Distention of placental intervillous spaces as a marker of thrombophiliaassociated complications of pregnancy
Aim. To investigate the course and outcomes of pregnancy in patients with massive placental structural changes with the distention of intervillous spaces (DIS) based on the correction of hypercoagulability.Chechneva M.A., Budykina T.S., Zakharov S.M., Biryukova N.V., Kulikova O.N., Ovchinnikova V.V.
Materials and methods. The study included 139 pregnant women with confirmed thrombophilia and placental structural changes detected by ultrasound as a massive DIS. During pregnancy, placental thickness and structure and pregnancy outcomes were analyzed depending on the treatment. Group 1 (n=64) did not receive or irregularly used anticoagulant or antiplatelet therapy. Group 2 (n=75) throughout pregnancy received treatment based on laboratory testing results.
Results. The study analyzed the course of pregnancy in patients with thrombophilia and structural changes in the placenta. In patients receiving continuous anticoagulant therapy based on laboratory testing results and achieving normal coagulation parameters, placental thickness decreased, followed by normalization of placental echotexture. Improvement in perinatal outcomes included a 4-fold reduction in missed miscarriage rate, a 3.5-fold decrease in placental abruption rate, and a 1.5-fold reduction in intrauterine growth restriction rate; the full-term delivery rate increased four-fold.
Conclusion. Placental echotexture assessment can be used as an additional diagnostic test for thrombophilia and hypercoagulability and a criterion for treatment effectiveness.
Keywords
Women with hypercoagulable states, including thrombophilia, have an increased risk of pregnancy complications, leading to preterm births, perinatal complications, and pregnancy losses.
Inherited thrombophilia is associated with an increased risk of venous thromboembolism (VTE) and poor pregnancy outcomes. However, there is limited evidence to guide screening for and management of these conditions in pregnancy. Screening all women for thrombophilias is not recommended given a low absolute risk of thromboembolism even with a thrombophilia [1, 2].
Adverse pregnancy outcomes include early recurrent spontaneous miscarriage and late thrombophilia-associated complications such as antenatal fetal death, preeclampsia, placental abruption, and intrauterine growth restriction. Simcox L.E. et al. reported a lack of strong evidence associated with adverse pregnancy outcomes and thrombophilia in pregnancy [3].
There continues to be a debate in the literature on whether to screen pregnant women for inherited thrombophilia, which would lead to significant material costs or test specific populations based on individualized risk assessment.
Thrombophilia testing is recommended in patients with a history of venous thrombosis or thrombosis episodes in female relatives [4]. According to Dłuski D. et al., thrombophilia testing is indicated before initiating anticoagulant prophylaxis in women who have a history of adverse pregnancy outcomes and consider pregnancy [5].
However, the need for anticoagulant therapy in pregnant women with thrombophilia to improve outcomes and prevent pregnancy complications is also a controversial and unresolved issue [6].
A 2016 meta-analysis of randomized controlled trials comparing the use of low molecular weight heparin (LMWH) in women with inherited thrombophilia and prior miscarriage showed no significant difference in live birth rates with the use of LMWH compared with no LMWH (relative risk, 0.81; 95% CI 0.55–1.19; P = 0.28) [7].
The 2019 review explored the evidence of the effect of inherited thrombophilia on recurrent miscarriage and other pregnancy complications, and whether antithrombotic treatment would modify pregnancy outcomes in women with inherited thrombophilia. The main conclusion is that inherited thrombophilia testing is not required for women with recurrent pregnancy losses or late pregnancy complications [8].
Early diagnosis and timely correction of hypercoagulable state could prevent pregnancy complications, including those leading to unfavorable perinatal outcomes. Currently, the clinical guidelines for thrombophilia recommend diagnostic measures in several specific situations. Patients with a history of VTE episodes associated with inherited or acquired high-risk thrombophilia are at a very high risk of subsequent VTE [9, 10].
