Manifestations of undifferentiated connective tissue dysplasia and prediction of recurrence after surgical correction of anterior pelvic organ prolapse in women
Marinkin I.O., Rakitin F.A., Volchek A.V., Soluyanov M.Yu., Kuleshov V.M., Makarov K.Yu., Sokolova T.M., Nimaev V.V., Aidagulova S.V.
Pelvic organ prolapse (POP) is a significant global health problem in women.
Objective: To examine the prevalence of clinical and phenotypic manifestations of undifferentiated connective tissue dysplasia (uCTD) in patients with anterior POP and investigate the recurrence rate after surgical treatment to develop a prognostic model for the selection of mesh or native implants.
Materials and methods: A study was conducted involving 460 patients aged 40–82 years who were diagnosed with anterior POP using the Pelvic Organ Prolapse Quantification system (POP-Q ISC, 1996). Patients were examined and underwent vaginal surgery using mesh or native implants in the gynecology department of the Research Institute of Clinical and Experimental Lymphology from 2015 to 2023. The patients were divided into two groups depending on the surgical treatment method for POP. Group 1 included 242 women aged 60 (56; 64) years, who underwent surgery using a mesh implant (transvaginal system cysto-swing, France), while Group 2 included 218 patients aged 66 (62; 68) years, who underwent surgery using native tissues.
Results: Among 328 patients in both groups, clinical manifestations of uCTD of moderate (50.87%) and severe (20.43%) grades prevailed. Pronounced manifestations of uCTD accounted for 71.3%, with no statistically significant differences in uCTD scores between groups 1 and 2. The most significant predictors of POP recurrence in patients with mesh implants, in descending order, were varicose veins (VV) and/or hemorrhoids that required surgical treatment, chronic bronchopulmonary diseases (CBPD), and vegetative-vascular dystonia (VVD). These manifestations of uCTD were also significant in group 2, with additional manifestations including hernias of various localizations, a tendency toward allergic and cold diseases, and an asthenic body type.
Conclusion: The modeling results identified three manifestations of uCTD that are universal predictors of the recurrence of anterior POP: VVD (a minor manifestation of uCTD), CBPD (a major sign of uCTD), and VV (severe manifestations of uCTD). If three additional manifestations of uCTD are identified, namely, an asthenic body type, a tendency toward allergic and cold diseases, and hernias of various localizations, it is advisable to opt for a mesh implant immediately.
Authors' contributions: Marinkin I.O., Aidagulova S.V. – conception and design of the study; Rakitin F.A., Soluyanov M.Yu., Kuleshov V.M., Makarov K.Yu., Sokolova T.M., Nimaev V.V. – collection and processing of primary material; Rakitin F.A., Aidagulova S.V. – literature analysis; Volchek A.V., Rakitin F.A. – statistical analysis; Aidagulova S.V., Marinkin I.O., Kuleshov V.M. – manuscript drafting, and editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Research Institute of Clinical and Experimental Lymphology – Branch of the Institute of Cytology and Genetics, Siberian Branch of Russian Academy of Sciences (Ref. No: 10 from 27.12.2012) and the Research Ethics Committee of the Novosibirsk State Medical University
(Ref. No: 115 from 28.03.2019).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Marinkin I.O., Rakitin F.A., Volchek A.V., Soluyanov M.Yu., Kuleshov V.M., Makarov K.Yu., Sokolova T.M., Nimaev V.V., Aidagulova S.V. Manifestations of undifferentiated connective tissue dysplasia and prediction of recurrence after surgical correction of anterior pelvic organ prolapse in women.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (12): 70-79 (in Russian)
https://dx.doi.org/10.18565/aig.2024.208
Keywords
Pelvic organ prolapse (POP) in women is a global health issue inextricably linked to an increase in life expectancy in economically developed countries. At the same time, POP also affects relatively young women and is associated with a decrease in quality of life and severe psychosomatic disturbances [1, 2]. The etiological factors of POP include age-related menopause, parity, high body mass index (BMI), pelvic surgery, and disorders leading to increased intra-abdominal pressure [3, 4].
