Гестационный сахарный диабет (ГСД) – заболевание, характеризующееся нарушением толерантности к глюкозе, впервые возникшее во время беременности и не соответствующее критериям манифестного диабета [1]. По данным Международной Федерации Диабета 2015 г., каждый седьмой ребенок рождается от матери, больной ГСД [2]. У женщин с ГСД повышен риск возникновения перинатальных осложнений, таких как преэклампсия (ПЭ), макросомия плода, дистоция плечиков плода, гипергликемия новорожденных [3].
Влияние микробного состава желудочно-кишечного тракта (ЖКТ) на здоровье человека становится все более очевидным. Показано, что пациенты с метаболическими нарушениями, такими как ожирение и сахарный диабет (СД) 2 типа, отличаются по составу микробиоты фекалий от здоровых людей [4, 5]. В экспериментальной модели на животных установлено, что снижение числа бифидобактерий приводит к увеличению продукции липополисахаридов, эндотоксемии, ожирению и инсулинорезистентности [6]. Prevotella copri и Bacteroides vulgatus являются основными видами микроорганизмов, которые определяют связь между биосинтезом аминокислот с разветвленной цепью, инсулинорезистентностью и нарушением толерантности к глюкозе [7].
Исследования микробиоты кишечника здоровых женщин выявили значительные изменения ее состава в зависимости от триместра беременности [8, 9]. Koren и соавт. установили, что с I по III триместр беременности снижается количество бактерий, продуцирующих бутират, и возрастает число бифидобактерий, протеобактерий и молочнокислых бактерий [8]. Работы, основанные на секвенировании 16S рРНК, выявили взаимосвязи между составом микробиома кишечника и метаболическим гормональным фоном у беременных с избыточной массой тела и ожирением на ранних сроках гестации [10]. Исследования свидетельствуют о том, что беременность связана с большими изменениями в микробиоте ЖКТ, которая играет важную роль в развитии субклинического воспаления и способствует возникновению ГСД [11].
Микробиота желудочно-кишечного тракта и ее влияние на организм человека
Человеческий организм является носителем уникальной микробной популяции, в которую входят бактерии, археи, грибы и вирусы. Состав микробиоты на каждом участке тела формируется, исходя из изменений условий окружающей среды, таких как уровень кислотно-основного состояния, насыщенность кислородом, наличие питательных веществ, влажность, температура. От перечисленных условий зависит взаимодействие различных популяций микроорганизмов и организма хозяина [12]. Микробные сообщества оказывают большое воздействие на здоровье организма путем вмешательства в его обменные процессы, иммунную систему и выработку гормонов [13]. Большинство микробов находятся в кишечнике, содержащем более сотни видов бактерий. Преобладающими являются бактерии типа Firmicutes и рода Bacteroides [14]. Кроме того, ротовая полость, кожа, влагалище являются важными нишами для определенных микробных сообществ, стимулирующих иммунную систему к защите от потенциальных патогенов [15].
Микробиота ЖКТ состоит из 500–1000 анаэробных видов бактерий, относящихся к нескольким семействам бактерий, плотность и разнообразие которых увеличиваются от проксимального к дистальному отделу ЖКТ [16]. Микробиота ЖКТ участвует в извлечении энергии и продукции короткоцепочечных жирных кислот (ЖК) из непереваренных углеводов [17, 18], биосинтезе витаминов [19], поддержании барьерной, эндокринной и иммунной функций кишечника [20–22]. Таким образом, микробиом ЖКТ рассматривается как «орган», контролирующий многие процессы в организме человека [23].
Существует гипотеза, что дисбактериоз играет ключевую роль в патогенезе многих острых и хронических состояний, включая метаболические заболевания, такие как ожирение, инсулинорезистентность, СД 1 и 2 типов. Выявлено несколько механизмов, связывающих дисбиоз с нарушенным метаболизмом: повышенная проницаемость стенок кишечника, увеличение абсорбции липополисахаридов (ЛПС), аномальная продукция короткоцепочечных ЖК, аберрантное превращение первичных желчных кислот во вторичные желчные кислоты, повышение эндотоксемии [24, 25]. Все эти изменения приводят к активации воспалительного и аутоиммунного путей, мимикрии аутоантигенов, стимуляции эндоканнабиноидной системы, нарушению секреции пептидов ЖКТ, нарушению сигнальной функции инсулина, увеличению энергопродукции и отложению жиров в организме [26, 27].
