Экстракты корней многолетней травы солодки (лат. Glycyrrhiza) в течение многих столетий используются различными системами народной медицины. Фармакологические данные, полученные в результате экспериментальных и клинических исследований, показывают, что экстракты солодки проявляют противовоспалительное, противовирусное, антимикробное, противодиабетическое, противоастматическое, антиоксидантное, иммуномодулирующее, противоопухолевое, гастропротекторное, гепатопротекторное, нейропротекторное и кардиопротекторное действия. За последние годы описаны основные действующие начала экстрактов солодки и предложен ряд соответствующих молекулярно-клеточных механизмов их действия [1].
Экстракт солодки содержит тритерпеновые сапонины, флавоноиды (флаваноны, халконы, изофлавены, флавоны, изофлавоны), кумарины и другие полифенольные соединения. Глицирризин является основным сапонином солодки, который обуславливает сладкий вкус лакричных экстрактов. Микробиота человека катализирует гидролиз глицирризина до глицирризиновой кислоты (эноксолона) – пентациклического тритерпеноида (рис. 1). Эноксолон проявляет противовирусные, противогрибковые, антипротозойные, антибактериальные свойства, заживляет повреждения желудка при язвенной болезни и способствует эрадикации H. pylori, может применяться как отхаркивающее и противокашлевое средство [2]. В дерматологии производные глицирризина используются по крайней мере с 1950-х гг. [3]. Глицирризин и его производные ингибируют продукцию простагландина E2, каскад NFκB, толл-рецепторы, подавляют продукцию ИЛ-4, ИЛ-5, ИЛ-13, что приводит к противовоспалительному действию [1].

В настоящей работе представлены результаты систематического анализа научной литературы по фармакологии глицирризина. По запросу «Glycyrrhizin OR glycyrrhizic OR glycyrrhizinic» в базе данных биомедицинских публикаций PubMed было найдено 3264 ссылки. Мы провели систематический компьютеризованный анализ этого массива публикаций с целью выявления основных направлений исследований глицирризина и его производных. Проведенный анализ литературы был осуществлен посредством современных методов анализа больших данных, развиваемых в рамках топологического и метрического подходов к задачам распознавания/классификации [4].
Результаты систематического компьютеризированного анализа литературы
В ходе систематического анализа литературы было выделено 52 информативных биомедицинских термина, отличающих публикации по глицирризину от публикаций в контрольной выборке. В качестве контрольной выборки публикаций использовались 3300 случайно выбранных статей из 494 103 найденных по запросу «(Humans [MeSH Terms] OR Rats [MeSH Terms] OR Mice [MeSH Terms] OR Disease Models, Animal [MeSH Terms] OR Cells, Cultured [MeSH Terms]) AND (Dose-Response Relationship, Drug [MeSH Terms] OR Plants, Medicinal [MeSH Terms] OR Chromatography, High Pressure Liquid [MeSH Terms] NOT Glycyrrhizin NOT glycyrrhizic NOT glycyrrhizinic)». Выбор перечисленных выше ключевых слов для формирования контрольной группы был сделан на основе наиболее часто встречающихся терминов в выборке публикаций по глицирризину.
Аннотация полученных терминов посредством референсных таблиц «SNAP» [5] позволила рубрицировать тексты исследований по соответствующим молекулярно-биологическим процессам в соответствии с международной номенклатурой GO (Gene Ontology) [6]. Экспертный анализ полученного списка рубрик GO позволил выделить 17 наиболее информативных рубрик, которые достоверно чаще встречались в выборке публикаций по глицирризину, чем в контроле (в 1,8–27 раз чаще, Р<0,05 для каждого из 17 терминов). В результате была получена своего рода «карта» молекулярно-физиологического действия глицирризина, включающая 17 молекулярных механизмов и 19 коморбидных патологий (рис. 2). На рисунке представлены диагнозы по МКБ-10 и отдельные симптомы и биологические процессы (коды в соответствии с международной номенклатурой GO приведены в тексте статьи). С использованием полученного списка наиболее информативных терминов было найдено 474 публикации и на основании экспертного анализа сформирована выборка из 52 репрезентативных публикаций, цитируемых в настоящей статье.
Анализ метрической диаграммы на рисунке 2 показывает, что наиболее информативные ключевые слова, описывающие фармакологию глицирризина, были сгруппированы в два кластера: кластер 1 «Активация Т-лимфоцитов» и кластер 2 «Хроническое воспаление и коморбидные патологии». Из диаграммы на рисунке 2 следует, что молекулярные механизмы действия глицирризина достаточно широки и включают регуляцию активности Т-лимфоцитов, тучных клеток, нейтрофилов, макрофагов, секреции провоспалительных и противовоспалительных цитокинов (интерферонов, интерлейкинов, С-С хемокинов), биосинтеза липоксинов и простагландинов и др.

