.5 days). All patients with the severe disease showed resolution of clinical signs and were transferred from the intensive care unit. All patients in this group had a high RR>30 breaths per min, decreased SpO2≤93%, and required oxygen therapy. The ventilation modes in patients of this group during treatment could repeatedly change due to a change in the clinical state. Upon admission to the intensive care unit and the first assessment of laboratory parameters, 2 (12.5%) and 2 (12.5%) patients were on invasive and non-invasive ventilation, eight patients (50%) received high-flow nasal oxygen therapy, four patients (25%) had high-flow (15 L/min) oxygen via face mask. The respiratory index PaO2/FiO2 ranged from 105 to 274 mm Hg (mean 152.5 mm Hg).
Six patients (36.5%) required mechanical ventilation during the further stay in the intensive care unit. A decrease in the level of consciousness was observed in 3 (18.75%) patients upon admission to the department and 8 (50%) patients during the entire period of management in the intensive care unit. Unstable blood pressure (systolic blood pressure less than 90 mm Hg) requiring vasopressor support was observed in 2 (12.5%) patients at the time of admission to the intensive care unit. It should be noted that 5 (31.25%) patients at the time of admission had high blood pressure (systolic blood pressure above 140 mm Hg). A decrease in urine output (less than 20 ml/hour) was observed in 1 (6.25%) patient. No patients required renal replacement therapy and efferent therapies. In all patients of this group, chest CT showed changes typical for a viral lesion: in 5 patients – CT-2, in 9 patients – CT-3, in 2 patients – CT-4. COVID-19 NEWS2 severity scores ranged from 2, reflecting a low risk of severe course, to 9 points, indicating a high disease risk. The mean COVID-19 NEWS2 score was 6, reflecting the average risk of a severe course of COVID-19. The mean qSOFA score was 1.3, indicating a low risk of severe COVID-19 at this stage of the disease. However, according to the results obtained during the calculation of the SOFA scores, the scores ranged from 3 to 6 (mean 4) (mortality at this value at the initial assessment is 20.2%), which characterizes this group of patients indicating possible risks of death. The risk of mortality in patients with a severe course of the disease was assessed using the three most studied scales (APACHE-II, SAPS-II, 4C Mortality Score for COVID-19). The mortality prognosis in patients of this group was evaluated using the APACHE-II scale for assessing acute physiological disorders and chronic disorders.
The scores ranged from 4 to 15 (mean 7.5), corresponding to an 8% risk of in-hospital mortality rate. The severity of the disease in this group of patients upon admission to the intensive care unit was measured by the SAPS II classification system. The scores ranged from 19 to 46 (mean 26), which corresponds to 7.2% of the risk of in-hospital mortality. The risk group for in-hospital mortality was determined using the 4C Mortality Score for COVID-19, specially designed for hospitalized patients with COVID-19. The scores ranged from 5 to 15 (mean 9), corresponding to a 31.4–34.9% risk of in-hospital mortality, indicating a severe course of the disease and a high risk of mortality. One death was recorded in this group after transfer to another health care facility. Comorbidities such as obesity, diabetes mellitus, hypertension were diagnosed in 4/16 (25%), 2/16 (12.5%), and 3/16 (18.75%) patients, respectively.
Complete blood count and blood chemistry tests showed that patients with severe COVID-19 (group 3) had higher counts of leukocytes, neutrophils, neutrophil-leukocyte index (NLI), and C-reactive protein (CRP), and also low relative and absolute lymphocyte counts (Table 2).
