Early predictors of preeclampsia
Objective. To investigate the clinical, anamnestic, and biochemical predictors of preeclampsia.Dubrovina A.V., Mutsalkhanova Yu.S., Vasilyeva V.V.
Subjects and methods. Clinical, anamnestic, and biochemical examinations were performed in pregnant women, who later formed two groups: pregnant women with late-onset moderate preeclampsia and patients without preeclampsia.
Results. The significant clinical markers for late-onset preeclampsia were shown to be increases in body mass index and mean blood pressure at 11-13 weeks’ gestation. The pregnant women with preeclampsia were found to have significantly higher serum levels of retinol-binding protein 4 and disintegrin and metalloproteinase 12. The anamnestic predictors of preeclampsia were ascertained to be hypertension in the history, chronic pyelonephritis, and preeclampsia in previous pregnancies.
Conclusion. A prognostic model based on the co-use of the studied predictors has been developed for the early prediction of late-onset moderate preeclampsia.
Keywords
Despite the achievements of medical science, preeclampsia remains one of the main reasons of maternal and infant morbidity and mortality in the present day obstetrics [1, 2]. However, currently we can observe a certain progress in the research of original mechanisms that may threatena gestation; this is connected with the change of understanding preeclampsia in the initial forming period [3–5]. In the latest works the concept of two clinical phenotypes of preeclampsia depending on the debut of the disease has been formed: the early onset (before 34 weeks) and the late onset (after 34 weeks) [6, 7]. The concept of early or late onset reflects the differences in pathophysiology of preeclampsia. Numerous works have demonstrated differences in the reasons of the disease origin: early preeclampsia is preconditioned by the placentation abnormalities, and the late one is connected, as a rule, with maternal factors [6, 7]. Early preeclampsia occurs more rarely, but has more negative consequences for mother and fetus [8].
Nevertheless, considerable achievements in the study of pathogeneses of the given complication of gestation have not changed the frequency of its occurrence so far. In practice there can be observed the absence of systematic approach to the prenatal care tactics towards women under the threat of preeclampsia with the very limited use of preeclampsia markers, developed in science. Besides, a lot of questions concerning ethiopathogenetic factors of preeclampsia still remain disputable. Thus, the priority task of modern obstetrics remains the search for early predictors of preeclampsia prognosis, which are efficient and available.
The aim of the current study is to define clinical, anamnestic and biochemical predictors of preeclampsia to improve its early prognosis and prevention methods.
Materials and Methods
This was a prospective analysis of clinical data of 25 women with preeclampsia who were chosen out of the total number of women (n = 645). They formed the first main clinical group and were observed during the whole gestation period. The patients taking part in the study suffered from moderately severe preeclampsia (ICD code О14.0), which appeared after 34 weeks of pregnancy (late preeclampsia). The second (control) group included 63 patients without preeclampsia, chosen by random numbers method.
All pregnant women underwent a complex of clinical, anamnestic and instrumental tests during the gestation period. Screening blood sampling was done within the period of 11–13 weeks of pregnancy. The women of the chosen groups underwent enzyme immunoassay to define biochemical markers in the blood serum: placental protein A (PAPP-А), associated with pregnancy, disintegrin and metalloproteinase 12 (ADAM12), retinol-connecting protein 4 (RBP4), b-subunit human chorionic gonadotropin (b-HCG) and cystatin C. The choice of the present array of biochemical parameters was made on the basis of literature data analysis [9–14].
The level of ADAM12 was established by test-systems Cloud-CloneCorp (USA), cystatin С – by test-systems BioVendor (Chech Republic), RBP4 – by test-systems AssayPro (USA), b-HCG and PAPP-А – by test-systems DELFIA PerkinElmer (Finland).
Statistic processing of the data was carried out using programs Statistica, version 12.5, Excel 2010, SPSS 24.002. Within the compared groups median values were assessed with 25 and 75% percentile calculation (1–3 quartile) for getting quantitative indicators. While comparing inter-group differences non-parametric criterion for the independent samples by Craskal-Wallis was used. Fisher’s criterion (φ) was used for comparing the occurrence frequency of the given indices of the two picks. Moreover, non-parametric correlation analysis with Spearmen criterion was applied as well as the “Solution trees” method was used to refer certain objects to one of the preliminarily familiar classes.