В кластер 1 «Активация Т-лимфоцитов» (GO:0002287 Активация α,β Т-клеток иммунного ответа) входят информативные термины, описывающие биологические процессы, относящиеся к функции Т-лимфоцитов (GO:0043378 CD8+ дифференцировка Т-клеток, GO:0002485 Презентация антигенов через MHC класса I, GO:0033371 Секреторные гранулы Т-клеток, GO:0032687 Ингибирование продукции интерферона-α, GO:0045358 Ингибирование продукции интерферона-β), тучных клеток (GO:0033364 Секреторные гранулы тучных клеток), нейтрофилов (GO:0043315 Дегрануляция нейтрофилов). Нарушения этих биологических активностей, регулируемых глицирризином, ассоциированы с аутоиммунной патологией. В регуляции Т-клеток также принимают участие полиненасыщенные жирные кислоты (ПНЖК), которые могут являться фармакодинамическим синергистом глицирризина (GO:0034626 Удлинение цепей ПНЖК).
С хроническим воспалением (кластер 2) ассоциирован несколько иной комплекс молекулярных механизмов, связанный с избытком активности макрофагов, нарушениями синтеза липоксинов и простагландинов (GO:0004051 5-липоксигеназа, GO:2001306 Биосинтез липоксина В4), цитокинов (GO:0016493 Рецептор С-С хемокинов, GO:0050720 Биосинтез ИЛ-1β, GO:0004920 Рецептор ИЛ-10) и интерферонов (GO:0004905 Рецепторы интерферона I). Глицирризин, способствуя снижению хронического воспаления через перечисленные выше механизмы, способствует торможению развития коморбидных хроническому воспалению патологий, в т.ч. заболеваний сосудов (атеросклероз, ишемия головного мозга), поджелудочной железы (K85 Острый панкреатит), печени (желтуха, лекарственный гепатит, K73.9 Хронический гепатит неуточненный, K75.4 Аутоиммунный гепатит, K76.9 Болезнь печени неуточненная), кожных покровов (атопический дерматит, L23 Аллергический контактный дерматит, L25 Контактный дерматит неуточненный, L40 Псориаз), астмы и конъюнктивита (H10 Конъюнктивит). Помимо противовоспалительного действия, глицирризин и его производные могут проявлять прямое антибактериальное и противовирусное действия (коронавирусные инфекции, B00 Инфекции, вызванные вирусом герпеса, B19 Вирусный гепатит неуточненный), противогрибковое (B37 Кандидоз), антилейшманийное (B55.0 Висцеральный лейшманиоз), в т.ч. при топическом применении.
«Внекластерные» термины, описывающие эффекты глицирризина (правая верхняя часть диаграммы на рисунке 2) включают различные типы клеток иммунной системы (лейкоциты, лимфоциты, эозинофилы), окулярный кератит (H16 Кератит), заболевания зубов (K02 Кариес зубов, K05.3 Хронический пародонтит) и сердечно-сосудистую патологию (ишемия миокарда, I10 Эссенциальная гипертензия).
Далее последовательно рассмотрены результаты исследований молекулярных механизмов противовоспалительного действия глицирризина, эффекты глицирризина и его производных на инсулинорезистентность и нарушения липидного профиля, результаты фундаментальных и клинических исследований по топическому применению глицирризина и его производных в терапии заболеваний, затрагивающих кожу и слизистые оболочки, в т.ч. бактериального, грибкового и вирусного происхождения.
Фундаментальные исследования молекулярных механизмов противовоспалительного действия глицирризина
Структура производных глицирризина аналогична структуре кортизона, что, с одной стороны, может быть одной из основ противовоспалительного действия глицирризина, с другой стороны, может вызвать псевдоальдостеронизм (при избыточном приеме внутрь или при приеме в сочетании с определенными препаратами) [7]. 5% раствор глицирризина показал сопоставимое с дексаметазоном (0,1%) местное противовоспалительное действие при воспалении конъюнктивы у кроликов [8]. Фундаментальные исследования продемонстрировали широкий спектр молекулярных механизмов: регулировку уровней провоспалительных цитокинов, сигнальные каскады HMGB1 и NFκB, активность толл-рецепторов [9].