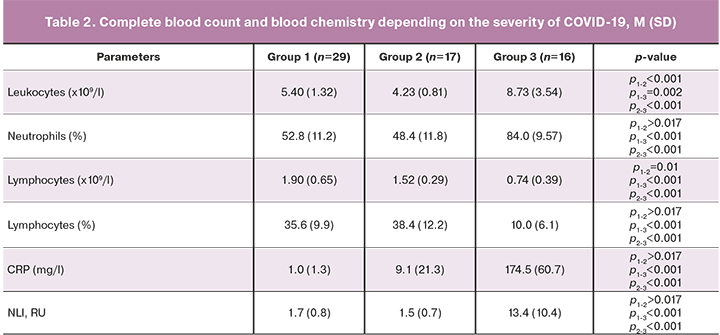
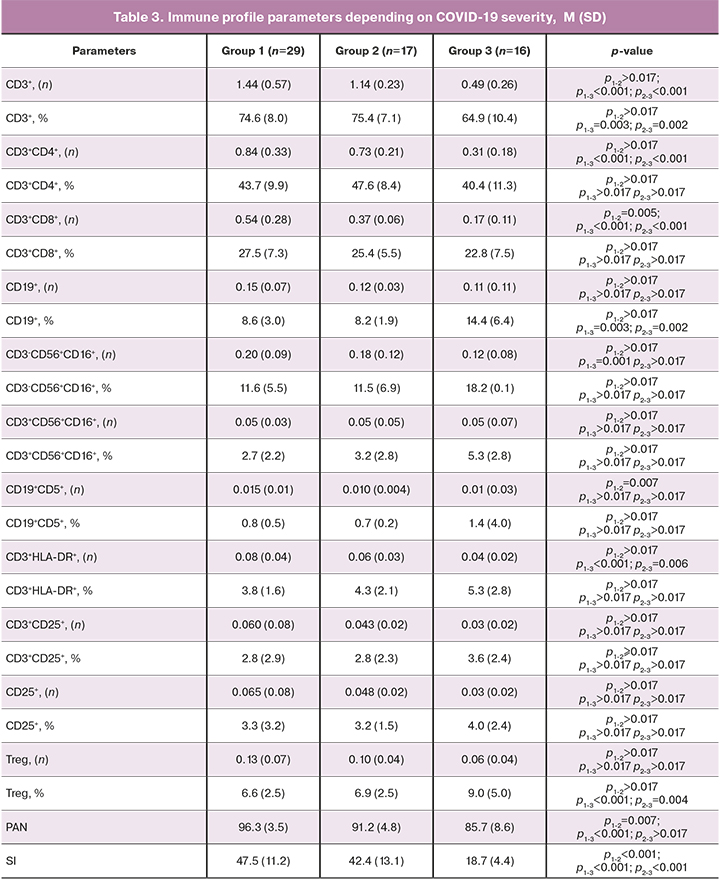
Patients with a severe form of COVID-19 (group 3) had low absolute counts of CD3+, CD3+CD4+, CD3+CD8+, T-lymphocytes expressing the activation marker HLA-DR (CD3+HLA-DR+), NK cells, PAN, and SI (Table 3). There was an increase in the relative count of Treg cells (Table 3).
ROC analysis was performed to identify lymphocyte subpopulations predictive for the development of severe forms of COVID-19. Its results are presented in Table 4.
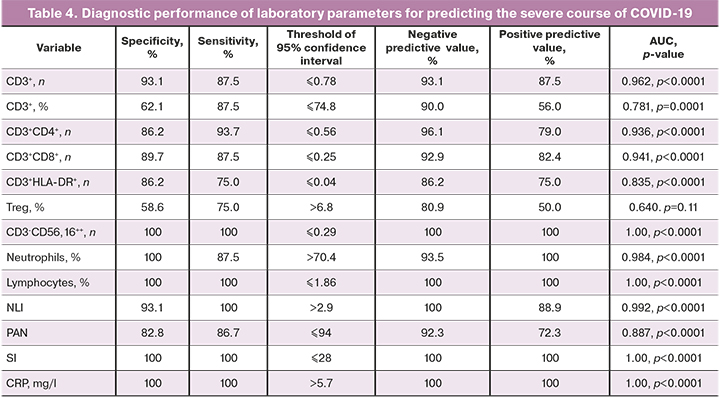
CRP, leukocytes, neutrophils, absolute NK cells, and T-lymphocytes counts (the total number and subpopulations and the number of T-lymphocytes with HLA-DR expression), the NLI, PAN, and SI had the best diagnostic performance. Therefore they can be used as predictors of an adverse disease outcome. All detected changes in the above immune profile parameters indicate the manifestation of the immune system dysfunction, leading to the development of a systemic inflammatory response [5].
To confirm the obtained result, concentrations of pro- and anti-inflammatory cytokines, chemokines, and growth factors were assessed in patients with severe and moderate forms of the disease. The findings are presented in Table 5, Figure.