In other clinical situations, blood coagulation measurements are not recommended. To conduct expensive testing, separate indications are needed, which may not be identified even with careful clinical observation of a pregnant woman within the existing protocol framework. In a previously conducted retrospective study, we showed a correlation between structural changes in the placenta with the detection rate of inherited thrombophilia and hypercoagulability and the rates of past adverse perinatal outcomes. It was proposed to consider massive structural changes in the placenta in the form of distention of intervillous spaces (DIS) as a marker of hypercoagulability in the index pregnancy [11]. The second part of the study is a prospective analysis of clinical observations of pregnant women who had massive DISs of the placenta detected by ultrasound examination.
The present study aimed to investigate the course and outcomes of pregnancy in patients with massive placental structural changes with the distention of intervillous spaces (DIS) based on hypercoagulability correction.
Materials and methods
The study included 139 pregnant women who had confirmed thrombophilia gene polymorphism. At the first antenatal appointment, they had no hypercoagulability but had placental structural changes detected by ultrasound as massive DISs. Patients of group 1 (n=64) either did not receive anticoagulant therapy or received it in short empirical courses without careful laboratory control. Patients in group 2 (n=75) after the first visit were examined for thrombophilia gene polymorphism, received anticoagulant or antiplatelet therapy according to a hematologist's recommendations throughout pregnancy based on laboratory testing results until normal coagulation was achieved. Inclusion criteria were massive placental structural changes detected at the first visit by ultrasound as DISs. Massive distentions of intervillous spaces were visualized as hypoechoic heterogeneous zones of irregular or conditionally triangular shape with indistinct outer contours located randomly or between the cotyledons of the placenta, in some cases with a detectable blood flow in the spaces (the total size of the areas of expansion of the MEP exceeded 1/3 of the sectional placenta area). Rounded anechoic zones in the center of cotyledons were not considered as DISs. Retrochorial and retroamnial hematomas were considered as complications of pregnancy. All participants provided signed informed consent to take part in the study.
Exclusion criteria were other structural changes in the placenta, gestational age >38 weeks (not suitable for laboratory testing and correction of the condition).
Research methods included 1) medical history, analysis of previous pregnancy complications, and the course of the index pregnancy until the consultation at the institute; 2) Ultrasound fetometry with percentile assessment of the data obtained. The WHO percentile values of the INTERGROWTH-21st Fetal Growth Standards were used. Fetal growth assessment and diagnosis of growth restriction were carried out according to international DELFI standards. Ultrasound examinations of the placenta were performed using a Samsung WS 80A or Voluson E 10 machine.
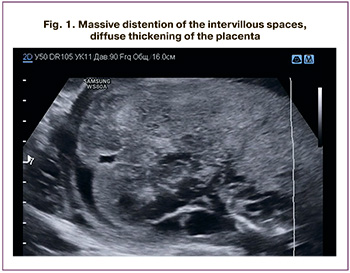 Ultrasound placentometry included measuring the thickness in the projection of the umbilical cord root (if possible). In cases with uneven placental thickness, extreme (maximum) values were measured. If technical capabilities were available, the placenta's linear dimensions were determined in two mutually perpendicular sections. In the case of massive DISs, the ultrasound report included a note for the attending physician «Structural changes are consistent with thrombophilia», and laboratory testing was recommended (Fig. 1).
Ultrasound placentometry included measuring the thickness in the projection of the umbilical cord root (if possible). In cases with uneven placental thickness, extreme (maximum) values were measured. If technical capabilities were available, the placenta's linear dimensions were determined in two mutually perpendicular sections. In the case of massive DISs, the ultrasound report included a note for the attending physician «Structural changes are consistent with thrombophilia», and laboratory testing was recommended (Fig. 1).
Blood coagulation testing, including activated partial thromboplastin time, prothrombin time, thrombin time, antithrombin III, fibrinogen, was performed on a Sysmex CS-2000i automated coagulometer.
To assess the pathogenetic role of thrombophilia, the patients underwent molecular genetic typing for the following polymorphisms: F5 169 G>A, F2:20210 G>A, MTHFR:677C>T, PAI1 SERPINE1 PAI-1): -675 5G>4G. DNA was isolated from whole blood using DiatomTM DNA Prep 200 kits. Production was carried out with DNA-Technology kits in real-time with a DT Prime 5 amplifier from DNA-Technology.