Глицирризин, дозозависимо активируя рецептор LXRα, ингибирует продукцию провоспалительных цитокинов ИЛ-6 и ИЛ-8 в фибробластах десны человека, которые были стимулированы бактериальными липополисахаридами [10]. Глицирризин ингибирует IFN-γ-индуцированный цитокин CXCL10, подавляя сигнальный путь JAK/STAT1 [11]. В имиквимодовой модели псориаза у мышей глицирризин ингибирует экспрессию цитокинов ИЛ-17A, IFN-γ, сигнального белка STAT3 и стимулирует экспрессию деацетилазы сиртуин-1 (ген SIRT1), способствующей выживанию клеток [12].
Рецептор HMGB1 (высокоподвижный рецептор конечных продуктов гликирования) предназначен для распознавания конечных продуктов гликирования (RAGE) и участвует в сигнальных путях толл-рецепторов и NFκB, которые избыточно активируются при патологиях, ассоциированных с хроническим воспалением. Например, на модели остеоартрита височно-нижнечелюстного сустава у крыс глицирризин ослаблял прогрессирование заболевания путем ингибирования сигнального пути HMGB1-RAGE/TLR4/NFκB/AKT [13].
Показана перспективность применения глицирризина при хроническом пародонтите, сопровождающемся повышенной экспрессией белка-рецептора HMGB1 и провоспалительных цитокинов ФНОα, ИЛ-1β и ИЛ-6. Глицирризин достоверно снижал уровни HMGB1 (от 5795,6±1121,5 до 586,4±436,8 пг/мл), ФНОα (от 421,8±93,7 до 87,9±21,6 пг/мл), ИЛ-6 (от 1423,8±235,2 до 622,6±176,1 пг/мл) и ИЛ-1β (от 1562,8±334,3 до 733,5±265,1 пг/мл) в жидкости десневой борозды [14]. Очевидно, что снижение локального воспаления при пародонтите соответствует торможению деструктивных процессов в ткани зуба и десны.
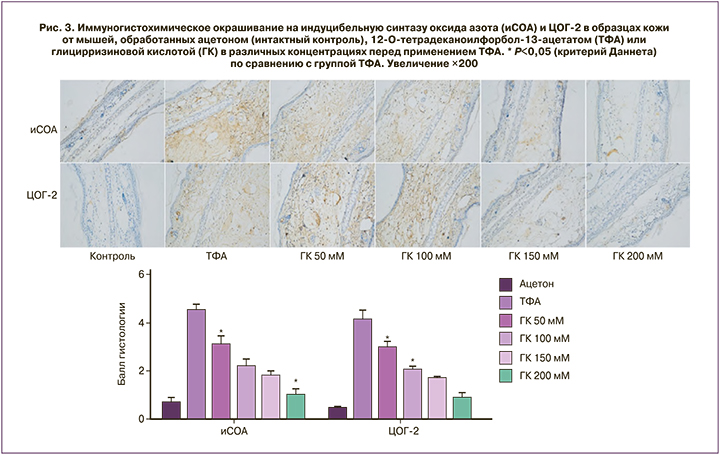
Топическое применение глицирризиновой кислоты подавляет воспалительные реакции в коже, вызванные 12-О-тетрадеканоил-форбол-13-ацетатом (ТФА). Противовоспалительное действие обусловлено блокированием NFkB-зависимых сигнальных путей MAPK и PI3K/Akt, индуцибельной синтазы оксида азота, циклооксигеназы-2 (ЦОГ-2) (рис. 3) и фосфорилирования IκBα, которое приводит к активации провоспалительного каскада NFκB (рис. 4) [15].

Бактериальные липополисахариды (ЛПС) активируют толл-рецепторы, что приводит к усилению хронического воспаления. Глицирризин ингибирует индуцированную ЛПС продукцию медиаторов воспаления в эпителиальных клетках эндометрия, существенно подавляя продукцию цитокинов ФНОα, ИЛ-1β, простагландина E2 и NO. Снижение экспрессии толл-рецептора TLR4 и уменьшение активации NFκB является важным фактором в терапии эндометриоза [16]. Глицирризин напрямую ослаблял активность толл-рецепторов типов 2 и 4 в модели экспериментального вазоспазма у крыс [17]. Таким образом, глицирризин и его производные снижают уровни провоспалительных цитокинов, ингибируют сигнальные каскады HMGB1, NFκB и толл-рецепторы.
Гепатопротекторные свойства глицирризина
Глицирризин усиливает продукцию ИЛ-10 дендритными клетками печени у мышей с моделью гепатита, вызванного конканавалином-А. Уровни ИЛ-10 снижаются при воспроизведении модели, а лечение глицирризином обращает этот эффект [18]. Метаанализ 6 рандомизированных исследований (n=608) подтвердил, что инъекции глицирризина улучшают функцию печени у детей с острым желтушным вирусным гепатитом А/В. Глицирризин в сочетании со стандартной терапией достоверно снижал аномально высокие уровни аланинаминотрансферазы (-24,09 ЕД/л, 95% ДИ -30,83–-17,34) и аспартатаминотрансферазы (-18,67 ЕД/л, 95% ДИ -21,88–-15,45). Ни в одном из 6 исследований не сообщалось о нежелательных явлениях терапии глицирризином [19].