The pathogenesis of POP involves both quantitative and qualitative changes in the connective tissue of the pelvic floor structures, particularly highlighting the role of matrix metalloproteinases [5–7]. Changes in structural proteins along with genetically determined deviations in fibrillogenesis in the extracellular matrix are also indicative of the heterogeneous clinical and phenotypic manifestations of undifferentiated connective tissue dysplasia (uCTD). The presence of uCTD in patients can manifest as POP, morphofunctional disorders of the visceral organs and musculoskeletal system, and other pathologies [8–10].
For the rehabilitation of patients with clinically severe POP, surgical methods employing various approaches and the use of synthetic mesh implants or native tissues can significantly enhance quality of life. However, despite their undeniable benefits, POP surgeries are commonly accompanied by side effects and recurrences [11, 12].
Following the introduction of synthetic materials and their increased use in pelvic floor pathology, there has been a decrease in the number of cases of vaginal mesh implantation, both in primary and recurrent POP [13]. In surgeries for anterior POP, one of its most common variants, anterior colporrhaphy with native tissue has traditionally been preferred, with a recurrence risk of approximately 10% [14, 15]. According to other data [16], the rate of recurrence requiring repeat surgery is 38% when POP is corrected through the vaginal approach using native tissues.
Thus, predicting the development of POP and making a scientifically based choice of surgical technique [17, 18], including an "individualized or standardized approach to the choice of surgical technique" [3], remains a relevant consideration. As nearly all women with POP exhibit manifestations of uCTD, evaluating these factors in patients before surgical treatment may help predict the risk of POP recurrence and select the optimal method of surgical correction.
This study aimed to examine the prevalence of clinical and phenotypic manifestations of uCTD in patients with anterior POP and subsequently develop a prognostic model for the selection of mesh or native implants, taking into account recurrence after surgical treatment.
Materials and methods
A study was conducted on 460 patients, aged 40–82 years, diagnosed with anterior pelvic organ prolapse according to the Pelvic Organ Prolapse Quantification System (POP-Q ISC, 1996). The patients underwent vaginal surgery with either mesh or native implants at the Gynecology Department of the RICEL Clinic between 2015 and 2023. All patients were assessed for medical history, reproductive function, concomitant somatic diseases, and the presence and age of menopause.
The inclusion criteria for the study were the presence of POP with a grade III defect of the anterior pelvic floor (POP-Q III (Ba)), requiring surgical correction, menopause, and recurrence of anterior POP with the development of cystocele. The exclusion criteria included active or latent infection of the genitourinary system, pregnancy, acute or chronic diseases in an acute stage, severe somatic diseases, and neoplastic processes.
The patients were divided into two groups based on the surgical treatment method for POP. Group 1 included 242 women aged 60 (56–64) years who underwent surgery with a mesh implant (Transvaginal System Cysto-Swing (France)). Group 2 included 218 patients aged 66 (62–68) years who underwent surgery using native tissue. The choice of surgical method was influenced by the preference for plastic surgery with autologous tissue due to mesh-related complications [13].
The results of the surgical treatment were evaluated through examinations in the early postoperative period and then annually for six years. Remote treatment outcomes were monitored using the POP-Q ISC system. In the remote postoperative evaluation, the absence of objective signs or grade I POP was considered a positive treatment outcome, whereas the development of grade II or higher POP was classified as a negative outcome.
During physical and ultrasound examination of the pelvic organs in patients, we focused on the manifestations of uCTD [8] across three categories: minor signs, major signs, and severe manifestations.
Minor signs included asthenic body type (ABT), skin striae (SS), refractive errors in individuals up to 40 years of age (RE), muscular hypotonia (MH), flattening of the arch of the foot (FAF), a tendency to form external hematomas and bleeding of the mucous membranes (EHB), vegetative-vascular dystonia (VVD), and heart rhythm and conduction disorders (HRC).
Major signs included scoliosis and kyphosis (SK), flat feet of 2–3 degrees (FF), skin elastosis (SE), hypermobility of joints with a tendency toward dislocations (JH), a tendency to allergic reactions and colds (TAC), chronic bronchopulmonary diseases (CBPD), varicose veins and hemorrhoids (VV), biliary dyskinesia (BD), and genital prolapse and hernias in first-degree relatives (PDR).