Методы исследования микробиоты желудочно-кишечного тракта
Методы исследования бактериальных сообществ ЖКТ включают в себя культуральный метод, посредством которого можно культивировать некоторые бактерии, полимеразную цепную реакцию (ПЦР) и наиболее часто используемый метод NGS (секвенирование нового поколения). С помощью NGS производят выделение 16S субъединицы рРНК. Таким образом, выделяется не весь геном, а лишь те его регионы, по которым возможно установить родовую или видовую принадлежность микроорганизмов. Данный метод дает информацию о разнообразии различных микроорганизмов, как внутри одного вида бактерий (α-разнообразие), так и между разными видами (β-разнообразие), а также об их соотношении. Дополнительные методы включают в себя метатранскриптомику, метаболомику и тестирование на животных. Метагеномное секвенирование показывает полный набор генов в микробиоме и может предоставить информацию об относительном количестве генов в функциональных цепочках на всех таксономических уровнях [12].
Изменения микробиоты желудочно-кишечного тракта во время беременности
Нормально протекающая беременность характеризуется увеличением бактериальной нагрузки и значительным изменением в составе микробиоты ЖКТ [28].
В исследованиях, ассоциированных с метаболическими нарушениями, оценивают взаимосвязи уровня гликемии, индекса инсулинорезистентности НОМА, индекса массы тела (ИМТ) с наличием тех или иных микроорганизмов в кишечнике.
В I триместре состав микробиоты ЖКТ не отличается от такового у небеременной женщины. В период с конца I по III триместр беременности состав микробиоты кишечника кардинально меняется. Увеличивается число представителей типов Actinobacteria и Proteobacteria, снижается внутривидовое разнообразие микрофлоры (α-разнообразие) [8]. В III триместре происходит значительное снижение числа бактерий рода Fecaliabacterium – бутират-продуцирующих микроорганизмов, обладающих противовоспалительной активностью особенно у пациентов с метаболическим синдромом [29]. Межвидовое разнообразие бактерий (β-разнообразие) в III триместре увеличивается [8].
Значительные гормональные, иммунологические, метаболические изменения, происходящие в III триместре беременности, ведут к прибавке массы тела, увеличению синтеза провоспалительных цитокинов и повышению инсулинорезистентности [29]. В одном из экспериментальных исследований мышам – гнотобиотам подсаживали образцы стула беременных женщин I и III триместров. Было показано, что у мышей, получивших микрофлору ЖКТ беременных поздних сроков, наблюдается прибавка массы тела, увеличение инсулинорезистентности и синтеза провоспалительных цитокинов [8]. Авторы делают вывод о том, что изменения микробиоты ЖКТ способствуют метаболическим сдвигам во время беременности. Бактериальный состав микрофлоры организма беременной в III триместре оказывает существенное положительное влияние на течение беременности и развитие плода. В норме все три фактора: прибавка веса, повышенная инсулинорезистентность и наличие субклинического воспаления – необходимы для роста плода. Одним из возможных механизмов влияния микробиоты ЖКТ на этот процесс является увеличение всасывания глюкозы и ЖК, повышение секреции ангиопоэтин-подобного белка 4 (ANGPTL-4), индуцирование катаболических путей и стимуляция иммунной системы [8, 30].
На микробиоту ЖКТ беременных влияют такие внешние факторы, как диета и окружающая среда. В исследовании на мышах показано, что соблюдение диеты до зачатия и во время беременности оказывает прямое воздействие на микробиом. Основную группу мышей кормили пищей с высоким содержанием жиров, у группы контроля питание было сбалансированным. В дальнейшем выявили, что в течение беременности у основной группы произошли значительные изменения в качественном составе микробиоты, в отличие от группы контроля [31].