Глицирризин при глюкозотолерантности, инсулинорезистентности и нарушениях липидного профиля
В эксперименте у мышей с диабетом, вызванным стрептозотоцином, глицирризиновая кислота проявляла гипогликемический эффект, приводя к повышению чувствительности к инсулину, снижению уровня глюкозы в крови натощак, уровней триглицеридов, общего холестерина, липопротеинов низкой плотности и к увеличению содержания холестерина липопротеинов высокой плотности [20]. Глицирризин снижал гиперинсулинемию (p<0,05), гипергликемию (p<0,05), глюкозотолерантность (p<0,01), гликирование гемоглобина (p<0,05), перекисное окисление липидов (p<0,05) и патологические изменения почек и печени [21]. У крыс с ожирением, вызванным диетой с высоким содержанием жиров, глицирризиновая кислота улучшала экспрессию липопротеинлипазы, чувствительность к инсулину, нормализовала концентрации липидов сыворотки крови (устранение гипертриглицеридемии, повышение уровня ЛПВП, снижение концентраций свободных жирных кислот, общего холестерина и ЛПНП в сыворотке крови), противодействуя развитию висцерального ожирения [22, 23].
В модели острого панкреатита у мышей применение глицирризина приводило к снижению уровней амилазы, ФНОα, ИЛ-6 и HMGB1 и степени поражения поджелудочной железы [24]. Глицирризиновая кислота оказывает защитное действие на повреждения клеток эпителия почечных канальцев, вызванные гипергликемией. Высокие концентрации глюкозы в крови увеличивают пролиферацию клеток, экспрессию TGF-β1 и снижают экспрессию цитопротекторных ферментов AMPK, SIRT1 и Mn-супероксиддисмутазы, тогда как глицирризиновая кислота предотвращает эти негативные изменения [25].
Топическое применение глицирризина и его производных в терапии заболеваний кожи
При топическом применении глицирризина и его производных в виде специальных фармацевтических форм непосредственно в локальном очаге достигается не только противовоспалительный, но и другие эффекты (в частности, противовирусный и антибактериальный). Топическое применение глицирризина апробировано при атопическом дерматите, псориазе и витилиго.
Атопический дерматит (АД) – мультифакториальное воспалительное заболевание кожи, характеризующееся зудом, хроническим рецидивирующим течением. АД встречается приблизительно у 20% детей, распространенность АД среди взрослых составляет 8%. В модели АД у мышей, вызванного 2,4-динитрохлорбензолом (ДНХБ), глицирризин облегчал симптомы АД путем ингибирования упоминаемых ранее сигнальных путей HMGB1, NFκB и воспалительных цитокинов (рис. 5) [26].
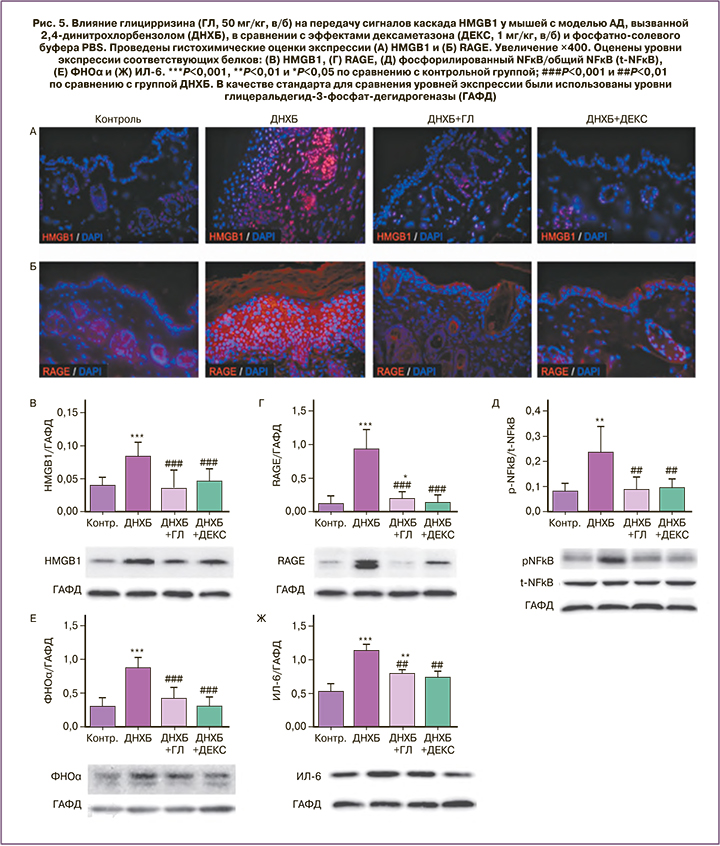
Показана перспективность использования глицирризината аммония в комплексной терапии пациентов с АД (n=48). Добавление глицирризината аммония (в виде крема для наружного применения) к стандартной терапии приводило к достоверному снижению индекса EASI на 76% (контроль: на 57%, p<0,05) и снижению интенсивности зуда до 2,3 балла (контроль: 4,1 балла, p<0,05). Уровень гидратации кожи в группе I составил 36,8 ед., за период лечения увеличившись на 80%, и был выше, чем в контроле (26,7 ед., p<0,05). Значение дерматологического индекса качества жизни при