Results and Discussion
The following facts have been discovered in the process of analyzing clinical and anamnestic data. While comparing height-weight indices of the chosen groups it has been found that body mass index (BMI) of the patients with preeclampsia within the period of 11–13 weeks of pregnancy is statistically much higher than that of the patients from the control group. Thus, it has been found out that BMI in the early periods of gestation for the patients with preeclampsia was 29 kg/m2, while for the patients from the control group it was 25 kg/m2 (р = 0.0001). One of the most large-scale recent studies [15], with the participation of 159,072 pregnant women, revealed that the risk factor for the preeclampsia development is not only obesity with BMI ≥ 30.0 kg/m2, but also weight of woman with BMI 25.0–29.9 kg/m2. Risk for preeclampsia development within such index is considerably higher than among women with BMI 20.0 kg/m2 [16]. Our study confirmed the role of BMI as an independent factor of preeclampsia development.
 Analysis of anamnestic data revealed that most women under study had the second pregnancy, both in control group and in the group of patients with preeclampsia, 49 (78%) and 20 (80%) women, relatively; р = 0.578. The number of women with the first pregnancy in the chosen groups was also similar, 14 (22%) and 5 (20%) women, respectively; р = 0.688. It is known that preeclampsia in the patient’s history is a prognostic factor of preeclampsia development in case of real pregnancy. In our study 5 (20%) pregnant women from the main group had preeclampsia in their histories, while in the control group there were 2 (3%) women with such anamnesis, p = 0.009. In the history of 4 (16%) patients with preeclampsia there was information about chronic endometritis, which is positively more frequent than in the control group – 2 (4%) women, р = 0.0001. Abortion in anamnesis was observed in 7 (28%) patients from the main group and 21 (33%) patients from the control group (р > 0.05). Analysis of occurrence of extra-genital pathology revealed considerable differences in chronic pyelonephritis, 6 (24%) and 8 (13%) women, relatively; р = 0.010) and arterial hypertension in anamnesis of 10 (40%) and 8 (13%) patients, relatively; p = 0.0001). According to the literature data [6, 17], there is a higher risk for preeclampsia during pregnancy for patients with arterial hypertension in anamnesis. The results of our study confirm the data of S. Iacobelli and co-authors, who demonstrated that aggravated somatic anamnesis including both arterial hypertension and kidney pathology is a risk factor for preeclampsia development. [17].
Analysis of anamnestic data revealed that most women under study had the second pregnancy, both in control group and in the group of patients with preeclampsia, 49 (78%) and 20 (80%) women, relatively; р = 0.578. The number of women with the first pregnancy in the chosen groups was also similar, 14 (22%) and 5 (20%) women, respectively; р = 0.688. It is known that preeclampsia in the patient’s history is a prognostic factor of preeclampsia development in case of real pregnancy. In our study 5 (20%) pregnant women from the main group had preeclampsia in their histories, while in the control group there were 2 (3%) women with such anamnesis, p = 0.009. In the history of 4 (16%) patients with preeclampsia there was information about chronic endometritis, which is positively more frequent than in the control group – 2 (4%) women, р = 0.0001. Abortion in anamnesis was observed in 7 (28%) patients from the main group and 21 (33%) patients from the control group (р > 0.05). Analysis of occurrence of extra-genital pathology revealed considerable differences in chronic pyelonephritis, 6 (24%) and 8 (13%) women, relatively; р = 0.010) and arterial hypertension in anamnesis of 10 (40%) and 8 (13%) patients, relatively; p = 0.0001). According to the literature data [6, 17], there is a higher risk for preeclampsia during pregnancy for patients with arterial hypertension in anamnesis. The results of our study confirm the data of S. Iacobelli and co-authors, who demonstrated that aggravated somatic anamnesis including both arterial hypertension and kidney pathology is a risk factor for preeclampsia development. [17].