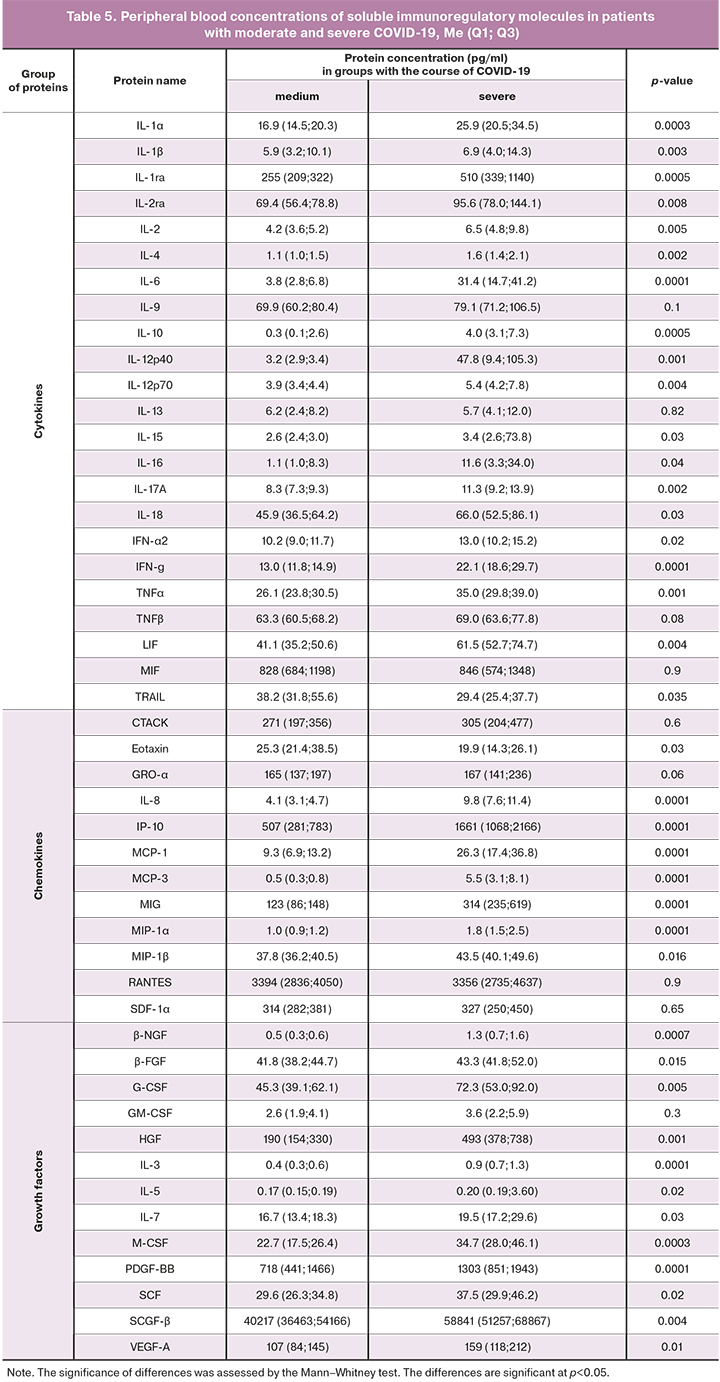
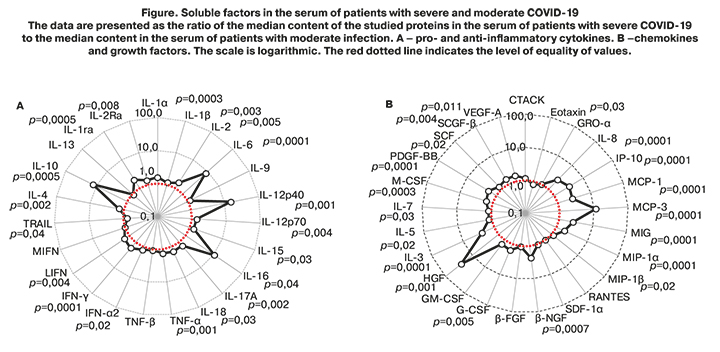
The peripheral blood plasma cytokine profile in patients with severe COVID-19 was characterized by changes in 39 of the 48 investigated soluble factors, except for CTACK, GRO-α, SDF-1α, GM-CSF, IL-13, MIF, RANTES, TNF-β, IL-9. Noteworthy is the high level of pro-inflammatory cytokines IL-6, IL-12p40, IL-16, and immunoregulatory IL-10, chemokines MCP-1, MCP-3, MIG, MIP-1α, MIP-β, IP-10, IL-8 and almost all studied growth factors (G-GSF, M-GSF, HGF, PDGF, SCF, SCGF, VEGF-A, β-NGF), which reflects the development of a phenomenon called "cytokine storm" in patients with severe disease associated with the destruction of various populations of cells in the body and causing the variety of clinical symptoms leading to poor outcomes.