применении глицирризината аммония составило 4,7 балла, что в 2 раза меньше, чем в контроле (9,2 балла, p<0,05). Терапия с использованием глицирризината приводила к снижению частоты обострений АД за 6 месяцев наблюдений до 28% (контроль: 63%, p<0,05) и увеличению средней продолжительности ремиссии АД на 4 недели по сравнению с контрольной группой (p<0,05). При этом средняя продолжительность обострения АД в группе лечения составила всего 1,6 недели и была в 3,5 раза короче по сравнению с контролем (p<0,05) [27].
Глицирризиновая кислота при использовании в виде трансдермального геля может проявлять антипсориатическое действие за счет ингибирования экспрессии ИЛ-17А в дерме и ИЛ-23 в эпидермисе [28]. Глицирризин ослаблял поражения кожи в модели псориаза, вызванного имиквимодом у мышей (линия BALB/c), посредством ингибирования ФНОα-зависимых сигнальных каскадов NFκB/MAPK [29]. У пациентов с псориазом глицирризин улучшает клиническую симптоматику посредством снижения дифференцировки клеток Th17 и концентраций соответствующих цитокинов (ИЛ-6, ИЛ-17, ИЛ-22 и TGF-β) в крови [30].
Метаанализ 11 рандомизированных исследований подтвердил положительные эффекты использования глицирризина в качестве дополнительной терапии псориаза. Добавление глицирризина к стандартной терапии увеличило количество пациентов, достигших индекса площади и тяжести псориаза PASI 60 (отношение шансов – ОШ 1,30; 95% ДИ 1,21–1,40) по сравнению с контролем. В обеих группах наблюдалось сопоставимое количество пациентов с побочными эффектами [31].
Комбинация оксиматрина и глицирризината диаммония значительно смягчает аллергический контактный дерматит мышей, вызванный динитрофторбензолом [32]. Глицирризин в сочетании с лазерной терапией и инъекциями ацетонида триамцинолона повышает эффективность лечения хронического витилиго [33].
Экстракты Glycyrrhiza glabra со стандартизированным содержанием глицирризиновой кислоты (порядка 20%) могут использоваться при кожных высыпаниях, включая дерматит, экзему, зуд. Пропиленгликоль был лучшим растворителем для экстракта, а Carbopol-940 в качестве гелеобразующего агента показал наилучшие результаты в конечных рецептурах. При использовании в течение 2 недель 2% гель с экстрактом солодки для местного применения был более эффективен, чем 1%, для сокращения площади эритемы и уменьшения отека и зуда (p<0,05) [34].
Глицирризин может ингибировать рост опухолей, вызванный проканцерогенным веществом ТФА у мышей. Воспаление в коже при воздействии ТФА опосредуется ЦОГ, липоксигеназами и орнитин- декарбоксилазой (ОДК). ТФА усиливает перекисное окисление липидов со снижением уровня ферментов-антиоксидантов каталазы, глутатиона, глутатионпероксидазы, глутатионредуктазы и глутатион-S-трансферазы, повышением активности ОДК и более интенсивным включением тимидина с радиоактивной меткой «[3H]» в ДНК клеток кожи. Профилактическое топическое применение глицирризина (2 или 4 мг/сут) приводило к значительному снижению микросомального перекисного окисления липидов (P<0,001), восстановлению содержания глутатиона (P<0,001) и повышению уровней антиоксидантных ферментов в коже [35].