Next comparison parameter was mean arterial pressure (MAP), which was calculated according to the formula: (2 х DBP + SBP)/3, where DBP – diastolic blood pressure, SPB – systolic blood pressure. Comparative analysis of MAP revealed much higher figures for the pregnant women with preeclampsia. Besides, a strong correlation between a group of signs (MAP, BMI) on the one hand and subgroups with preeclampsia and nominally healthy people on the other hand was revealed (Table 1). Indicators of MAP were considerably higher in patients with preeclampsia (84 [75; 93] mmHg) in comparison with patients from the control group (78 [73; 83] mmHg, р = 0.0082). According to the data, provided by D. Gallo with co-authors [2], based on observation of 17,383 pregnant women, MAP indicators also had high prognostic efficiency concerning preeclampsia.
Our study revealed high frequency of termination of current pregnancy threat for women with preeclampsia in comparison with the patients from the control group, 12 (48%) and 13 (21%), respectively; р = 0.010), and also the increase of frequency of caesarean section for the pregnant with preeclampsia, 14 (56%) and 9 (14%), respectively; р = 0.010). According to the literature data, the threat of pregnancy termination in the first trimester is connected with pathological progress of gestation, since the development of pregnancy initially takes place in the unfavorable condition and is accompanied by the defect of chorion invasion [18].
Thus, analysis of clinicaland anamnestic data allowed us to determine a number of characteristics, which are statistically considerably different in the patients from the chosen clinical groups, namely: presence of preeclampsia, chronic pyelonephritis and arterial hypertension, high indices for BMI in the beginning of pregnancy, MAP indicators in the early stages of pregnancy, complications of current pregnancy, such as pregnancy termination threat. Analysis of the data received allowed us to recommend the indicated factors, which predict development of the late preeclampsia of medium severity, for further use. The advantage of the given screening is the absence of additional temporary and financial expenses for the examination and the simplicity of its performance. With the combination of the offered parameters doctors will be able to estimate the risk for preeclampsia development and make decisions about the necessity of applying additional predictors.
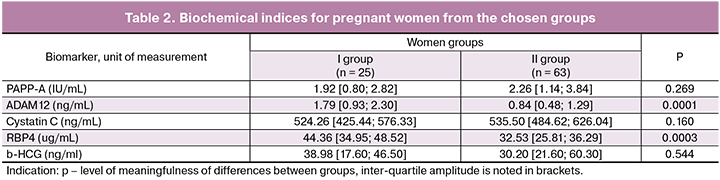
The next task of our work was to identify early biochemical correlators of preeclampsia. Currently, despite the scientists’ efforts, there are not any biochemical markers matching the definition of the World Healthcare Organization for the selection of biomarkers. They could either forecast or make a diagnosis of preeclampsia. We carried out an analysis of concentration of PAPP-A, ADAM12, RBP4, b-HCG and cystatin C in the venous blood of patients from the chosen groups as possible predictors of preeclampsia (Table 2).
One of the possible correlators of preeclampsia has been PAPP-A, used as a biochemical marker for chromosome anomalies. Besides, according to different works, PAPP-A is used for prognosis of unfavorable outcomes of pregnancy, intrauterine growth restriction, premature delivery, stillbirth [9]. Comparative analysis of PAPP-A among pregnant women from the chosen groups revealed absence of substantial differences, 1.92 [0.80; 2.82] IU/mL and 2.26 [1.14; 3.84] IU/mL; р = 0.269. The received results correlate with the data of some authors [10, 11], who deny prognostic value of PAPP-A among patients with late preeclampsia.