Severe manifestations of uCTD include hernias at various locations (HVL), mitral valve prolapse (MVP), varicose veins and hemorrhoids requiring surgery (VVHS), habitual joint dislocations (HDD), diverticula and dolichosigma (DD), polyvalent allergy, and anaphylaxis (PAA).
Signs in the first category were assigned 1 point each, those in the second category were assigned 2 points, and those in the third category were assigned 3 points. The severity of uCTD was determined by the total points accumulated: mild uCTD was classified as up to 9 points, moderate as 9–16 points, and severe as >16 points. The surgical treatment outcomes in both groups were evaluated according to the degree of uCTD.
Statistical analysis
Statistical analysis involved testing the normality of continuous variable distributions using the Shapiro–Wilk test and assessing the equality of general variances using the Fisher test. We employed contingency table analysis and nonparametric Kruskal–Wallis rank analysis of variance. Spearman’s rank correlation was used to evaluate the correlation between continuous variables that were not normally distributed.
Logistic regression was used to build a prognostic model for genital prolapse recurrence after plastic surgery using native tissues. In our study, the dependent variable was the recurrence of genital prolapse, which had two possible categorical values: "yes" or "no." Independent variables included the presence or absence of signs of connective tissue disorder (CTD), as well as various medical history, and anthropometric data. For the binary variables, tetrachoric correlations were calculated to exclude interrelationships. Prior to the modeling stage, the recurrence probabilities for each group (minimum prediction accuracy) were calculated. The p-values obtained from Wald tests were used to analyze the significance of the independent variables. Variables with a p-value less than 0.05 were considered statistically significant at a 5% significance level. Before modeling, the entire sample was randomly split into a training sample (400 out of 460, 87% of observations) and a test sample (60 out of 460, 13% of observations). The model was trained on the training sample using the maximum likelihood method, and the gradient descent method was employed to optimize the loss function. Model performance was assessed using accuracy, recall, contingency tables, goodness-of-fit tests, and area under the ROC curve (AUC) metrics. The Henley–McNeil method was used to calculate the AUC. The sample parameters presented in the tables below have the following designations: n is the size of the analyzed subgroup, M is the mean, m is the error of the mean, and p is the achieved significance level. In the absence of a normal distribution, the data are presented as follows: Me is the median, (Q1; Q3) is the interquartile range, and Min–Max denotes the minimum-maximum. The χ² test was used to compare the groups based on qualitative characteristics. Statistical analysis was performed using the MedCalc Statistical Software, version 18.9.1 (http://www.medcalc.org; 2018) and the RStudio package (http://www.rstudio.com/).
Informed consent for examination and treatment of POP was obtained from patients in accordance with European Community Directives (86/609/EEC) and the Helsinki Declaration, in compliance with the "Ethical Principles for Conducting Medical Research Involving Human Subjects" and the "Rules of Clinical Practice in the Russian Federation." The study was reviewed and approved by the Research Ethics Committee of the Research Institute of Clinical and Experimental Lymphology – Branch of the Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Sciences, and Research Ethics Committee of Novosibirsk State Medical University (Ref. No: 10 from 27.12.2012).
Results
A total of 460 patients with grade III anterior POP were randomly divided into two groups that did not differ in uCTD manifestations and parity. However, they differed significantly in age (and, accordingly, in the duration of menopause) and body mass index (BMI) (Table 1). Our study focused on immediate and long-term outcomes to evaluate the efficacy of surgical treatment with mesh and native implants and to develop a personalized approach to choosing the surgical treatment method based on the frequency and nature of clinical and phenotypic manifestations of uCTD in patients with anterior POP.
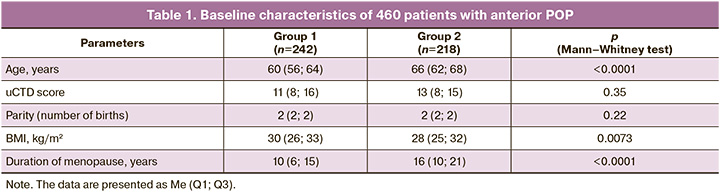
We evaluated the results of two surgical treatment methods for anterior POP in the early and late postoperative periods.