Найдены многочисленные корреляционные связи между конкретными представителями бактерий и изменениями процесса обмена веществ в организме во время беременности. Прямая зависимость выявлена между бактериями рода Colinsella и содержанием циркулирующего в крови инсулина, триглицеридов и липопротеидов низкой плотности (ЛПНП); бактериями рода Sutterella и содержанием в крови С-реактивного белка; соотношением бактерий Ruminococcaceae/Lachnospiraceae и уровнем лептина; бактерий семейства Bacteroidaceae и содержанием грелина; бактерий рода Coprococcus и глюкозозависимым инсулинотропным полипептидом (GIP). Кроме того, существует обратная связь между: бактериями рода Blautia и уровнем инсулина; соотношением бактерий Faecalibacterium/Fusobacterium и содержанием глюкозы крови; бактериями вида Odoribacter и артериальным давлением крови; бактериями семейства Ruminococcaceae и GIP; бактериями семейства Prevotellaceae и содержанием грелина в плазме крови [11:10]. В одном из исследований было показано, что существует связь между ИМТ женщины до беременности и составом микробиоты ЖКТ новорожденного, родившегося через естественные родовые пути [32]. На данный момент еще не изучены многие механизмы влияния микробиоты ЖКТ на метаболические изменения в организме беременной, в связи с этим требуются дальнейшие исследования.
Микробиота желудочно-кишечного тракта. Потенциальные механизмы возникновения метаболических нарушений во время беременности
Нарушения процессов обмена веществ и изменения состава микробиоты ЖКТ во время беременности взаимосвязаны между собой и могут влиять друг на друга посредством нескольких механизмов (рисунок). Например, короткоцепочечные ЖК являются результатом брожения непереваренных полисахаридов [17, 18], а количество и тип продуцируемых ЖК частично зависят от состава микробной флоры кишечника [32]. Помимо энергетической функции, ЖК стимулируют секрецию глюкагон-подобного пептида-1 L-клетками тонкого кишечника [20]. Таким образом, это ведет к увеличению стимулированной глюкозой секреции инсулина и уменьшению системного воспаления [33, 34].
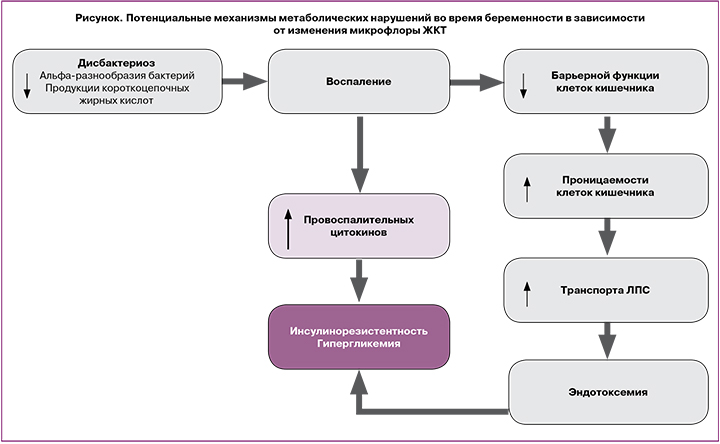
Состав кишечной микрофлоры также влияет на межклеточные соединения в слизистом слое тонкого кишечника, тем самым предотвращая перенос провоспалительных эндотоксинов из просвета кишки в кровоток [35]. Лактулозоманнитоловый тест показал, что беременные женщины имеют большую проницаемость кишечника, чем небеременные, что влияет на состояние метаболизма во время гестации [36].
Таким образом, дисбаланс микробиоты ЖКТ, связанный с нарушением обмена веществ во время беременности, может привести к воспалению, изменению транспорта глюкозы посредством нарушения продукции короткоцепочечных ЖК и секреции гормонов ЖКТ и/или несостоятельности барьерной функции кишечника.
Изменения микробиоты желудочно-кишечного тракта при гестационном сахарном диабете
Во второй половине беременности формируется инсулинорезистентность, которая значительно повышена при ГСД. Обнаружено, что повышение резистентности к инсулину может быть связано с увеличением соотношения бактерий Fermicutes/Bacteroidetes и уменьшением разнообразия бутират-продуцирующих бактерий, таких как Roseburia и Faecalibacterium prausnitzii [17, 18 32].
