Development and validation of a questionnaire for assessing androgen deficiency in women (FAD – Female Androgen Deficiency) of reproductive age
Relevance: The early manifestations of androgen deficiency begin in women after the age of 35, particularly in infertile patients with diminished ovarian reserve (DOR). Their detection can be used in clinical practice to assess the degree of androgen deficiency and to predict possible outcomes of assisted reproductive technology (ART) (IVF/ICSI) programs. Objective: To develop and validate a Female Androgen Deficiency (FAD) questionnaire for assessing androgen deficiency in infertility women for further use in clinical practice. Materials and methods: We developed the FAD questionnaire in accordance with international and Russian standards and tested it with a focus group of women with presumed androgen deficiency including infertile women with diminished ovarian reserve. After that we assessed the results of psychometric testing including reliability, validity and sensitivity of the developed questionnaire. Results: The results of FAD testing with the participation of patients and expert clinical opinion confirmed the acceptability of the FAD questionnaire, its compliance with the environment, and high indicators of content and external validity. The stable structure of the instrument was shown, indicating satisfactory construct validity of the questionnaire. The α-Cronbach's coefficient was equal to 0.837, indicating the internal consistency of the FAD questionnaire. An analysis of the correlations between the total FAD score and the number of symptoms in infertile women with DOR revealed a statistically significant strong negative relationship (r=-0.76; p<0.001), which reflects good criterion validity. The factor analysis showed the adequacy of the questionnaire's factor structure according to the relationships between variables and its stable construct validity. The total quality of life score on the questionnaire was lower in healthy women than in women with DOR [31.7 (6.2) vs 18.4 (4.1)], demonstrating satisfactory discriminant validity of the questionnaire. The correlation analysis of the FAD and FSFI showed satisfactory convergent validity of the FAD questionnaire. Conclusion: The study results testify to the reliability, validity, and sensitivity of the FAD questionnaire and its use for both clinical practice and for scientific purposes in obstetrics and gynecology, in particular, gynecological endocrinology and reproductive medicine.Chausov A.A., Gavisova A.A., Dolgushina N.V., Nazarenko T.A., Gardanova Zh.R.
Keywords
The problem of androgen deficiency in women of reproductive age has not been sufficiently explored and, despite the latest clinical and research advances in this condition in postmenopause it remains open for discussion. In modern scientific and clinical practice, the criteria for the diagnosis of androgen deficiency are not defined, nor is their contribution to the quality of life of a woman of reproductive age [1]. Since there is a current trend of late reproductive age childbearing, the problem of studying androgen deficiency seems extremely relevant, both from the point of view of reproductive achievements and the quality of life and health of women. The decision to have a child at a late reproductive age, 35 years and more is made already against the backdrop of changes in the concentration of reproductive indices, long before menstruation rhythm abnormalities, which becomes clinically pronounced at 40 years of 40 and is associated with changes in folliculogenesis [2]. Numerous studies have identified the impact of androgens and their metabolites as dominant on sexual dimorphism, cognitive, psychoemotional spheres, metabolic processes and sexual functioning. Detection of changes in these functions is not always possible on the basis of laboratory data alone, since the clinical diagnosis is retrospective in nature [1]. Difficulties in diagnosing androgen deficiency are further complicated by the lack of normal reference ranges of androgens for women, including by age group, and difficulties in determining the concentration of testosterone as a molecule with a low molecular weight, which makes it difficult to detect changes associated with a decrease in androgen levels. All the above suggests the need to develop additional diagnostic research methods and create a psychometric construct.
Androgen deficiency was defined in 2002 by the Princeton consensus in women with physiological menopause or prolonged severe hypoestrogenism in the presence of the following symptoms: decreased libido, impaired general well-being, dysphoric disorders, asthenia, a feeling of constant and unexplained fatigue, subdepressive mood, reduced bone mass and muscle strength, thinning hair and impaired cognitive and mental functions [3]. Such symptoms and manifestations are explained by the distribution of androgen receptors (AR) which expression is observed in various tissues in reproductive and nonreproductive organs, in particular in the central nervous system, which indicates the role of androgens in cognitive and sexual functioning. Thus, gonadectomy alters hippocampal neuroplasticity with subsequent depression and decreased sexual motivation and attraction. It has been established that the brain determines secondary sexual traits during development and sexual maturation, maintains its functional state throughout adult life, and models sexual behavior, which is a target not only for estradiol, which initiates sexual desire [4, 5], but also for testosterone, which influences neural and behavioral functions through genomic and non-genomic effects [6].
The functioning of key brain areas responsible for motivation and pleasure depends on testosterone concentration and is mediated mainly by AR activation. ARs are highly affine to dihydrotestosterone in the medial preoptic region of the hypothalamus, a key brain region that regulates sexual behavior. The influence of testosterone on sexual functioning is based on neuroendocrine mechanisms. However, the mechanisms for achieving the effects are still under study and are a topic of active debate whether it occurs through direct AR stimulation or through its conversion to estrogens and subsequent binding to estrogen receptors [7].