Statistical analysis
All results were entered into MS Excel 2016 spreadsheets. These data included age, parity, gestational age at the time of the examination, medical history, general clinical examination, and ultrasound findings, including fetometry and placentometry. Coagulation tests results were coded as hypercoagulation «1» and normocoagulation «0». Statistical analysis was based on nonparametric mathematical statistics methods with the calculation of the median and quartile for measurements of the placental thickness. Based on the normality of the data, continuous variables were evaluated with a Student’s t or Mann–Whitney U-test for independent variables and Wilcoxon's test two paired groups. Quantitative variables showing normal distribution were expressed as means (M), standard error of the mean (m), and standard deviation (SD). Continuous variables were compared with Student’s t-test.
Results
Patients in group 1 (n=64) underwent a total of 117 ultrasound examinations. The number of ultrasound examinations during the observation ranged from 1 to 7. The mean age of pregnant women was 33.4 (5.8) years. Eighteen women were primigravida, of which 10 had IVF pregnancies.
Patients in group 2 (n=75) underwent a total of 161 ultrasound examinations. The number of ultrasound examinations during the observation ranged from 2 to 5. The mean age of pregnant women was 31.6 (6.1) years. Twenty-three women were primigravida, of which 12 had IVF pregnancies.
There was a statistically significant difference in the past number of venous thrombosis; no other differences were found (Table 1).
According to the gynecological anamnesis, significant differences were only in primary infertility rates (Table 2). The obstetric history deserves special attention.
Characteristics of the obstetric history in group 1
Forty-six women were multigravida, of which 31 (67.4%) had a second pregnancy. A history of placental abruption, chorionangioma, intrauterine growth restriction, missed miscarriage, antenatal fetal death, and intrapartum fetal death were reported in 2 (4.3%), 1 (2.2%), 4 (8.6%), 4 (8.6%), 2 (4.3%), and 1 (2.2%) cases, respectively. In 48% of cases, there was a complicated obstetric history, and 52% of women had previous pregnancy ended with the birth of a healthy baby.
Twelve women had a third pregnancy. A history of antenatal fetal death, intrapartum fetal death, preterm operative delivery due to severe preeclampsia, and missed miscarriage were reported in 2 (16.7%), 1 (8.3%), 6 (50%), and 6 (50%) cases, respectively. Out of 24 pregnancies, only ten ended in childbirth, and only in 4 patients, there was full-term childbirth with a healthy baby (16.6%).
One patient had the fourth pregnancy (two missed miscarriages and one preterm birth). One patient had the fifth pregnancy (two missed miscarriages and two preterm births), and the patient had the seventh pregnancy (4 missed miscarriages and two antenatal fetal deaths at 27 and 32 weeks).
Out of 68 multigravida women in group 1, in 34 (50%) pregnancy resulted in a live birth, and 34 (50%) had an unfavorable outcome.
Gestational age at the time of seeking medical care at the MRRIOG ranged from 5 to 38 weeks, including 10 (15.6%), 5 (7.8%), 38 (59.4%), and 11 (17.2%) women in the first trimester, at 11–14 weeks, at 15 to 26 weeks, and in the III trimester, respectively. Six (9.4%) patients were not examined for gene polymorphism for thrombophilia, and 58 (90.6%) were examined. Thrombophilia was not detected in 2 (3.1%) patients with gestational diabetes. In 96.9%, various hemostasis gene polymorphism variants were identified, which are presented in Table 3.
Two (3.1%) patients from the group refused treatment for religious reasons. One patient, according to the attending physician, did not require therapy. Fifty-three (82.8%) pregnant women received short-course therapy with periodic discontinuation of anticoagulants.