В своем исследовании Koren и соавт. [8] выявили, что у женщин, у которых впоследствии развился ГСД, внутривидовое разнообразие (α-разнообразие) микроорганизмов ЖКТ в I триместре беременности было снижено; при этом не было существенных различий межвидового разнообразия (β-разнообразие) состава микробиоты в сравнении с микробиотой здоровых женщин. У пациенток с ГСД, в отличие от здоровых женщин, было обнаружено увеличение содержания в микрофлоре бактерий видов Parabacteroides disnis, Klebsiella variicola [20], родов Ruminococs, Eubacterium, Prevotella [37], Collinsella, Rothia, Desulfovibrio, типов Actinobacteria [34], Firmicutes и снижение бактерий видов Methanobrevibactermithii, Alistipes species, Bifidobacterium species, Eubacterium species [20], родов Akkermansia, Bacteroides, Parabacteroides, Roseburia и Dialister [37]. В микробиоте ЖКТ пациенток с ГСД отмечается увеличение мембранного транспорта веществ и их катаболизма, повышение активности липолисахаридных и фосфотрансферазных систем, снижение метаболизма аминокислот [35].
Вопрос о том, являются ли изменения микробиома ЖКТ причиной или следствием ГСД, остается открытым. Возможно, что снижение разнообразия микроорганизмов с увеличением доли бактерий семейства Ruminococcaceae является причиной повышения энергетического обмена, начальных проявлений субклинического воспаления и нарушения выработки инсулина [35].
В исследовании Kuang Y.S. и соавт. [38] отмечено, что рост разнообразия микробного состава групп метагеномной связи у женщин с ГСД и у группы контроля прямо пропорционален уровню толерантности к глюкозе. Установлено, что системы биосинтеза и экспорта ЛПС участвуют в регуляции уровня гликемии. По мнению авторов, количество ЛПС влияет не только на уровень глюкозы крови, но и на повышение проницаемости кишечника, изменение соотношения грамотрицательных и грамположительных бактерий, а также на развитие субклинического хронического воспаления [39, 40]. Происходит снижение грамположительных бутират-продуцирующих бактерий, в клеточной стенке которых практически не содержатся ЛПС (Clostridium), и увеличение грамотрицательных условно-патогенных микроорганизмов (Bacteroides, Proteobacteria), содержащих ЛПС. В итоге у пациентов с ГСД повышается мембранный транспорт, энергетический метаболизм и количество фосфотрансферазных переносчиков, которые катализируют захват углеводов и отвечают за перенос глюкозы через внешние и внутренние мембраны клеток. Таким образом, увеличение количества этих переносчиков приводит к повышенному использованию глюкозы в качестве источника энергии [36].
Согласно другим исследованиям, данные анализа микробиоты через 3–16 месяцев после родов показали, что имеется различие между женщинами с нормальным гликемическим статусом (1-я группа) и ГСД в анамнезе (2-я группа). Во 2-й группе обнаружено преобладание бактерий семейства Prevotellaceae, родов Collinsella, Olsenella, Clostridium и сниженное количество бактерий типа Firmicutes, родов Fusobacterium и Ruminococcus [34, 36].
Интересно, что при повторном исследовании у этих женщин через 5 лет после родов различий в составе микробиоты ЖКТ не обнаружено [39].
Влияние приема пробиотиков на состав микробиоты желудочно-кишечного тракта
Профилактика и лечение дисбактериоза кишечника необходимы для снижения риска развития ГСД во время беременности, а также перинатальных и неонатальных осложнений [41]. Согласно некоторым исследованиям, пробиотики способны повысить чувствительность к инсулину и улучшить метаболизм холестерола [42, 43]. Применение пробиотиков оказывало положительные эффекты в случаях ожирения, СД 2 типа, неалкогольной жировой болезни печени и других патологиях [44].
Пробиотическими микроорганизмами являются определенные штаммы бактерий, принадлежащие к роду Lactobacillus, Bifidobacterium, Enterococcus, а также бактерии рода Propionibacterium и некоторые дрожжи S. Boulardii [45]. У пробиотиков четыре основных механизма действия: 1) конкуренция с патогенными бактериями за питательные вещества и места прикрепления к стенке кишечника; 2) улучшение барьерной функции эпителия кишечника; 3) иммуномодулирующее действие; 4) положительное влияние на другие органы и системы посредством иммуномодулирующего действия и системы нейротрансмиттеров (гамма-аминомасляная кислота, серотонин) [46]. Эти эффекты, в основном, обеспечиваются бактериями рода Lactobacillus путем продукции молочной кислоты и перекиси водорода, а также через активацию клеток в Пейеровых бляшках, изменения экспрессии Toll-like рецепторов и увеличение продукции слизи в бокаловидных клетках [47]. Bifidobacterium animalis – это еще один штамм бактерий, используемый в качестве пробиотиков. Данный вид мало восприимчив к средам с низким содержанием кислорода и высоким содержанием кислоты [48].