As women age, there is a progressive decrease in follicular reserve in the ovaries, which between 30 and 40 years of age correlates with a decrease in fertility. Along with the decrease in the number of antral follicles (CAF), the quality of oocytes also decreases [5]. The main risk factor for diminished ovarian reserve (DOR) is known to be age [6]. This occurs not only due to a decrease in the pool of follicles in the ovaries [7], but also as a result of decreased sensitivity of the ovaries to follicle stimulating hormone (FSH) [8] and decreased levels of androgen precursors [dehydroepiandrosterone (DHEA), DHEA-sulfate (DHEA-S), androstenedione] and bioactive androgen – testosterone [9, 10].
According to the results of the assisted reproductive technology (ART) program conducted in the V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation from 2014 to 2021, there was an increase in the number of women with DOR (Fig. 1).
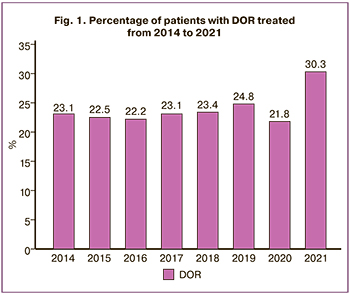
The gold standard for determining blood hormone concentrations is high-performance liquid chromatography mass spectrometry. The use of psychometric testing can be a method of assessing early manifestations of androgen deficiency before hormonal examination in women of reproductive age in order to correct them.
In scientific and clinical practice, one of the methods for diagnosing cognitive disorders and sexual functioning is psychometric testing using a questionnaire survey [10]. The use of information obtained directly from the patient when analyzing the clinical manifestations of the disease and assessing the dynamics of symptoms during the course of treatment contributes to the implementation of a patient-centered approach in the treatment of women with infertility and DOR.
In modern medical practice, the most widely used international questionnaire to detect age-related androgen deficiency in men is Aging Males Symptoms [11, 12]. While in women there is no questionnaire to detect early changes in androgen deficiency in the available literature. The Kupperman Index, a scale for assessing estrogen deficiency in menopause, is widely used for women [13]. For a detailed assessment of female sexual functioning, the FSFI (Female Sexual Function Index) questionnaire is used [14, 15].
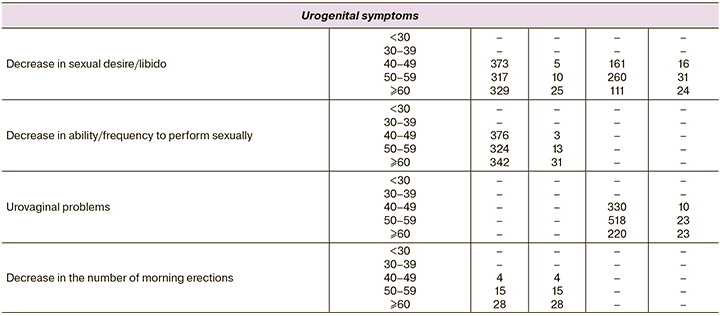
When developing the AGMS questionnaire for men, German researchers analyzed 6 databases of men and women distributed by age (Appendix 1). The analysis showed an increase in complaints with increasing age, an increase in the proportion of patients with complaints in the age range of 30–39 years and 40–49 years for women, indicating the impact of ageing on overall well-being. This is especially true for women after age 35 and older. The association with androgens and androgen deficiency has been seen in a decrease in levels of androstenedione and DHEA-C as precursors of steroid hormones after age 30 years [16].
Women most commonly experience symptoms that may be attributable to androgen deficiency after the age of 35, when functional activity of sex glands – ovaries decreases and becomes even more pronounced after the age of 40. Uniform distribution and occurrence of complaints both in men and women once again confirmed the age-associated decrease of sex steroids. It is reasonable to consider the manifestations of the syndrome complex when several complaints are combined. In menopause, the manifestations observed most frequently on all three scales used to assess severity were psychoemotional, somatic-vegetative, and genitourinary. Considering the important role of sex steroids, estrogens, and androgens in the functional activity of the reproductive system in both men and women, we can assume that sex steroid deficiency affects both sexes, but the severity of their manifestations manifests clinically differently. Moreover, women's reproductive activity is reduced in time, unlike men, which is explained by physiological features on the one hand, and by high concentrations of androgens in the body; whereas in women the main circulating hormone in the body is estradiol and its metabolites, but androgens are precursors of estrogen synthesis. In hyperestrogenism, there is no increase in the ovarian follicular pool, while an increase in androgens results in adrenal hyperandrogenism and polycystic ovarian syndrome. However, an age-dependent decrease in both follicular reserve and androgens with different clinical manifestations depending on their initial values was observed after the age of 35. All of the above suggests the need to develop and validate the androgen deficiency questionnaire for women.
We have developed and validated the Female Androgen Deficiency (FAD) questionnaire (Appendix 2) for the early diagnosis of manifestations even before laboratory changes in androgens are detected. This questionnaire makes it possible to assess the severity of psychological, somatic, and sexual disorders caused by age-related androgen reduction not only in postmenopause but also in the reproductive period, which is relevant in women with infertility.
The present study aimed to develop and validate a Female Androgen Deficiency (FAD) questionnaire for assessing androgen deficiency in infertility women for further use in clinical practice and in scientific research.
Materials and methods
The study was carried out from 2019 to 2022 at the 1st Gynecological Department, V.I. Kulakov NMRC for OG&P. All patients included in the study received FAD and FSFI questionnaires at a one-step consultative appointment, followed by a clinical and laboratory examination; The interview was selectively performed as described below. The FAD questionnaire was administered one month after the consultation (patients who received therapy completed one month after androgen priming).