Therefore, a higher level of leukocytes, neutrophils, CRP, NLI, low relative and absolute lymphocyte counts, pronounced changes in immunological parameters, a systemic inflammatory reaction associated with the release of mediators ("cytokine storm") predispose to severe COVID-19.
Discussion
Cytokines and chemokines play an essential role in the pathogenesis of viral infections. Neutrophils also play a crucial role in innate immunity, the first line of defense against viral infections. Therefore, an imbalance or overreaction of the immune system can lead to immunopathology [6–8]. Although there is no direct evidence of the participation of proinflammatory cytokines and chemokines in the development of lung pathology during infections caused by other strains of coronaviruses with the formation of SARS and MERS syndromes (2002 and 2012), there is evidence of hyper-inflammatory reactions in the pathogenesis of acute respiratory syndrome in patients with severe disease. High serum levels of pro-inflammatory cytokines (IFN-γ, IL-1, IL-6, IL-12, and TGF-β) and chemokines (CCL2, CXCL10, CXCL9, and IL-8) have been found in SARS patients with severe disease compared with that in patients with uncomplicated ARVI [9–12].
In contrast, these patients had very low levels of the anti-inflammatory cytokine IL-10 [9]. In addition to proinflammatory cytokines and chemokines, individuals with fatal SARS had increased levels of IFN (IFN-α and IFN-γ) and IFN-stimulated genes (ISG) (CXCL10 and CCL-2) compared with healthy individuals from the control group or people with mild to moderate disease [13–16]. These results first suggested a possible role for IFN and ISG in the immuno-pathogenesis of SARS in humans. Therefore, dysregulation and/or an increase in cytokine and chemokine responses from SARS-CoV-infected dendritic cells and macrophages may play an essential role in the pathogenesis of SARS. A change in PAN is characterized by the production of reactive oxygen species by neutrophils, that is, an oxidative burst and its intensity, expressed in SI, which may be an indicator of reduced resistance to infectious and inflammatory agents. PAN reflects the ability of blood microphages (one of the primary cells capable of phagocytosis) to absorb any pathogenic agents (bacteria, viruses, affected cells).
A decrease in PAN can be observed in chronic infectious diseases, immunodeficiencies, neoplasms, the use of immunosuppressants (excluded in our study), as well as in congenital defects of the phagocytic system, malabsorption, malnutrition, etc. In MEDLINE to date, there are only sporadic reports investigating the relationship between PAN and COVID-19 [17]. A decrease in the activity of innate immunity in the form of impaired phagocytosis by neutrophils of pathogens can adversely affect the development of any infectious and inflammatory process, including those caused by SARS-CoV-2. Besides, extracellular traps of neutrophils, extracellular networks released by neutrophils, can promote the release of cytokines and contribute to forming a "cytokine storm" in this disease [18].
Our study confirmed that a decrease in PAN, SI, as well as neutrophilia and lymphopenia, are characteristic of patients with a severe form of the disease, which may indicate a high intensity of the inflammatory process and a disruption of immune system function by a viral infection in patients with a severe course of the disease. Therefore, immune profile parameters such as the absolute count of T-lymphocytes, a subpopulation of T cells, NK cells, PAN, SI, and NLI can be reliable prognostic indicators for the development of severe forms of COVID-19 [19, 20].
Analysis of lymphocyte subpopulation composition showed a reduction in the T-cell immunity with increased counts of Treg-cells with natural regulatory activity. The latter suggests the formation of immune system imbalance, which, along with the development of hyper-inflammatory reactions, is manifested by an increase in CRP, a decrease in NK-cells, and, at the same time, a "cytokine storm." "Cytokine storm" is usually associated with unusually high production of cytokines triggering immunopathological reactions and reflecting immune response dysregulation in patients with SARS-CoV-2 [5, 21]. Murakami M., Kamimura D., Hirano T. [22] suggested a potential mechanism of cytokine storm associated with the activation through the signaling pathways NF-κB and STAT3 of the production of various proinflammatory cytokines and chemokines. This results in pleiotropic effects on cells of acquired and innate immunity: the weakening of the T-cell link of immunity in response to SARS-CoV-2 and uncontrolled disturbances in the inflammatory reactions of the natural immune system to SARS-CoV-2 lead to a "cytokine storm," which is consistent with the results obtained in this work.