Next biomarker of comparison is ADAM12, which participates in breakdown of protein. The protein unites insulin-like growth factor [12]. The examined factor indirectly influences the interaction in the intercellular space between embryo trophoblast and maternal decidual cells while placenta is formed and functions, and, thus, makes its contribution to the mechanism of regulation of the fully functional vascular system of chorion. The level of ADAM12 in women with preeclampsia was considerably higher, 1.79 [0.93; 2.30], (р < 0.001), than in the control group, 0.84 [0.48; 1.29]. The study of E. Matwejew with co-authors revealed the increased levels of ADAM12 among women with preeclampsia in comparison with the control group [13]. The results of our study confirmed the meaningfulness of ADAM12 as possible early marker of the development of late preeclampsia of medium severity. It is known that RBP4 along with PAPP-A participates in the placenta formation, and its alterations and malfunction may be essential for the development of preeclampsia [19]. Besides, the so called adipokines, RBP4 being one of them, play a crucial role in the development of resistance towards insulin, metabolism of lipids, atherosclerosis and system inflammatory response. Identifying RBP4 in our work demonstrated that pregnant women with late preeclampsia of medium severity were characterized by higher level of this parameter, 44.36 [34.95; 48.52] ug/mL in comparison with the women from the control group, 32.53 [25.81; 36.29] ug/mL. Earlier Р. Mendola with co-authors [20] identified the increase of RBP4 level by preeclampsia (р = 0.022). Besides, the authors discovered that the level of RBP4 is considerably higher among pregnant women with severe preeclampsia (р = 0.046). However, in another study [21] the role of RBP4 for early diagnosing of preeclampsia was not proved. The authors connected the received results with the fact that all women in the study had normal BMI. Some studies revealed that placental expression of cystatin C increased by preeclampsia, which lead to the increase of this marker level in blood plasma. Our work demonstrated that the level of cystatin C in the blood of patients with preeclampsia did not differ substantially from the data of the control group (р = 0.160). Similar results with the absence of statistical difference between patients of both groups (р = 0.544) were received for the level of b-HCG. According to the literature data combined screening with the application of maternal factors and serum b-HCG increases the efficiency of identifying early preeclampsia, but not preeclampsia after 37 weeks [22].
Under the design of our study, we made a correlation analysis of each indicator in the group of pregnant women with preeclampsia. Its results identified statistically meaningful direct correlation of interaction between ADAM12 and RBP4 (p = 0.02), and also between cystatin C and RBP 4 (p = 0.04). For women from the control group we found a meaningful correlation between levels of ADAM12 and RBP4 (р = 0.03). Thus, the received results allowed us to suppose that biomarkers ADAM12 and RBP4 are diagnostically important for early predictors of late preeclampsia of medium severity.
In order to identify the most precise and specific indicators we used the “Decisions trees” method (with the division of drawing into educational and verifying) with the “exhaustive CHAID” construction method, which lead to identifying a meaningful correlation between the group of indicators (RBP4, ADAM12) on the one hand and the subgroups with preeclampsia and the nominally healthy women on the other hand. Thus, in case of concentration of RBP4 > 87.90 ug/mL, pregnant women should be referred to the high risk of preeclampsia group. Also, if pregnant women show RBP4 ≤ 87.90 ug/ml and simultaneously ADAM12 > 2.33 ng/ml, they should be referred to the high risk of preeclampsia group as well. The forecast of preeclampsia within the framework of this method is done with the sensitivity of 87.5% and specificity of 85% with the odds ratio 39.667 [3.498; 49.83].
Conclusion
Preeclampsia is a threatening complication of pregnancy which affects 3% of all pregnant women. According to modern national classification, the condition can be divided into early (before 34 weeks) and late (after 34 weeks) preeclampsia. Search for parameters, at the early stages of gestation indicating the development of late preeclampsia and its morbidity, remains a perspective direction of medical science. Analysis of literature data demonstrated that the best predictive meaningfulness belongs to the combination of clinical data and biochemical characteristics. And today the efforts of scientists are aimed at the development and improvement of multi-factor models of preeclampsia forecasting.
Our study demonstrated the possibility of predicting late preeclampsia of medium severity at an early stage, on the basis of combination of maternal factors (BMI and MAP). However, a more precise prediction can be made only after identifying biochemical markers RBP4 and ADAM12. The rise of these markers concentration accompanied by the increase of BMI and MAP within the period of 11–13 weeks gestation indicates the high level of possibility of late preeclampsia development after 34 weeks of medium severity (method with the sensitivity of 87.5% and specificity of 85%). Important anamnestic markers of preeclampsia are arterial hypertension in case history, chronic pyelonephritis and also preeclampsia in previous pregnancies.