For linguistic validation and elimination of double interpretation of the questions, the test version of the FAD was first tested on a population of 20 women from different socioeconomic groups by individual interviewing. The FAD questionnaire was tested by the same method on 10 patients with established androgen deficiency and on 10 patients without androgen deficiency.
The key aspects when interviewing patients included the evaluation of correctness of the language and accessibility of perception; comfort of choosing an answer; and ease of assessing a problem. This phase also tested the validity of the questionnaire based on patients' opinions and the range of possible problems included in the study.
In the second stage of the study, we tested the FAD questionnaire in a group of women with infertility and DOR, whose laboratory findings revealed androstenedione <7.0 nmol/L and FSH <8.6 mEU/L with evaluation of the psychometric properties of the instrument.
Women of reproductive age (18 to 42 years) were included in the study if they had indications for the ART and DOR programs, provided consent to participate in the study, and were able to complete the questionnaires. Written informed consent was obtained from each patient enrolled in the study.
Exclusion criteria were surgical menopause (bilateral oophorectomy or hysterectomy), hormone-producing tumors, body mass index (BMI) ≥30 kg/m2 and ≤18 kg/m2, HIV infection and other immunodeficiency conditions, rheumatic diseases, immunomodulatory therapy, administration of glucocorticoids, combined oral contraceptives, other hormonal drugs, intrauterine contraception, and cancer.
The study initially enrolled 600 women, 300 per group. Patients who did not meet the inclusion criteria and did not provide complete information about their medical history and laboratory tests were excluded or refused to participate further in the study. The 496 patients included in the study were examined according to Order 803H of the Ministry of Health of 31 the Russian Federation of July 2020. The following factors were registered when taking the medical history: the patient's age, somatic diseases, smoking, BMI, sexual activity, age of menopause in the woman's mother.
Two groups of patients were formed according to the state of ovarian reserve defined by the Bologna criteria. Group 1 included 256 women with DOR (AMH≤1.2 ng/mL and CAF≤5 on day 3 of the menstrual cycle), Group 2 included 240 women with normal ovarian reserve (NOR) (AMH≥1.2 ng/mL and CAF≥5 on day 3 of the menstrual cycle).
Validation of the developed FAD questionnaire included assessment of several parameters including reliability, validity, and sensitivity to changes over time. Validity assessment included construct validity, discriminant validity, criterion validity, and convergent validity. Reliability was analyzed by assessing internal consistency by calculating the Cronbach's α coefficient (internal consistency coefficient). The construct validity of the questionnaire was tested by examining its structure by factor analysis. Discriminant validity was evaluated using the known group method, comparing the total score of the questionnaire in women with infertility and DOR in ART programs with oocyte donors. To assess convergent validity, we analyzed the correlations between the total score and the number of symptoms experienced. Criterion validity was assessed using a correlation analysis between the FAD total score and the scales of the FSFI questionnaire, which was considered the standard for assessing changes in sexual functioning in women. The androgen deficiency assessment questionnaire FAD characterized three groups of symptoms with four items in each group and was helpful in detecting the decrease in androgen concentration and the manifestations of androgen deficiency taking into consideration the prevalence of AR in a woman's organism.
Group 1 – psychological factors, including sleep problems, nervousness, loss of motivation, and depression.
Group 2 – somatic factors, including deterioration of well-being and general conditions, pain and aches in muscles and joints, sweating and decreased muscle strength.
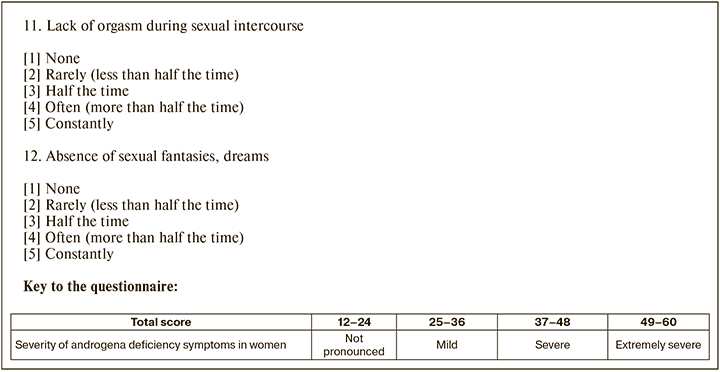
Group 3 – sexual factors included decreased frequency of sexual intercourse, decreased sexual desire/libido, lack of orgasm during sexual intercourse, and lack of sexual fantasies and dreams.
Each item on the questionnaire was rated according to five degrees of symptom severity: none – 1 point, rarely (less than half time) – 2 points, half time – 3 points, often (more than half the time) – 4 points, constantly – 5 points.
For each group of factors, the severity was calculated by summing up the scores received for the corresponding questions. The total score characterizing the severity of androgen deficiency symptoms in women was calculated by summing the scores of all parameters.
To confirm the result, all women answered the questionnaire twice, one month apart.
To assess the sensitivity of the questionnaire to changes over time, women with androgen deficiency were treated with androgen-containing drugs (oral DHEA and testosterone-containing gel) for priming purposes. The results of the questionnaire before and after therapy were assessed both as a total score and for the three components separately.