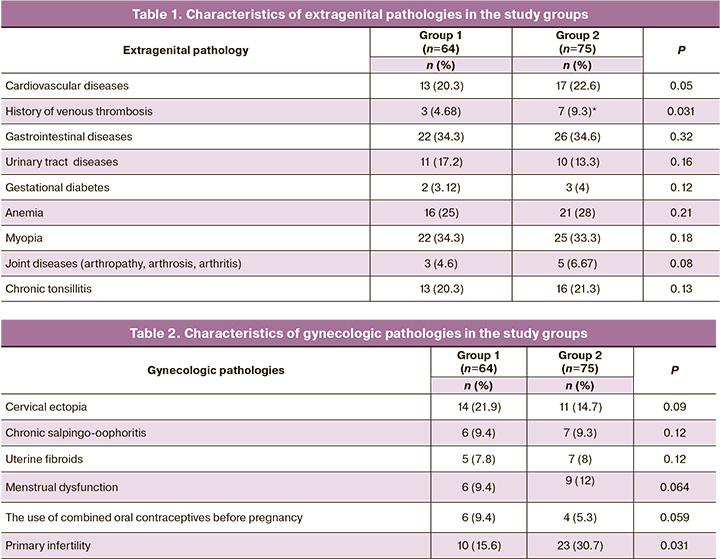
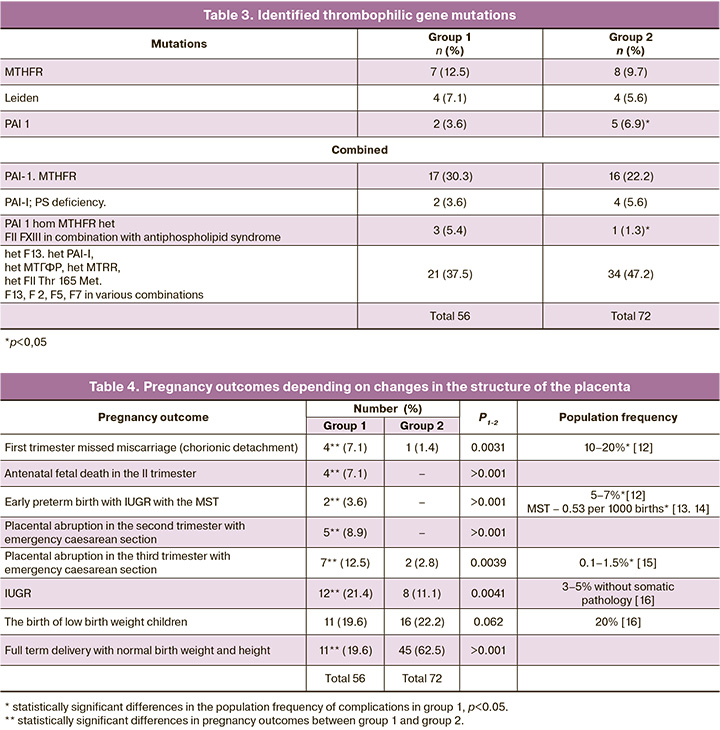
Among patients who did not receive therapy or received irregular or intermittent treatment without proper laboratory control, 5 (9.4%) and 3 (5.6%) patients were found to have early aging of the placenta and placental hypoplasia, respectively. Repeat examinations in this group of patients did not reveal normalization changes in the placental structure (Fig. 2, 3). In 2 (3.6%) cases, the progression was observed with the formation of massive subchorial thrombosis (MSCT), which caused premature placental abruption and preterm birth (at 28 weeks with a birth weight of 640 g and 26 weeks with a birth weight of 820 g).
Pregnancy outcomes in patients of group 1 are presented in Table 4.
Characteristics of the obstetric history in group 2
Group 2 included 75 pregnant women, including 52 (69.33%) multigravida women, of which 46 (61.3%) and 6 (8%) patients had the second and the third pregnancy. Among 52 multigravida women, 32 (61.5%) pregnant women had a complicated obstetric history including missed miscarriage [19 (36.5%)], placental abruption in the second trimester of pregnancy with antenatal fetal death [4 (7.6%)], placental abruption in the third trimester of pregnancy, requiring an emergency cesarean section [4 (7.6%)], and giving birth to a child with IUGR [5 (9.6%)].