Generalization of the received results allowed us to define the following quantitative criteria and practical recommendations. In case of having MAP>98 mmHg and BMI > 30.5 kg/m2 at 11–13 weeks of pregnancy with the notification of preeclampsia in previous pregnancies in anamnesis, chronic arterial hypertension and chronic pyelonephritis, pregnant women can be referred to the high risk group of preeclampsia development after 34 weeks of pregnancy . If the level of RBP4 is identified higher than 87.90 mkg/ml and ADAM12 is higher than 2.33 ng/ml is it advisable to designate the optimal place and date of delivery well in advance (not later than 34 weeks).
References
1. Duhig K.E., Shennan A.H. Recent advances in the diagnosis and management of pre-eclampsia. F1000 Prime Reports. 2015; 7: 24.
2. Gallo D., Poon L.C., Fernandez M., Wright D., Nicolaides K.H. Prediction of preeclampsia by mean arterial pressure at 11-13 and 20-24 weeks’ gestation. Fetal Diagn. Ther. 2014; 36(1): 28-37.
3. Сухих Г.Т., Ванько Л.В. Иммунные факторы в этиологии и патогенезе осложнений беременности. Акушерство и гинекология. 2012; 1: 128-36. [Sukhikh G.T., Vanko L.V. Immune factors in the etiology and pathogenesis of pregnancy complications. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2012; 1: 128-36. (in Russian)]
4. Фомина М.П. 3D-допплерометрия плацентарного кровотока в прогнозировании синдрома задержки роста плода. Журнал акушерства и женских болезней. 2013; 62(2): 160-5. [Fomina M.P. 3D dopplerometry of placental blood flow in predicting fetal growth retardation syndrome. Zhurnal akusherstva i zhenskikh bolezney. 2013; 62(2): 160-5. (in Russian)]
5. Zenclussen A.C. Adaptive immune responses during pregnancy. Am. J. Reprod. Immunol. 2013; 69(4): 291-303.
6. Bakker R., Steegers E.A., Hofman A., Jaddoe V. Blood pressure in different gestational trimesters, fetal growth, and the risk of adverse birth outcomes: the generation R study. Am. J Epidemiol. 2011; 174(7): 797-806.
7. Bateman B.T., Bansil P., Hernandez-Diaz S., Mhyre J.M., Callaghan W.M., Kuklina E.V. Prevalence, trends, and outcomes of chronic hypertension: a nationwide sample of delivery admissions. Am. J. Obstet. Gynecol. 2012;206(2): 134-8.
8. Baschat A.A., Magder L.S., Doyle L.E., Atlas R.O., Jenkins C.B., Bilizer M.G. Prediction of preeclampsia utilizing the first trimester screening examination. Am. J. Obstet. Gynecol. 2014; 211(5): 514. e1-7.
9. Sung K., Roh A.J., Eoh K., Kim E. Maternal serum placental growth factor and pregnancy-associated plasma protein A measured in the first trimester as parameters of subsequent preeclampsia and small-for-gestational-age infants: A prospective observational study. Obstet. Gynecol. Sci. 2017;60(2): 154-62.
10. Giguere Y., Masse ́J., Theriault S., Bujold E., Laford J., Rosseau F. et al. Screening for preeclampsia early in pregnancy: performance of a multivariable model combining clinical characteristics and biochemical markers. BJOG. 2015; 122(3): 402-10.
11. O’Gorman N., Wright D., Poon L., Rolnik D., Syngelaki A., de Alvarado M. et al. Multicenters creening for preeclampsia by maternal factors and biomarkers at 11-13 weeks’ gestation: comparison to NICE guidelines and ACOG recommendations. Ultrasound Obstet. Gynecol. 2017; 49(6): 756-60.