The androgen profile was also assessed by mass spectrometry and chemiluminescent immunoassay.
Statistical analysis
Statistical analysis was performed using the Statistica V10 software packages (StatSoft Inc., USA) and SPSS Statistics V17 (USA). The distribution of continuous variables was tested for normality using the Kolmogorov–Smirnov test. Quantitative variables showing normal distribution were expressed as means (M) and standard deviation (SD), and the presented as M (SD) and groups were compared using parametric tests (Student’s t test to compare data between two groups, to compare paired samples, the paired t test was used). Nonnormally distributed variables were presented as median with interquartile range Me (Q1; Q3). Differences between two groups with non-normally distributed data were analyzed using the Mann–Whitney U-test, Wilcoxon test was used to compare paired samples. Chi-square (χ2) test were used to compare qualitative variables presented as counts and percentages. We also used Pearson correlation analysis (for normally distributed variables) and nonparametric Spearman correlation analysis. Cronbach's α-coefficient was calculated to assess the reliability of the questionnaire. Factor analysis using principal components analyses with varimax rotation was applied according to the stochasticity criterion. Differences between were considered statistically significant at p<0.05.
The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P (Ref. No: 2 of February 07, 2019).
Results
When creating the preliminary test version of the questionnaire, the optimal wording was chosen, which was proposed taking into account the cultural characteristics of the population. Women with infertility and DOR between the ages of 18 and 43 were included in the interviewing procedure to test for FAD. During the interviewing process, all patients noted the clarity of the presentation and comprehensibility of the meaning of the test-version questions, their positive impression of the proposed way of assessing quality-of-life problems, and the relevance of the questionnaire questions to actual problems in women with infertility and DOR. On average, it took the patient 3 minutes to complete the questionnaire. Based on the results of FAD testing with patients, three indicators of external validity were determined - comprehensibility, ease, and convenience of assessment (for each symptom and for the instrument as a whole), and completeness of assessment (for the instrument as a whole). Each factor is expressed as a score from 0 to 1; the higher the score, the better the external validity of the instrument in this category. On the whole, we got high values of external validity for FAD: understandability – 1,0 points, ease and convenience of evaluation – 0,91 points, completeness of evaluation – 0,87 points. The overall (average) external validity score was 0.88. The comments of the patients on some questions revealed during the test were, in general, insignificant and were most likely caused by the individual characteristics of the patients and external factors.
Therefore, according to the results of the FAD testing with patient participation, as well as during peer review, clinicians confirmed the acceptability of the FAD questionnaire, its compliance with the environment, and high content and external validity.
Women of reproductive age [mean 37.3 (2.4) years] with infertility and DOR, who referred for ART at the V.I. Kulakov NMRC for OG&P participated in the testing of the FAD questionnaire.
All patients had a regular menstrual cycle with a mean duration of 27.4 (2.1) days. In mothers of patients in the group of women with DOR the age at menopause was 45.6 (2.6) years.
Each third patient had salpingitis and adnexitis in their history of pelvic inflammatory and infectious diseases. Surgical interventions, such as appendectomy or diagnostic laparoscopy due to tubal-peritoneal factor, were mentioned by 30% of the patients. A history of adhesive disease was often observed, which was probably associated with a higher frequency of surgical interventions. There was an average of 2.7 (1.2) previous IVF attempts that failed at the stage of hormonal verification of pregnancy. The duration of infertility was 6.8 (5.9) years, of which 347/496 (70%) patients had primary infertility. A history of pregnancy that ended in childbirth was observed in 164/496 (33%) patients, medical abortion for various reasons in 94/496 (19%), early termination of pregnancy before 6–7 weeks of gestation was reported by173/496 (35%) patients.
When assessing anthropometric data, we found a tendency towards a higher BMI in patients with DOR, which is 24.6 (5.4) kg/m², although there was no statistically significant difference.
Let us consider the results of testing the FAD questionnaire in the focus group of women with infertility and DOR. In general, the questionnaire was understandable and did not cause difficulties in completing in the vast majority of patients.
Construct validity
Factor analysis using principal component analyses with varimax rotation was used according to the stochasticity criterion (Fig. 2).
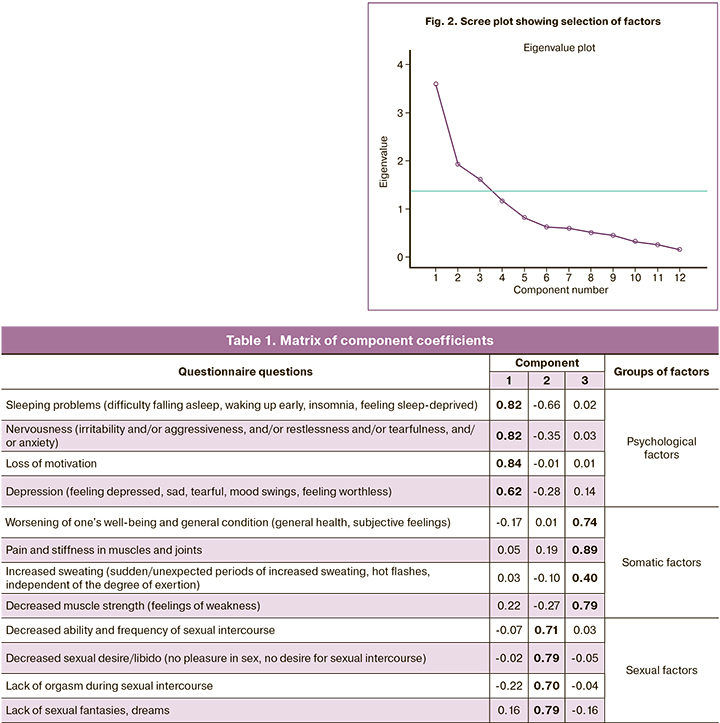
As a result of our analysis, we selected 3 factors with a commonality value greater than 1. After the varimax rotation by Kaiser normalization, we obtained the results (Table 1).