During the first year after surgery, four recurrences (1.7%) were recorded in patients in group 1, compared to 34 recurrences in group 2 (15.6%), which was significantly lower (p<0.0001) when comparing the groups using the χ2 test (Table 2). The risk of recurrence of anterior POP during the first year after surgery is more than 8 times lower in group 1 than in group 2 – 8.44 (95% CI 2.00–35.61), p=0.0027.
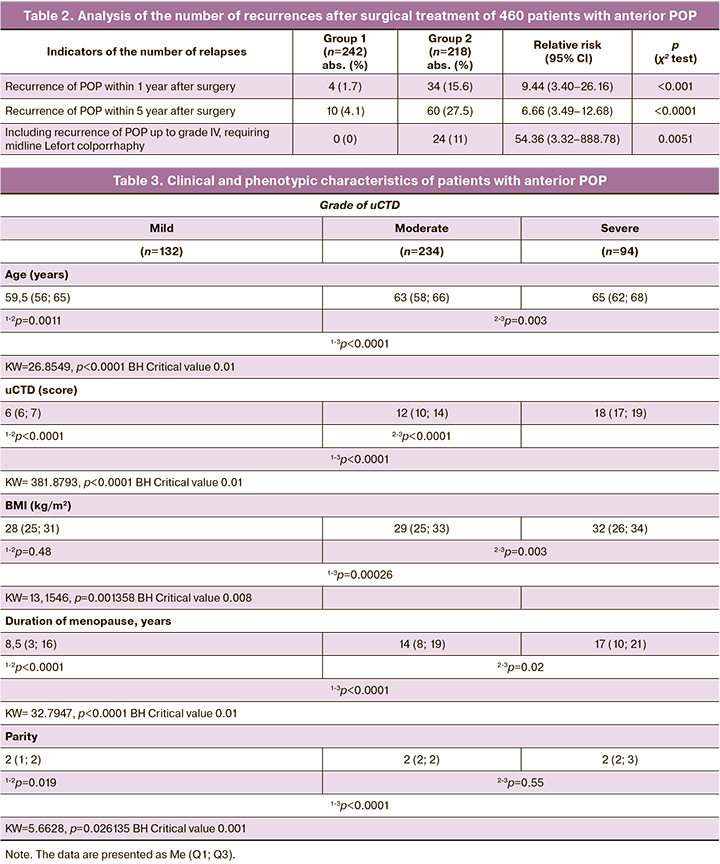
Recurrences during the follow-up of patients from 1 year to 5 years were also less frequent in group 1 than in group 2: only 10 recurrences in patients in group 1 with mesh implants compared to 60 recurrences in group 2 (4.1% in group 1 versus 27.5% in group 2), which was statistically significantly (p<0.0001) lower when comparing groups using the χ2 test (Table 2).
A longer follow-up period of five years revealed an increase in the number of recurrences of anterior POP; however, the risk of recurrence in group 1 remained statistically significantly lower (6 times) than in group 2 – 6.00 (95% CI 2.42–14.90), p=0.0001.
In the long-term follow-up, 5 years after surgical correction of anterior POP, 24/218 (11%) patients in group 2 (all over 70 years old) showed progression of POP to grade IV, which required midline Lefort colporrhaphy. The risk of requiring such an operation in group 2 was 54.37 times higher than that in group 1 (54.37; 95% CI 3.32–888.78), emphasizing the role of age and duration of menopause in women, not only in the development of severe POP but also in the rate of recurrence of the pathological process during plastic surgery with native tissues. This is consistent with data [19] on POP recurrence 2 years after vaginal reconstructive surgery using native tissues.
We then analyzed the initial clinical and phenotypic manifestations of uCTD in 460 patients who underwent surgery using the two methods for anterior POP, which formed the basis for developing prognostic models for anterior POP recurrence after surgical treatment (Tables 3, 4, and 5).