Therefore, out of 58 pregnancies in this subgroup, only 50% achieved an uncomplicated, full-term delivery. In the first trimester, structural changes in the chorion were detected in 8 (10.7%) patients, 54 (72%), and 13 (17.3%) patients presented for the first time in the II and the III trimester, respectively. Among patients presenting in the first trimester at 5 - 14 weeks, 7 (87.5%) out of 8 had threatened miscarriage in the form of the detachment of the ovum with the formation of retrochorial hematoma.
During baseline examination at 15 to 26 weeks, all pregnant women showed diffuse placental thickening measuring a maximum of 46 mm, massive structural changes in the form of DIS. In 4 (7.4%) cases, placental abruption with the formation of retroplacental hematomas was found. Two (3.7%) and 11 (20.3%) patients had oligohydramnios and early fetal IUGR, respectively. Among eight patients presenting for the first time in the third trimester with a characteristic structure of the placenta, 3 (37.3%) and 2 (25%) pregnant women were diagnosed with gestational diabetes and IUGR.
Of the 75 patients in group 2, 72 (96%) were examined for thrombophilia gene polymorphism. The results are shown in Table 3.
As shown from the table, a statistically significant difference was noted only in the detection rate of antiphospholipid syndrome in patients of group 1 and PAI 1 in patients in group 2. In our earlier study, there was a correlation (r=0.63) between the detection of DIS and the diagnosis of thrombophilia and between changes in the placenta and the form of mutation (r= 0.18), that is, a very weak positive correlation [11].
In 72 (96%) patients with thrombophilia, standard coagulation test results suggested hypercoagulability, which served as an indication for the administration of anticoagulant or antiplatelet therapy under dynamic laboratory and ultrasound control.
During dynamic observation against the therapy background, no changes were noted in the placental structure in 10 (13.9%) patients. An increase in the placental thickness and an increase in the DIS area were reported in 12 (16.7%) patients. A decrease in the structure's thickness and normalization was observed in 50 (69.4%) pregnant women (Fig. 3).
The normalization of the placenta structure appears to reflect the normalization of intraplacental blood flow, which leads to a significant reduction in adverse obstetric outcomes. Comparative data are presented in Table 4.
Conclusion
Placental echotexture can be used as an additional diagnostic test to identify pregnant women at risk of severe pregnancy complications. However, it cannot be a criterion for making a clinical diagnosis. Drobinskaya A.N. and co-authors presented morphological changes in the placenta in thrombophilia as foci of fibrinoid deposition in the intervillous space with bricking of villi, pseudo-infarctions, blood clots, hemorrhages in the decidual basal plate; an increase in the density of intervillous fibrinoid; a decrease in the thickness of terminal villi; a reduction in the density of the basal plate [17]. Logic dictates that adequate anticoagulant therapy can lead to changes in the structure of DISs, improvement of intra-placental hemodynamics. These processes are reflected in the placenta's ultrasound image in the form of a decrease in the thickness and normalization of the structure, which is demonstrated by the findings of 72 patients of group 2. In turn, the normalization of placental function can reduce the number of adverse perinatal outcomes associated with hypercoagulability. Undoubtedly, further research is needed, but even now, the normalization of the placental echotexture may be proposed as one of the criteria for anticoagulant therapy's sufficiency.
References
1. Croles F.N., Nasserinejad K., Duvekot J.J., Kruip M.J., Meijer K., Leebeek F.W. Pregnancy, thrombophilia, and the risk of a first venous thrombosis: systematic review and bayesian meta-analysis. BMJ. 2017; 359: j4452. https://dx.doi. org/10.1136/bmj.j4452.
2. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. ACOG Practice Bulletin No. 197: Inherited T hrombophilias in Pregnancy. Obstet. Gynecol. 2018; 132(1): e18-34. https:// dx.doi.org/10.1097/AOG.0000000000002703.
3. Simcox L.E., Ormesher L., Tower C., Greer I.A. Thrombophilia and preg nancy complications. Int. J. Mol. Sci. 2015; 16(12): 28418-28. https:// dx.doi.org/10.3390/ijms161226104.