Kaiser–Meyer–Olkin sampling adequacy criterion (0.72) and Bartlett's sphericity criterion (p<0.001) indicate acceptable adequacy of factor analysis with the sample. The calculated commonality coefficients exceeded 1 for all. Therefore, the factor analysis showed the adequacy of the grouping of the questionnaire questions by implied factors and stable construct validity.
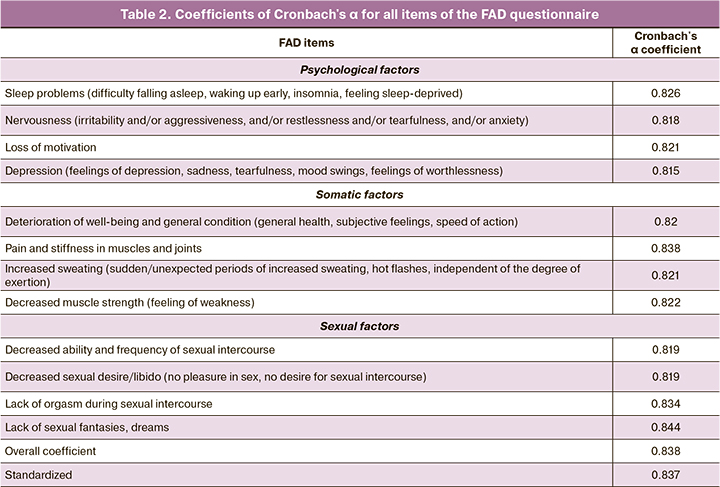
Reliability
When assessing internal consistency, a value of the Cronbach's α coefficient of 0.837 was obtained for the 12 questions related to the manifestations of androgen deficiency. This value indicates the internal consistency of the FAD questionnaire. Table 2 shows the values of the α-Cronbach's coefficient when the questionnaire items were removed one after another. It can be concluded that the instrument used allows a fairly accurate assessment of the symptoms/problems that form with androgen deficiency in women of reproductive age at an early stage.
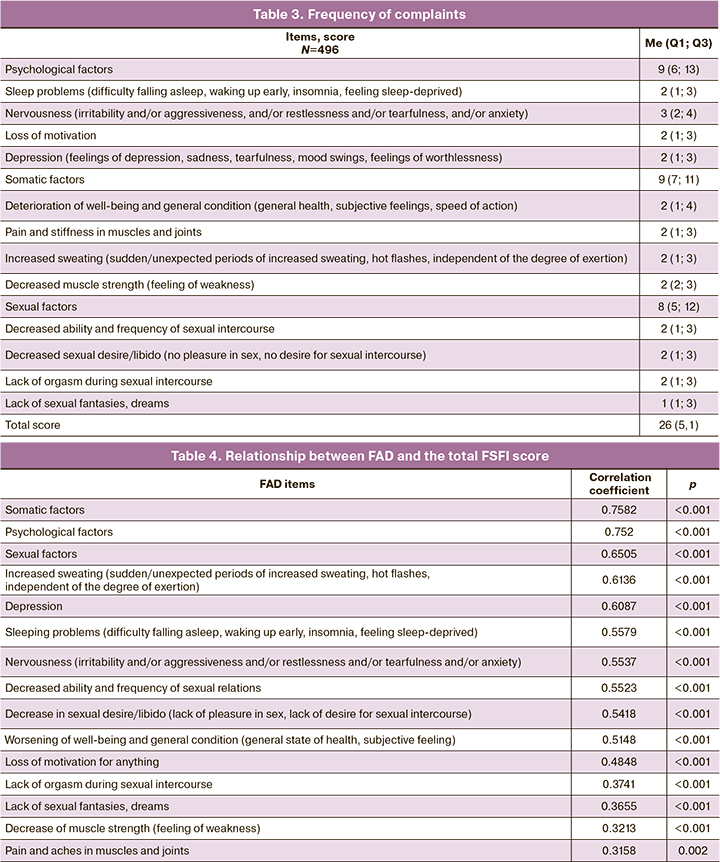
Convergent validity
Analysis of the correlation between the total score of the questionnaire and the number of complaints and symptoms due to the forming symptom complex - androgen deficiency showed that the most frequent symptoms were nervousness (3.4 (1.4) points), decreased ability and frequency of sexual relations (2.9 (1.2) points), worsened well-being and general well-being (2.8 (1.3) points), decreased sexual desire/libido (2.8 (1.1) points). An analysis of the correlations between the total FAD score and the number of symptoms in women with infertility and DOR revealed a strong negative statistically significant relationship (r=-0.76; p<0.001). The more symptoms and problems associated with the disease a patient experience, the more pronounced are the manifestations of androgen deficiency. These findings reflect the good criterion validity of the FAD questionnaire (Table 3).