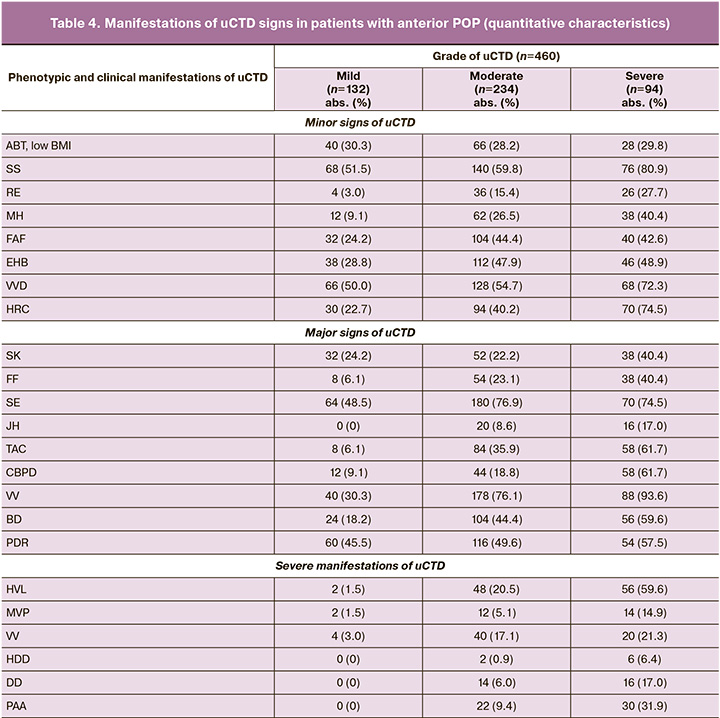
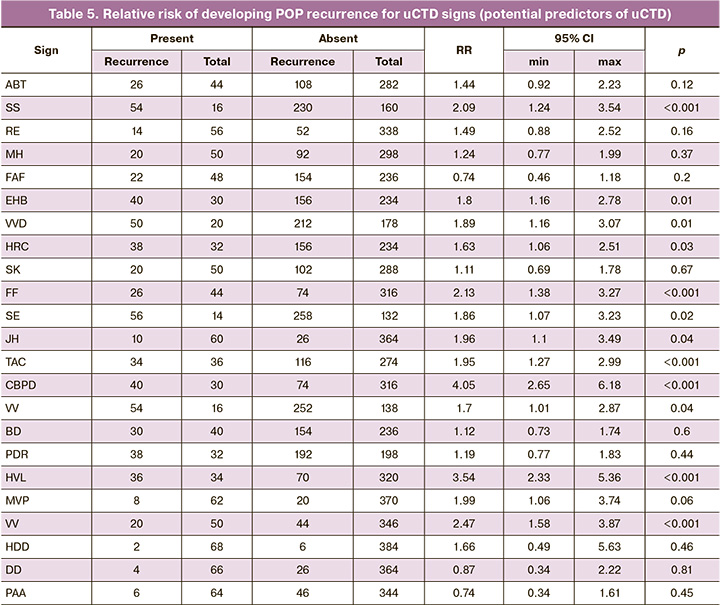
The analysis of the clinical and phenotypic manifestations of uCTD in patients from the two groups confirmed the hypothesis regarding the influence of the degree of uCTD on the severity of POP. In patients with anterior POP grade III requiring surgical correction, clinical manifestations of uCTD of moderate and severe degrees were predominant, with 328 (71%) patients affected. The presence of a conjugacy of uCTD symptoms was observed in patients from both groups, allowing for the prediction of one symptom from a pair, with a probability of over 95%; if one of the uCTD symptoms from the pair is present, the other symptom will also be present, with a probability exceeding 95%.
There were no isolated signs of uCTD in patients; each patient had an average of 3 signs of uCTD – 3 (2; 8). A significant number of statistically significant intersystem correlations were noted between the signs of the uCTD: RE, ABT, MH, and HRC.
In 328 patients from both groups, clinical manifestations of moderate (50.87 %) and severe (20.43 %) uCTD were predominant. Thus, pronounced manifestations of uCTD were observed in 71.3% of the cases, while no statistically significant differences in uCTD scores were found between the 1st and 2nd groups (Figure).