Глицирризин при воспалении слизистых оболочек
Местное применение глицирризина (микроклизмы) облегчало течение экспериментального колита у крыс. Модель колита воспроизводилась посредством перорального введения декстрансульфата натрия. По сравнению с плацебо лечение глицирризином значительно уменьшало степень колита, снижало уровни экспрессии провоспалительных цитокинов и хемокинов ИЛ-1β, ИЛ-6, ФНОα, индуцированного цитокинами нейтрофильного хемоаттрактанта CINC-2 и моноцитарного хемоаттрактантного белка MCP-1 в слизистой оболочке кишечника. Кроме того, глицирризин ингибировал окислительную активность миелопероксидазы слизистой оболочки кишки [36].
Глицирризин ингибирует воспалительную реакцию в эпителиальных клетках молочной железы в модели мастита у мышей и ослабляет гистопатологические изменения тканей, активность миелопероксидазы, инфильтрацию нейтрофильных гранулоцитов, также подавляя экспрессию провоспалительных цитокинов ФНОα, RANTES, ИЛ-1β и ИЛ-6, вызванную ЛПС [37].
Глицирризин при местном нанесении на слизистую носовых ходов подавляет сигнальный путь NFκB, тем самым ослабляя продукцию воспалительных цитокинов, опосредованную гистамином активацию белка муцина 5AC (MUC5AC) в эпителиальных клетках. Этот противовоспалительный эффект способствует облегчению аллергического ринита [38].
Глицирризин в терапии бактериальных и грибковых инфекций
Глицирризиновая кислота активирует макрофаги и усиливает их способность уничтожать болезнетворные микроорганизмы (в частности, сальмонеллы [39]). Показана эффективность глицирризиновой кислоты в лечении лейшманиоза у мышей линии BALB/C [40].
Глицирризиновая кислота подавляет ЦОГ-2-опосредованные провоспалительные реакции, возникающие при инфекции Leishmania donovani у мышей, усиливая ответы макрофагов за счет ингибирования ЦОГ-2-опосредованного высвобождения простагландина Е2 в макрофагах, инфицированных лейшманиями, снижала количество паразитов в печени и селезенке и увеличивала пролиферацию Т-клеток [41].
Глицирризин оказывает бактериостатическое действие на некоторые штаммы Pseudomonas aeruginosa, вызывающие бактериальный кератит при ношении контактных линз [42]. Местное применение глицирризина может быть рекомендовано при кератитах, вызванных P. aeruginosa. Глицирризин значительно снижал бактериальную нагрузку роговицы и уровни провоспалительных цитокинов CXCL2, ИЛ-1β если лечение начато не позднее чем через 24 ч после заражения [43].
Важным антибактериальным свойством глицирризина и его производных является ингибирование бактериальных пленок (биопленок) – конгломераций бактерий, характеризующихся повышенной устойчивостью к антибиотикам и даже к антисептикам. Трудность лечения так называемых «больничных инфекций» обусловлена, в частности, преобладанием бактериальных штаммов, способных образовывать биопленки. Глицирризин снижал экспрессию бактериальных белков, отвечающих за устойчивость к антибиотикам и образование бактериальных биопленок P. aeruginosa, подавлял адгезию штаммов P. aeruginosa с множественной лекарственной устойчивостью. Минимальная ингибирующая концентрация глицирризина для биопленок P. aeruginosa составила 0,25 мг/мл, что является терапевтически значимой концентрацией [44].
Угнетающее воздействие глицирризина на биопленки P. aeruginosa обуславливает перспективность применения стандартизированных экстрактов корня солодки как эффективной альтернативы антибиотикам в лечении пациентов с бактериальными поражениями кожи, слизистых, роговицы. Эффективность глицирризина в подавлении роста биопленок P. aeruginosa увеличивается с повышением концентрации от 100 до 800 мг/мл. Глицирризин блокирует проницаемость биопленок P. aeruginosa, что нарушает их питание и приводит к уничтожению бактериальных конгломератов [45].
Модулируя T-клеточный ответ 2-го типа, глицирризин повышает устойчивость мышей, инфицированных вирусом мышиного лейкоза LP-BM5, к оппортунистической инфекции Candida albicans. Инфицирование вирусом LP-BM5 снижает Т-клеточный иммунитет и повышает восприимчивость мышей к C. albicans в 20–100 раз. Глицирризин, индуцируя CD4+ T-клетки, подавляющие продукцию цитокинов 2-го типа, повышает устойчивость мышей к кандидозу до уровня контроля [46].