Discriminant validity
To determine the sensitivity of the FAD questionnaire, we performed an analysis comparing the total score in the groups of women with infertility and DOR with the group of women with NOR and oocyte donors by "known groups" method: the total FSFI score was lower in healthy women than in women with DOR (31.7 (6.2) points versus 18.4 (4.1) points, Student's test, p<0.001). Thus, the ability of the instrument to determine the degree of severity of androgen deficiency manifestations based on DOR was demonstrated. These data characterize the satisfactory discriminant validity of the questionnaire.
Criterion validity
The correlation analysis revealed statistically significant positive correlations between the total FAD and FSFI scores: a strong correlation between the total score and the psychological factors, r=0.752 (Table 4, Fig. 3).
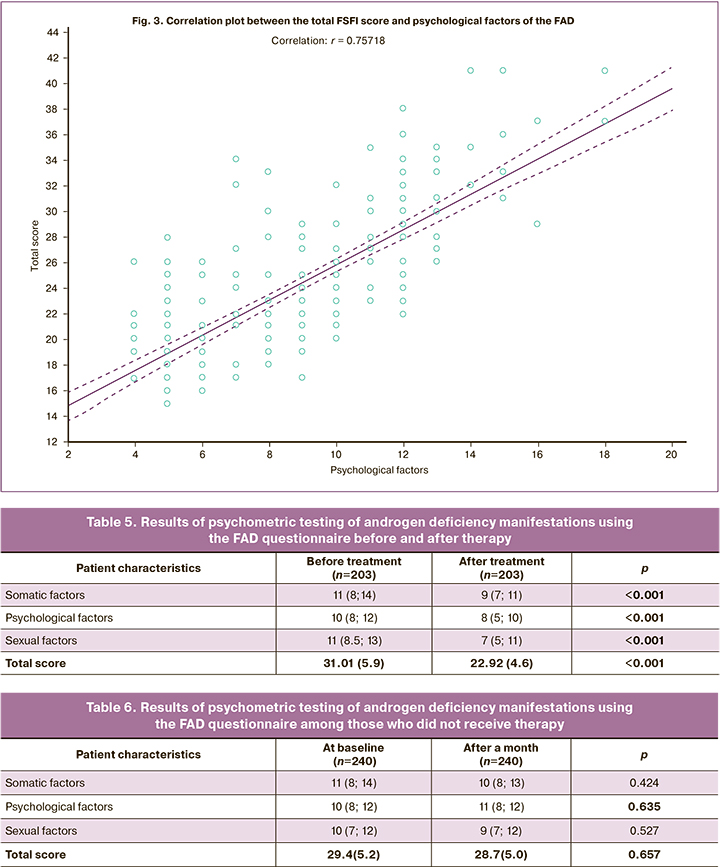
Therefore, satisfactory convergent validity of the FAD questionnaire was shown.
Sensitivity to change
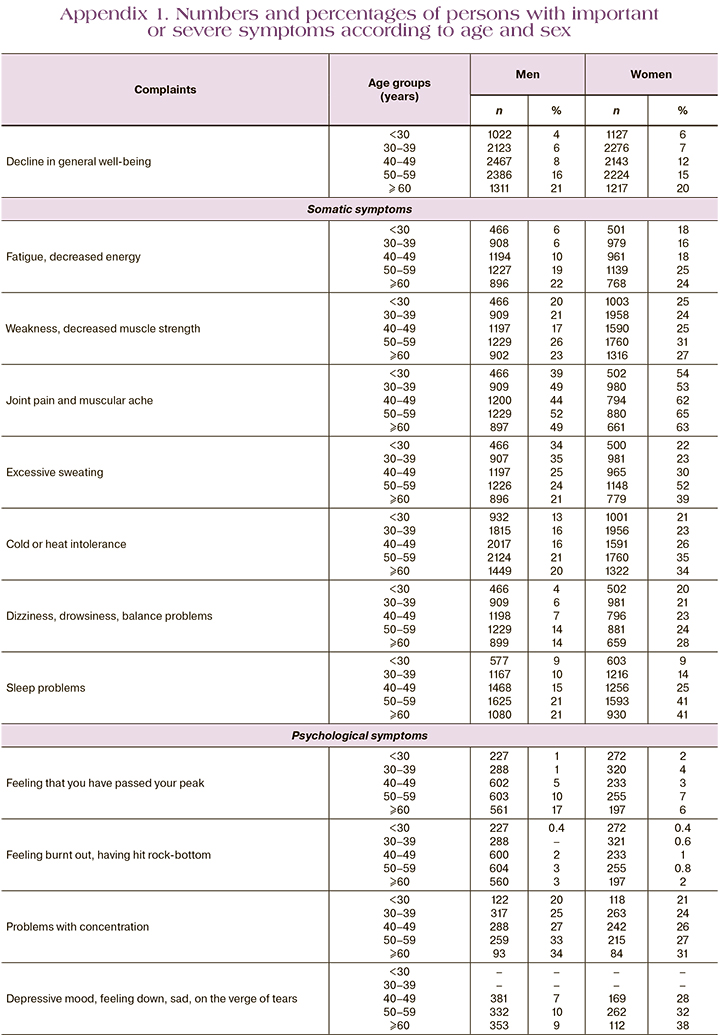
The sensitivity of the questionnaire to changes over time was analyzed by estimating changes in the total score for the groups of women who completed the questionnaire one month after androgen priming (n=203); those who did not receive androgen therapy completed the questionnaire again one month after the first questionnaire. Against the background of androgenic priming treatment, a statistically significant decrease in the total score was registered, from 31.01 (5.9) to 22.92 (4.6) (p<0.001), which is a sign of a significant improvement of the state and resolution of androgenic deficiency manifestations in women of childbearing age against the background of treatment. There was a statistically significant improvement in some components of androgen deficiency, including physical, from 10,51 (3,75) to 8,6 (2,83) points (p<0,001), somatic, from 10,28 (2,44) to 7,54 (2,16) and sexual, from 10,23 (2,79) to 6,78 (1,84) points (Table 5).