4. Stevens S.M., Woller S.C., Bauer K.A., Kasthuri R., Cushman M., Streiff M. et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J. Thromb. Thrombolysis. 2016; 41(1): 154-64. https:// dx.doi.org/10.1007/s11239-015-1316-1.
5. Dłuski D., Mierzyński R., Poniedziałek-Czajkowska E., Leszczyńska-Gorzelak B. Adverse pregnancy outcomes and inherited thrombophilia. J. Perinat. Med. 2018; 46(4): 411-7. https://dx.doi.org/10.1515/jpm-2017-0059.
6. Middeldorp S. Inherited thrombophilia: a double-edged sword. Hematology Am. Soc. Hematol. Educ. Program. 2016; 2016(1): 1-9. https://dx.doi.org/ 10.1182/ asheducation-2016.1.1.
7. Skeith L., Carrier M., Kaaja R., Martinelli .I, Petroff D., Schleußner E. et al. A meta-analysis of low-molecular-weight heparin to prevent pregnancy loss in women with inherited thrombophilia. Blood. 2016; 127(13): 1650-5. https:// dx.doi.org/10.1182/blood-2015-12-626739.
8. Arachchillage D.R.J., Makris M. Inherited thrombophilia and pregnancy complications: Should we test? Semin. Thromb. Hemost. 2019; 45(1): 50-60. https://dx.doi.org/10.1055/s-0038-1657782.
9. Сухих Г.Т., Филиппов О.С., Белокриницкая Т.Е., Бицадзе В.О., Гурьянов В.А., Долгушина Н.В., Калинина Б.А., Кан Н.Е., Кирющенков П.А., Кириенко А.И., Корнеева И.Е., Леваков С.А., Леонтьев С.Г., М акацария А.Д., Павлович С.В., Пырегов А.В., Рунихина Н.К., Тютюнник В.Л., Федорова Т.А., Ходжаева З.С., Шмаков Р.Г., Явелов И.С. Профилактика венозных тромбоэмболических осложнений в акушерстве и гинекологии. Клинические рекомендации. Протокол. М.; 2014. [Sukhih G.T., Filipov O.S. et al. Prevention of venous thromboembolic complications in obstetrics and gynecology. Clinical recommendations. Protocol. Moscow; 2014. (in Russian)].
10. Момот А.П., Николаева М.Г., Сердюк Г.В., Елыкомов В.А., Мамаев А.Н., Романов В.В., Фадеева Н.И., Кудинова И.Ю., Белозеров Д.Е., Трухина Д.А., Максимова Н.В., Вахлова Ж.И. Оценка состояния системы гемостаза при физиологически протекающей беременности. Методические рекомендации (проект). Российский вестник акушера-гинеколога. 2018; 18(3, вып. 2). [Momot A.P., Nikolaeva M.G., Serdiuk G.V., Elykomov V.A. et al. Evaluation of haemosthasis system in physiological pregnancy. Methodological recommendations (project). Russian Bulletin of obstetrician-gynecologist. 2018;№3; 2. (in Russian)].
11. Чечнева М.А., Будыкина Т.С., Бирюкова Н.В., Захаров С.М., Торшина З.В., Овчинникова В.В. Эхоструктура плаценты как маркер гиперкоагуляции. Российский вестник акушера-гинеколога. 2020; 20(3): 78-84. [Chechneva M.A., Budykina T.S., Biryukova N.V., Zakharov S.M., Torshina Z.V., Ovchinnikova V.V. Echostructure of the placenta as a marker of hypercoagulation. Russian Bulletin of obstetrician-gynecologist. 2020; 20(2) (in Russian)].
12. Сухих Г.Т., Серов В.Н., Адамян Л.В., Филиппов О.С., Баев О.Р., Клименченко Н.И., Тетруашвили Н.К., Тютюнник В.Л., Ходжаева З.С., Холин А.М. Преждевременные роды – клинические рекомендации (протокол). М.; 2014. [Premature birth – clinical recommendations (a protocol) (in Russian)].