Глицирризин в терапии вирусных инфекций
Производные глицирризина могут использоваться для лечения вирусных инфекций, вызываемых ДНК- или РНК-содержащими вирусами, включая различные штаммы Herpes simplex, Varicella zoster, папилломы человека, аденовирусов, цитомегаловирусов. Штаммы вирусов, резистентные к ацикловиру и йодоуридину, зачастую высокочувствительны к глицирризиновой кислоте. Иммуномодулирующий, противовоспалительный потенциалы глицирризиновой кислоты, наряду с прямым противовирусным действием, обуславливают активность этого соединения по отношению к вирусам гепатита А, В и С, везикулярного стоматита, простого герпеса, гриппа А, коронавирусов, респираторно-синцитиального вируса, вируса коровьей оспы, арбовирусов, энтеровируса человека 71 и др. Противовоспалительный механизм реализуется через снижение уровней цитокинов (ФНО-α, ИЛ-4, 5, 6, 8, 10, 12, 17), молекул межклеточной адгезии-1, Р-селектина, NFκB, STAT-3, 6, блокады синтеза простагландина Е2 и ингибирования толл-рецепторов [47].
Глицирризин ингибирует экспрессию провоспалительных цитокинов и хемокинов в макрофагах человека, индуцированных высокопатогенным вирусом гриппа А (штамм H5N1). Глицирризин (100 мкг/мл, терапевтически достижимая концентрация) снижал продукцию CXCL10, ИЛ-6 и CCL5, индуцированную H5N1, и снижал апоптоз клеток, вызванный вирусом гриппа. Концентрации глицирризина, ингибирующие H5N1-индуцированную экспрессию провоспалительных генов, не влияли на цитолитическую активность естественных клеток-киллеров [48].
Показаны противовирусные эффекты комбинации глицирризина (50 мг/кг) и рибавирина (40 мг/кг) против штамма H1N1 вируса гриппа A на мышиной модели вирусной пневмонии. Применение смеси глицирризина и рибавирина приводило к значительному падению титра вируса в легочном лаваже (P<0,01) и к ингибированию продукции провоспалительных цитокинов ИЛ-6 (P<0,01), ФНОα (P<0,01) и ИЛ-1β (P<0,01) [49].
Особый интерес представляют результаты исследований противовирусных эффектов глицирризина по отношению к вирусу SARS-CoV-2, вызывающему COVID-19. Хемореактомный скрининг 2700 препаратов из списка АТХ показал, что глицирризин является одной из перспективных молекул-кандидатов, которая может ингибировать репликацию вирусов in vitro на 65–70%, характеризуясь при этом весьма низкой частотой побочных эффектов (не более чем у 9% пациентов) [50]. Молекулярно-биологические и биофизические исследования показали, что глицирризин может ингибировать РНК-полимеразу [51] и вирусную протеазу Mpro [52] вируса SARS-CoV-2, блокировать прикрепление вируса к клеткам посредством шиповидного белка оболочки вируса [53]. Ингибируя вирусную протеазу Mpro, глицирризин тормозит репликацию вируса SARS-CoV-2 [54]. Противовоспалительная и антиоксидантная активность глицирризина, его противовирусные свойства, наряду с отсутствием каких-либо побочных эффектов при топическом применении, делают глицирризин перспективным средством для топического применения (нанесение на слизистую носовых ходов и конъюнктивы глаз) с целью профилактики COVID-19 [55]. Включение глицирризина в комплексную терапию COVID-19 средней степени тяжести приводило к сокращению времени выздоровления (7±1 день, плацебо: 12,5±4 дня, p=0,0001), более низкой смертности (0%, плацебо: 20%, p=0,0035) и снижению уровней провоспалительных С-реактивного белка и ферритина [56]. Гепатопротекторные эффекты глицирризина важны для лечения поражений печени, вызванных лекарствами, применяемыми для борьбы с вирусом SARS-CoV-2 [57].
Глицирризинат аммония может успешно использоваться в терапии контагиозного моллюска (вирусный дерматоз, наиболее часто встречающийся у детей). Монотерапия с использованием средства на основе аммония глицирризината не уступала по эффективности и срокам лечения комплексной терапии с дополнительной механической деструкцией элементов контагиозного моллюска [58].
Вирус простого герпеса приводит к усилению межклеточной адгезии между эндотелием сосудов и полиморфноядерными лейкоцитами (P<0,01). Промывание клеточных культур раствором глицирризина значительно снижало силу адгезии между клетками (P<0,01) [59].
Вирус Varicella zoster (VZV) вызывает ветряную оспу, остается в состоянии покоя в ганглиях задних корешков спинномозговых нервов и может реактивироваться в результате снижения VZV-специфического клеточного иммунитета, что приводит к опоясывающему герпесу. Сравнение эффективности лечения пациентов с опоясывающим герпесом глицирризином, ацикловиром, гамма-глобулином и бета-интерфероном показало, что ацикловир и глицирризин сильнее снижают симптомы боли и лучше, чем гамма-глобулин или бета-интерферон, регулируют экспрессию антигенов HLA-DR на CD8-позитивных клетках периферической крови [60].