The results of a follow-up survey of women who did not receive therapy (n=240, including women without androgen deficiency and with androgen deficiency) are presented in Table 6. Due to the fact that these patients did not receive any therapy to correct androgen deficiency, the absence of statistically significant differences in the dynamics is logical.
In general, based on the study findings, we can conclude that the FAD questionnaire is sensitive to changes in women's condition and can be used for practical purposes as an additional criterion for the effectiveness of treatment.
Discussion
As a result of our study, we developed and validated the FAD questionnaire for assessing androgen deficiency in women to detect manifestations of androgen deficiency affecting psychoemotional background and sexual functioning in women of reproductive age with infertility and DOR. The comprehensibility of the wording and ease of answering the question by the patients was shown.
The key psychometric characteristics investigated when testing the FAD questionnaire to assess the suitability of its use in clinical practice were its reliability, i.e., its ability to accurately assess the manifestations of androgen deficits, and the instrument's ability to reflect clinical differences in patients' condition (discriminant and criterion validity) and sensitivity to laboratory methods of androgen profile assessment. An important result of the study is the demonstrated stability of the instrument's structure, which indicates satisfactory construct validity and substantially determines the acceptability of its use for the assessment of androgen deficiency manifestations. The results of the assessment of the internal consistency of the instrument indicate the reliability of the FAD questionnaire. The obtained value of the Cronbach's α coefficient (0.83) characterizes a high internal constancy of its Russian version, i.e., a high accuracy of the results.
The tissues expressing AR are the most sensitive to changes in androgen concentration, including the nervous system, which is responsible for clinical manifestations of androgen deficiency, including general well-being deterioration, cognitive function, asthenia, sub-depressive and depressive mood, feeling of fatigue and lower motivation, hair loss and muscle activity decrease [17] and disorders of female sexual functioning disorders.
An age-related decrease in androgen levels makes a significant and possibly key contribution to changes in psychoemotional and sexual functioning, which is explained by a decrease in androgen synthesis, and consequently in estrogen synthesis. It is also necessary to take into account the probable decrease in the concentration of sex receptors as a factor of narrowing the window of therapeutic possibilities.
Taking into account the significance of androgen deficiency manifestations in the overall well-being of a woman, it is necessary to include this syndrome in the differential diagnosis of pathological conditions even in women of reproductive age with preserved menstrual cycle rhythm.
Conclusion
Considering the results of our study it is possible to conclude that the validated androgen deficiency questionnaire for women in accordance with international and national standards is a reliable, valid, and sensitive instrument for assessing early manifestations of androgen deficiency in women with infertility and DOR and allows evaluation of the effectiveness of therapy with androgen-containing drugs, which can be used in clinical practice and in scientific research in reproductive medicine.


References
- Bachmann G., Bancroft J., Braunstein G., Burger H., Davis S., Dennerstein L. et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil. Steril. 2002; 77(4):660-5. https://dx.doi.org/10.1016/s0015-0282(02)02969-2.
- Butler L., Santoro N. The reproductive endocrinology of the menopausal transition. Steroids. 2011; 76(7): 627-35. https://dx.doi.org/10.1016/j.steroids.2011.02.026.
- Davison S.L., Bell R. Androgen physiology. Semin. Reprod. Med. 2006; 24(2): 71-7. https://dx.doi.org/10.1055/s-2006-939565.
- Dennerstein L., Randolph J., Taffe J., Dudley E., Burger H. Hormones, mood, sexuality, and the menopausal transition. Fertil. Steril. 2002; 77(Suppl. 4):S42-8. https://dx.doi.org/10.1016/s0015-0282(02)03001-7.
- Roney J.R., Simmons Z.L. Hormonal predictors of sexual motivation in natural menstrual cycles. Horm. Behav. 2013; 63(4): 636-45.https://dx.doi.org/10.1016/j.yhbeh.2013.02.013.
- Bramen J.E., Hranilovich J.A., Dahl R.E., Chen J., Rosso C., Forbes E.E. et al. Sex matters during adolescence: testosterone-related cortical thickness maturation differs between boys and girls. PLoS One. 2012; 7(3): e33850. https://dx.doi.org/10.1371/journal.pone.0033850.
- Cappelletti M., Wallen K. Increasing women's sexual desire: the comparative effectiveness of estrogens and androgens. Horm. Behav. 2016; 78: 178-93. https://dx.doi.org/10.1016/j.yhbeh.2015.11.003.