Conclusion
Summing up the study results, it can be concluded that the identified clinical and laboratory differences between clinical forms of COVID-19, including the immune profile parameters and cytokines, can be predictive for severe infection and used in clinical practice to predict the development of the disease. An uncontrolled systemic inflammatory response manifested in developing a "cytokine storm" represents an underlying mechanism of adverse outcomes. Therefore, promising new treatments may be developed based on the use of immunomodulatory therapy and cytokine antagonists.
References
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020; 382(18): 1708-20. https://dx.doi.org/10.1056/NEJMoa2002032.
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020; 395(10223): 507-13. https://dx.doi.org/10.1016/S0140-6736(20)30211-7.
- Wolfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Muller M.A., Niemeyer D. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020; 581(7809): 465-70. https://dx.doi.org/10.1038/s41586-020-2196-x.
- Министерство здравоохранения Российской Федерации. Временные методические рекомендации «Профилактика, диагностика и лечение новой коронавирусной инфекции (COVID-19).» Версия 11 (07.05.2021). [Temporary guidelines "Prevention, diagnosis and treatment of new coronavirus infection (COVID-19) Version 11" (approved by the Ministry of Health of the Russian Federation on May 7, 2021 1. (in Russian)]. https://base.garant.ru/400738625/.
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y. et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020; 71(15): 762-8. https://dx.doi.org/10.1093/cid/ciaa248.
- Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., Perlman S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016; 19(2): 181-93. https://dx.doi.org/10.1016/j.chom.2016.01.007.
- Davidson S., Maini M.K., Wack A. Disease-promoting effects of type I interferons in viral, bacterial, and coinfections. J. Interferon Cytokine Res. 2015; 35(4): 252-64. https://dx.doi.org/10.1089/jir.2014.0227.
- Shaw A.C., Goldstein D.R., Montgomery R.R. Age-dependent dysregulation of innate immunity. Nat. Rev. Immunol. 2013; 13(12): 875-87. https://dx.doi.org/10.1038/nri3547.
- Chien J.Y., Hsueh P.R., Cheng W.C., Yu C.J., Yang P.C. Temporal changes in cytokine/chemokine profiles and pulmonary involvement in severe acute respiratory syndrome. Respirology. 2006; 11(6): 715-22. https://dx.doi.org/10.1111/j.1440-1843.2006.00942. x.
- Wang C.H., Liu C.Y., Wan Y.L., Chou C.L., Huang K.H., Lin H.C., Kuo H.P. Persistence of lung inflammation and lung cytokines with high-resolution CT abnormalities during recovery from SARS. Respir. Res. 2005; 6(1): 42. https://dx.doi.org/10.1186/1465-9921-6-42.
- Wong C.K., Lam C.W.K., Wu A.K.L., Ip W.K., Lee N.L.S., Chan I.H.S., Sung J.J.Y. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004; 136(1): 95-103. https://dx.doi.org/10.1111/j.1365-2249.2004. 02415.x.
- Zhang Y., Li J., Zhan Y., Wu L., Yu X., Zhang W. et al. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect. Immun. 2004; 72(8): 4410-5. https://dx.doi.org/10.1128/IAI.72.8.4410-4415.2004.
- Cameron M.J., Bermejo-Martin J.F., Danes A., Muller M.P., Kelvin D.J. Human immunopathogenesis of severe acute respiratory syndrome (SARS). Virus Res. 2008; 133(1): 13-9. https://dx.doi.org/10.1128/IAI.72.8.4410-4415.2004.
- Cameron M.J., Ran L., Xu L., Danesh A., Bermejo-Martin J.F., Cameron C.M. et al. Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J. Virol. 2007; 81(16): 8692-706. https://dx.doi.org/10.1128/JVI.00527-07.
- Huang K., Su I.J., Theron M., Wu Y.C., Lai S.K., Liu C.C., Lei H.Y. An interferon‐γ‐related cytokine storm in SARS patients. J. Med. Virol. 2005; 75(2): 185-94. https://dx.doi.org/10.1002/jmv.20255.
- Theron M., Huang K.J., Chen Y.W., Liu C.C., Lei H.Y. A probable role for IFN-γ in the development of a lung immunopathology in SARS. Cytokine. 2005; 32(1): 30-8. https://dx.doi.org/10.1016/j.cyto.2005.07.007.
- Merad M., Martin J.C. Author Correction: Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020; 20(7): 448. https://dx.doi.org/10.1038/s41577-020-0353-y.