12. Myatt L., Clifton R.G., Roberts J.M., Spong C.Y., Hauth J.C., Varner M.W. et al.; Eunice Kennedy Shriver National Institute of Child Health and Human Development [NICHD] Maternal-Fetal Medicine Units [MFMU] Network. First-trimester prediction of preeclampsia in nulliparous women at low risk. Obstet. Gynecol. 2012; 119(6): 1234-42.
13. Matwejew E., Cowans N.J., Stamatopoulou A., Spencer K., von Kaisenberg C.S. Maternal serum ADAM-12 as a potential marker for different adverse pregnancy outcomes. Fetal Diagn. Ther. 2010; 27(1): 32-9.
14. Wright D., Syngelaki A., Akolekar R., Poon L.C., Nicolaides K.H. Competing risks model in screening for preeclampsia by maternal characteristics and medical history. Am. J. Obstet. Gynecol. 2015; 213(1): 1-10.
15. Zhang J.Z., He J. Risk factors of recurrent preeclampsia and its relation to maternal and offspring outcome. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2015;44(3): 258-63.
16. Itoh H., Kanayama N. Obesity and risk of preeclampsia. Med. J. Obstet. Gynecol. 2014; 2(2): 1024.
17. Iacobelli S., Bonsante F., Robillard P.Y. Comparison of risk factors and perinatal outcomes in early onset and late onset preeclampsia: A cohort based study in Reunion Island. J. Reprod. Immunol. 2017; 123: 12-6.
18. Воднева Д.Н., Шмаков Р.Г., Щеголев А.И. Роль маркеров инвазии трофобласта в развитии преэклампсии и опухолевой прогрессии. Акушерство и гинекология. 2013; 11: 9-12. [Vodneva D.N., Shmakov R.G., Shchegolev A.I. Role of markers for trophoblast invasion in the development of preeclampsia and tumor progression. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2013; 11: 9-12. (in Russian)]
19. Metcalfe A., Langlois S., Macfarlane J., Vallance H., Joseph K.S. Prediction of obstetrical risk using maternal serum markers and clinical risk factors. Prenat. Diagn. 2013; 34(2): 172-9.
20. Mendola P., Ghassabian A., Mills J.L., Zhang C., Tsai M.Y., Liu A., Yeung E.H. Retinol-binding protein 4 and lipids prospectively measured during early to mid-pregnancy in relation to preeclampsia and preterm birth risk. Am. J. Hypertens. 2017; 30(6): 569-76.
21. Al-Kholy A., Abadier M., Rageh E., El-Kallaf H. Serum levels of placental growth factor and retinol-binding protein-4 in pregnancy-induced hypertensive women. J. Am. Sci. 2010; 6(12): 448-55.
22. Bredaki F.E., Mataliotakis M., Wright A., Wright D., Nicolaidse K.H. Maternal serum alpha-fetoprotein at 12, 22 and 32 weeks’ gestation in screening for pre-eclampsia. Ultrasound Obstet. Gynecol. 2016; 47(4): 466-71.
Received 06.02.2018
Accepted 02.03.2018
About the Authors
Dubrovina Svetlana, MD, PhD, Professor, the main scientific researcher, Rostov State Medical University.344012, Russia, Rostov-on-Don, Mechnikova str. 43/38/2. Tel.: +79185585113. E-mail: s.dubrovina@gmail.com
Muzalchanova Ulduz, postgraduate student, Rostov State Medical University.
344012, Russia, Rostov-on-Don, Mechnikova str. 43/38/2. Tel.: +79280500333. E-mail: yulduzka111@mail.ru
Vasil’eva Valentina, PhD, assoc. prof., leading researcher, Rostov State Medical University. 344012, Russia, Rostov-on-Don, Mechnikova str. 43/38/2. Tel.: +79094080159. E-mail: v.vasiljeva1965@mail.ru
For citations: Dubrovina A.V., Mutsalkhanova Yu.S., Vasilyeva V.V. Early predictors of preeclampsia. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; (10): 47-51. (in Russian)
https://dx.doi.org/10.18565/aig.2018.10.47-51