- Nastri C., Lara L.A., Ferriani R.A., Rosa-E-Silva A.C., Figueiredo J.B., Martins W.P. Hormone therapy for sexual function in perimenopausal and postmenopausal women. Cochrane Database Syst. Rev. 2013; (6): CD009672. https://dx.doi.org/10.1002/14651858.CD009672.pub2.
- Davis S.R., Goldstat R., Papalia M.A., Shah S., Kulkarni J., Donath S., Bell R.J. Effects of aromatase inhibition on sexual function and well-being in postmenopausal women treated with testosterone: a randomized, placebo-controlled trial. Menopause. 2006; 13(1): 37-45. https://dx.doi.org/10.1097/01.gme.0000168061.32917.83.
- Graziottin A. The biological basis of female sexuality. Int. Clin. Psychopharmacol. 1998; 13(Suppl. 6): S15-22. https://dx.doi.org/10.1097/00004850-199807006-00004.
- Heinemann L.A., Saad F., Zimmermann T., Novak A., Myon E., Badia X. et al. The Aging Males' Symptoms (AMS) scale: update and compilation of international versions. Health Qual. Life Outcomes. 2003; 1: 15.https://dx.doi.org/10.1186/1477-7525-1-15.
- Heinemann L.A.J., Zimmermann T., Vermeulen A., Thiel C. A New ‘Aging Male’s Symptoms’ (AMS) rating scale. Aging Male. 1999; 2(2): 105-14.http://dx.doi.org/10.3109/13685539909003173.
- Davis S.R. The Kupperman Index undressed. Maturitas. 2019; 26: 90-1.https://dx.doi.org/10.1016/j.maturitas.2019.04.219.
- Rosen R., Brown C., Heiman J., Leiblum S., Meston C., Shabsigh R. et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J. Sex MaritalTher. 2000; 26(2): 191-208. https://dx.doi.org/10.1080/009262300278597.
- Стеняева Н.Н., Хритинин Д.Ф., Чаусов А.А. Гинекологические заболевания как предикторы женской сексуальной дисфункции. Гинекология. 2021; 23(2): 149-54. https://dx.doi.org/10.26442/20795696.2021.2.200784. [Stenyaeva N.N., Chritinin D.F., Chausov A.A. Gynecological diseases as predictors of female sexual dysfunction. Gynecology. 2021; 23(2): 149-54.(in Russian)]. https://dx.doi.org/10.26442/20795696.2021.2.200784.
- Heinemann L.A., Thiel C., Assmann A., Zimmermann T., Hummel W., Vermeulen A. Sex differences in 'climacteric symptoms' with increasing age?A hypothesis-generating analysis of cross-sectional population surveys.Aging Male. 2000; 3(3): 124-31. https://dx.doi.org/10.1080/13685530008500334.
- Poletti A., Martini L. Androgen-activating enzymes in the central nervous system. J. Steroid Biochem. Mol. Biol. 1999; 69(1-6): 117-22.https://dx.doi.org/10.1016/s0960-0760(98)00150-2.
Received 26.10.2022
Accepted 16.11.2022
About the Authors
Andrey A. Chausov, Head of the Information and Analytical Center of the Department of Regional Cooperation and Integration, V.I. Kulakov NMRC for OG&P,Ministry of Health of Russia, a_chausov@oparina4.ru, https://orcid.org/0000-0002-3094-7209, 117997, Russia, Moscow, Oparina str., 4.
Alla A. Gavisova, PhD, Senior Researcher at the 1st Gynecological Department, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(916)829-05-90,
gavialla@yandex.ru, 117997, Russia, Moscow, Akademika Oparina str., 4.
Nataliya V. Dolgushina, Dr. Med. Sci., Professor, Deputy Director – Head of the Department for Scientific Projects Administration, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia, n_dolgushina@oparina4.ru, https://orcid.org/0000-0003-1116-138X, 117997, Russia, Moscow, Academician Oparin str., 4.
Tatiana A. Nazarenko, Dr. Med. Sci., Professor, Director of Institute of Reproductive Medicine, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
+7(495)531-44-44, t_nazarenko@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Zhanna R. Gardanova, Dr. Med. Sc., Professor, Senior Researcher, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia; Head of the Department of Psychotherapy, N.I. Pirogov RNRMU, Ministry of Health of Russia, +7(926)538-00-83, zanna7777@inbox.ru, https://orcid.org/0000-0002-9796-0846,
117997, Russia, Moscow, Ac. Oparina str., 4.
Authors' contributions: Chausov A.A. – conception and design of the study, statistical analysis; Gavisova A.A. – review of the relevant literature, material collection, manuscript drafting, final approval of the manuscript; Dolgushina N.V. – conception and design of the study, editing the article; Nazarenko T.A., Gardanova Zh.R. – editing the article.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P (Ref. No: 2 of February 07, 2019).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Chausov A.A., Gavisova A.A., Dolgushina N.V., Nazarenko T.A., Gardanova Zh.R. Development and validation of a questionnaire for assessing androgen deficiency in women (FAD – Female Androgen Deficiency) of reproductive age.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 11: 75-89 (in Russian)
https://dx.doi.org/10.18565/aig.2022.11.75-89