13. Баринова И.В., Кондриков Н.И. Массивный субхориальный тромбоз. Архив патологии. 2012; 74(6): 55-8. [Barinova I.V., Kondrikov N.I. Massive subchorial thrombosis. Archives of pathology. 2012; 6: 57-60. (in Russian)]
14. Петрухин В.А., Реброва Т.В., Чечнева М.А., Баринова И.В., Мельников А.П., Малова А.Н. Массивный субхориальный тромбоз. Российский вестник акушера-гинеколога. 2015; 15(4): 49-54. https://dx.doi.org/10.17116/ rosakush201515449-54. [Petrukhin V.A., Rebrova T.V., Chechneva M.A., Barinova I.V., Melnikov A.P., Malova A.N. Massive subchorial thrombosis. Russian Bulletin of obstetrician-gynecologist. 2015; 15(4): 49-54 (in Russian)]. https://dx.doi.org/10.17116/rosakush201515449-54.
15. Жаркин Н.А., Лавенюкова Е.М., Мирошников А.Е. Преждевременная отслойка нормально расположенной плаценты. Эпидемиология, факторы риска, прогнозирование, исходы. Российский вестник акушера-гинеколога. 2018; 18(3): 20-4. [Zharkin N.A., Lavenyukova E.M., Miroshnikov A.E. Premature detachment of a normally located placenta. Epidemiology, risk factors, prognosis, outcomes. Russian Bulletin of obstetrician-gynecologist. 2018; 18(3): 20-24 (in Russian)]. https://dx.doi.org/10.17116/rosakush201818320-24.
16. Levine T.A., Grunau R.E., McAuliffe F.M., Pinnamaneni R., Foran A., Alderdice F.A. Early childhood neurodevelopment after intrauterine growth restriction: a systematic review. Pediatrics. 2015; 135(1): 126-41. https:// dx.doi.org/10.1542/peds.2014–1143.
Received 12.05.2020
Accepted 08.06.2020
About the Authors
Marina A. Chechneva, Dr. Med. Sci., Head of the Department of Ultrasound Diagnostics, MRRIOG.Tel.: +7(926)600-39-66. E-mail: marina-chechneva@yandex.ru. ORCID: 0000-0001-8117-9054. 101000, Russia, Moscow, Pokrovka str., 22a.
Tat’yana S. Budykina, Dr. Med. Sci., Head of Clinical Diagnostic Laboratory, MRRIOG.
Tel.: +7(916)116-13-55. E-mail: budyt@mail.ru. ORCID: 0000-0001-9873-2354. 101000, Russia, Moscow, Pokrovka str., 22a.
Saveliy M. Zakharov, Ph.D. Student, MRRIOG. Tel.: +7(926)707-78-06. E-mail: crazyfilin@yandex.ru. ORCID: 0000-0001-8117-9054. 101000, Russia, Moscow, Pokrovka str., 22a.
Vladlena V. Ovchinnikova, Ph.D., Researcher at the MRRIOG. Tel.: +7(916)732-99-45. E-mail: vlada.ov777@mail.ru. ORCID: 0000-0002-6611-2510. 101000, Russia, Moscow, Pokrovka str., 22a.
Natalia V. Birukova, Ph.D., Researcher at the Obstetric Observatory Department, MRRIOG.
Tel.: +7(916)949-54-16. E-mail: n-biryukova@bk.ru. ORCID: 0000-0001-9486-3630. 101000, Russia, Moscow, Pokrovka str., 22a.
Olga N. Kulikova, Researcher at the Clinical Diagnostic Laboratory, MRRIOG. ORCID: 0000-0002-8383-3758. 101000, Russia, Moscow, Pokrovka str., 22a.
For citation: Chechneva M.A., Budykina T.S., Zakharov S.M., Biryukova N.V., Kulikova O.N., Ovchinnikova V.V. Distention of placental intervillous spaces as a marker of thrombophilia-associated complications of pregnancy. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2020; 10: 63-70 (in Russian)
https://dx.doi.org/10.18565/aig.2020.10.63-70