Глицирризиновая кислота ингибирует проникновение вируса Эпштейна–Барр в клетки, ограничивает рост опухолевых клеток и индуцирует апоптоз в различных клеточных линиях рака. Глицирризиновая кислота, не влияя на реактивацию вируса, создавала микроокружение опухоли, при котором снижается способность вируса инфицировать соседние клетки. Глицирризиновая кислота может быть потенциальным терапевтическим препаратом для повышения эффективности лечения лимфоидных злокачественных новообразований, ассоциированных с вирусемией Эпштейна–Барр [61].
Вирус папилломы человека (ВПЧ) может поражать кожу и слизистые оболочки, в т.ч. влагалища и шейки матки. Лечение плоскоклеточного интраэпителиального поражения, вызванного ВПЧ (n=62, возраст 27,8±9,5 года), посредством топического использования глицирризиновой кислоты позволило устранить поражения, трудно поддающиеся лечению в рамках стандартной терапии. Через 12 недель терапии улучшение было достигнуто у 74% пациентов, использовавших глицирризиновую кислоту (P<0,001) [62].
Остроконечные кондиломы представляют собой доброкачественные разрастания кожи и слизистых оболочек влагалища, вызванные ВПЧ. Кондиломы – одно из самых распространенных венерических заболеваний в мире, заболеваемость которым увеличилась в 5–10 раз за последние три десятилетия. Описаны перспективы использования глицирризина для лечения кондилом [63].
Для применения в гинекологии и при заболеваниях урогенитальной и аногенитальной области разработан препарат «Эпиген Интим» спрей 0,1% на основе глицирризината аммония для местного, наружного и интравагинального использования. Несмотря на то что препарат относится к группе ATX D06BB «Противовирусные препараты», глицирризинат аммония обладает комплексным терапевтическим воздействием (противовирусным, противовоспалительным, иммуностимулирующим, противозудным и регенерирующим). При этом мутантные штаммы вирусов, резистентные к ацикловиру, высокочувствительны к препарату, как и не мутантные штаммы. Иммуностимулирующий эффект глицирризината аммония проявляется повышением числа и активности Т-лимфоцитов, увеличением концентраций иммуноглобулинов А, М и индукцией выработки эндогенных интерферонов. «Эпиген Интим» спрей рекомендуется для лечения вирусных инфекций, вызванных папилломавирусами, герпесвирусами, цитомегаловирусами человека, в составе комплексной терапии; состояний, сопровождающихся снижением местного иммунитета, в т.ч. неспецифического вульвовагинита, кандидозного вульвовагинита и бактериального вагиноза, в составе комплексной терапии; при явлениях дискомфорта в области половых органов, сопровождающихся зудом, жжением и сухостью слизистых оболочек, в т.ч. при недостаточности функции яичников.
Заключение
Глицирризин и его производные (глицирризиновая кислота и др.) относятся к действующим началам препаратов, характеризующихся, прежде всего, высокой безопасностью применения и внутрь, и местно (гели, мази, растворы). Местное применение особенно важно в дерматологии, проктологии, аллергологии, оториноларингологии и гинекологии. Глицирризин и его производные успешно используются в терапии заболеваний кожи (псориаза, атопического дерматита, витилиго, опоясывающего лишая) и воспалительных заболеваний с поражением слизистых оболочек (вызванных как условно-патогенными возбудителями, так и вирусами). Представленные в настоящей работе результаты фундаментальных и клинических исследований указывают на перспективы топического применения глицирризина в гинекологической практике.
Во-первых, следует обратить внимание на антибактериальное и противогрибковое действия глицирризина. Для лечения вагинальных инфекций особое значение имеет антикандидозная активность глицирризина, его влияние на биопленки и иммуностимулирующее действие.
Во-вторых, противовирусные и иммуностимулирующие свойства глицирризина важны для лечения герпесвирусной и папилломавирусной инфекций. Например, топическое использование глицирризиновой кислоты позволяет улучшать результаты лечения ВПЧ-инфекции, которая трудно поддается стандартной терапии.
В-третьих, глицирризин отличается весьма многогранным противовоспалительным действием, включающим регуляцию активности Т-лимфоцитов, тучных клеток, нейтрофилов, макрофагов, секреции провоспалительных и противовоспалительных цитокинов, биосинтеза липоксинов и простагландина Е2. Противовоспалительное действие глицирризина важно для заживления микротрещин, снижения зуда, жжения и для сохранения и восстановления нормального биоценоза влагалища.