В литературе имеется мало данных о влиянии использования пробиотиков на микрофлору ЖКТ при ГСД. По данным проведенного рандомизированного клинического исследования (РКИ) показано, что введение пробиотиков в рацион питания беременной женщины снижает частоту возникновения ГСД на 60% [49]. Первое РКИ включало в себя 256 женщин. У 66% из них, получавших бактерии видов Lactobacillus rhamnosus GG, Bifidobacterium lactis BB-12 и консультирование по питанию с ранних сроков беременности, не развился ГСД по сравнению с теми, кто получал плацебо + консультирование или только плацебо [50]. Во втором исследовании участвовали 70 женщин, которые употребляли в пищу йогурт, обогащенный Lactobacillus acidophilus LA5 и Bifidobacterium animalis BB-12 в течение 9 недель. В результате в III триместре беременности наблюдались небольшие колебания уровня инсулина в крови и индекса инсулинорезистентности НОМА [36]. Также было проведено исследование, в которое входило 138 женщин с ожирением. В течение 4 недель (с 24 по 28 неделю беременности) половине из них давали капсулы с пробиотиком, содержащим Lactobacillus salivarius UCC118, другим – плацебо. В результате не было обнаружено достоверных различий уровня гликемии, риске возникновения ГСД или весе новорожденного [51]. Полученные данные указывают на то, что положительный результат зависит от длительности приема препарата, и от того, какие штаммы пробиотических бактерий используются.
Помимо этого, пробиотики рассматривают как средство лечения ГСД. В исследовании Lindsay и соавт. [52] участвовали149 женщин с ГСД. Половине из них были назначены пробиотики, содержащие Lactobacillus salivarius UCC118, другой половине – плацебо. Применение пробиотиков не повлияло на гликемический профиль, необходимость терапии ГСД фармакологическими препаратами, вес плода при рождении, но снизило уровень общего холестерина и ЛПНП в III триместре беременности. В другом РКИ приняли участие 64 женщины с ГСД. Часть из них получила пробиотик, состоявший из комбинации бактерий Lactobacillus acidophilus LA-5,Bifidobacterium BB-12, Streptococcus thermophilus STY-31 и Lactobacillus delbrueckiibulgaricus LBY-27, другая – плацебо в течение 8 недель от момента постановки диагноза. В результате у первой группы уменьшилась прибавка в весе, значительно снизился уровень гликемии и инсулинорезистентности [53]. Данные исследования показывают, что прием пробиотиков во время беременности оказывает положительное влияние на организм женщины, предотвращая метаболические нарушения [54].
Заключение
Проведенный анализ данных показал, что микробиота ЖКТ играет важную роль в развитии метаболических нарушений в организме женщины во время беременности. Исследования говорят о том, что количество и разнообразие микрофлоры ЖКТ оказывает влияние на течение беременности, новорожденного и период раннего детства. Во время беременности в организме женщины происходит смена гормонального фона. Микробиота ЖКТ способна оказывать влияние на секрецию гормонов кишечника и, таким образом, воздействовать на эндокринную систему организма-хозяина. Помимо этого, существует взаимосвязь между микрофлорой ЖКТ и иммунной системой. Измененная микробиота ЖКТ у беременной стимулирует выработку провоспалительных цитокинов, что способствует развитию локального воспалительного процесса в стенке кишечника. Альтерация энтероцитов обуславливает нарушение всасывания глюкозы, жирных кислот, ЛПС, токсинов. Совокупный процесс нарушений проницаемости клеточных мембран энтероцитов и гиперэкспрессии провоспалительных цитокинов может приводить к повреждению функции инсулиновых рецепторов, что обуславливает развитие инсулинорезистентности. Это является важным этапом патогенеза ГСД.
Методом коррекции нарушенной микрофлоры ЖКТ является прием пробиотиков. Пробиотики способствуют уменьшению реакций субклинического воспаления, восстановлению баланса всасываемых веществ. Это, вероятно, снижает риск развития ГСД. Использование определенных пробиотических добавок во время беременности может служить профилактикой ГСД, однако требуются дальнейшие исследования данного вопроса.