- Hussman J.P. Cellular and molecular pathways of COVID-19 and potential points of therapeutic intervention. Front. Pharmacol. 2020; 11: 1169. https://dx.doi.org/10.3389/fphar.2020.01169.
- Долгушина Н.В., Кречетова Л.В., Иванец Т.Ю., Вторушина В.В., Инвияева Е.В., Климов В.А., Сухих Г.Т. Влияние иммунного статуса на тяжесть течения COVID-19. Акушерство и гинекология. 2020; 9: 129-37. [Dolgushina N.V., Krechetova L.V., Ivanets T.Yu., Vtorushina V.V., Inviyaeva E.V., Klimov V.A., Sukhikh G.T. The impact of the immune status on COVID-19 severity. Obstetrics and Gynecology. 2020; 9: 129-37. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.9.129-137.
- Liu J., Liu Y., Xiang P., Pu L., Xiong H., Li C. et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J. Transl. Med. 2020; 18(1): 206. https://dx.doi.org/10.1186/s12967-020-02374-0.
- Wang F., Nie J., Wang H., Zhao Q., Xiong Y., Deng L. et al. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J. Infect. Dis. 2020; 221(11): 1762-9. https://dx.doi.org/10.1093/infdis/jiaa150.
- Murakami M., Kamimura D., Hirano T. Pleiotropy and specificity: insights from the interleukin 6 family of cytokines. Immunity. 2019; 50(4): 812-31. https://dx.doi.org/10.1016/j.immuni.2019.03.027.10.1016/j.immuni.2019.03.027.
Received 16.06.2021
Accepted 05.07.2021
About the Authors
Lubov V. Krechetova, Dr. Med. Sci., Head of the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(495)438-11-83. E-mail: l_krechetova@oparina4.ru. ORCID: 0000-0001-5023-3476. 117997, Russia, Moscow, Oparina str., 4.Eugenia V. Inviyaeva, Ph.D. (bio.sci.), Senior Researcher at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel: +7(495)438-11-83. E-mail: e_inviyaeva@oparina4.ru. ORCID: 0000-0001-9878-3637. 117997, Russia, Moscow, Oparina str., 4.
Valentin F. Sadykov, Anesthesiologist-Intensivist at the Department of Anesthesiology and Critical Care, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
E-mail: v_sadykov@oparina4.ru. ORCID: 0000-0002-3511-5292. 117997, Russia, Moscow, Oparina str., 4.
Valentina V. Vtorushina, Ph.D., Immunologist at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(495)438-11-83. E-mail: v_vtorushina@oparina4.ru. ORCID: 0000-0002-8406-3206. 117997, Russia, Moscow, Oparina str., 4.
Tatiana Yu. Ivanets, Dr. Med. Sci., Head of the Clinical Diagnostic Laboratory, V.I. Kulakov NMRC for OG&P, Ministry of Healthcare of the Russian Federation.
Tel.: +7(495)438-25-66. E-mail: t_ivanets@oparina4.ru. ORCID: 0000-0002-7990-0276. 4 Oparin str., 117997, Moscow, Russia.
Denis N. Silachev, Dr. Bio. Sci., Head of the Cell Technologies Laboratory, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
E-mail: d_silachev@oparina4.ru. ORCID: 0000-0003-0581-9755. 117997, Russia, Moscow, Ac. Oparina str., 4.
Aleksey V. Pyregov, Dr. Med. Sci., Professor, Director of the Institute of Anesthesiology, Intensive Care and Transfusiology, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia. E-mail: a_pyregov@oparina4.ru. ORCID: 0000-0001-8382-9671. 117997, Russia, Moscow, Ac. Oparina str., 4.
Nataliya V. Dolgushina, Dr. Med. Sci., Professor, Deputy Director – Head of the Department of Research Research and Development, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel: +7(495)438-49-77. E-mail: n_dolgushina@oparina4.ru. ORCID: 0000-0003-1116-138X. 117997, Russia, Moscow, Ac. Oparina str., 4.
Gennady T. Sukhikh, Dr. Med. Sci., Professor, Academician of the RAS, Director of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(495)438-18-00. E-mail: g_sukhikh@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
For citation: Krechetova L.V., Inviyaeva E.V., Sadykov V.F., Vtorushina V.V., Ivanets T.Yu., Silachev D.N., Pyregov A.V., Dolgushina N.V., Sukhikh G.T.
Immune status of COVID-19 patients with different disease severity.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 8: 75-85 (in Russian)
https://dx.doi.org/10.18565/aig.2021.8.75-85