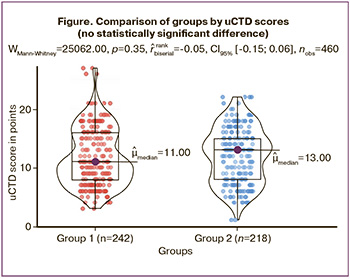
Having confirmed the absence of differences in uCTD manifestations between the two groups, we built models to predict POP recurrence in 460 patients who underwent surgery using two different methods. The dataset mainly contained dichotomous variables indicating the presence or absence of a pathological sign (recurrence), as well as rank variables (age, disease duration, and uCTD grade). Before applying logistic regression, the data were prepared and cases with missing values and outliers in the rank variables were removed. To assess the performance of the model, the data were randomly divided into training and testing sets at a 70/30 ratio, preserving the variance of the rank variables. The logistic regression model was built using the stats 4.2.2 library (R Stats Package). The model was trained on the training set using the log-likelihood method. Model performance was assessed by creating contingency tables, calculating quality metrics, and analyzing the area under the receiver operating characteristic curve (ROC) using the pROC 1.18.5 package. Regression coefficients were interpreted in terms of odds ratios (OR). The p-values obtained from Wald tests were used to analyze the significance of the independent variables. Variables with a p-value of less than 0.05 were considered statistically significant at a 5% significance level and were included in the model.
In group 1, three uCTD manifestations were significant: VVD, CBPD, and VV (Table 6).
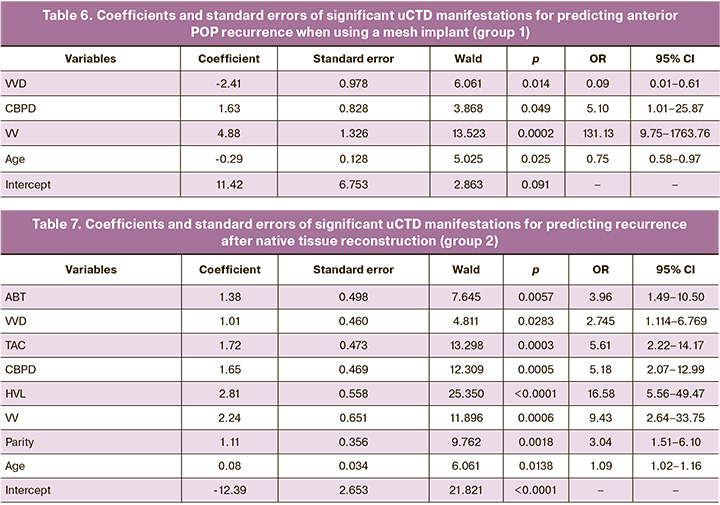
The most significant predictors of POP recurrence in patients in group 1 (after using mesh implants) are, in descending order: VV (OR=131.13; 95% CI 9.75–1763.76), CBPD (OR=5.10; 95% CI 1.01–25.87), and VVD (OR=0.09; 95% CI 0.01–0.61). Thus, the model for predicting immediate and late recurrences in group 1 for surgical treatment of anterior POP using mesh implants is as follows:
logit(p) = 11.42 – 2.41×VVD + 1.63×CBPD + 4.88×VV – 0.29×Age,
where p is the probability of recurrence, which can be calculated using the following formula:
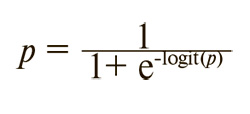
If the logit(p) value is greater than 2, the probability of recurrence approaches 100%; if the logit(p) value is 0 or less, the probability of recurrence is less than 50%.
The construction of ROC curves showed high sensitivity and specificity of the obtained model; the area under the ROC curve (AUC) was 0.970, the root mean square error was 0.011, and the 95% confidence interval (CI) was 0.940–0.987.
In the second group, modeling the prediction of recurrence after plastic surgery using native tissues revealed that six clinical and phenotypic manifestations of uCTD (ABT, VVD, TAC, CBPD, HVL, and VV), age, and parity (Table 7), were important for inclusion in the model. It is noteworthy that the three manifestations of uCTD were universal in both the first and second groups; however, for predicting recurrence following plastic surgery with native tissues, three additional manifestations of uCTD were significant.
The most significant predictors of POP recurrence in group 2 (after native tissue reconstruction) were, in descending order: the presence of HVL (OR=16.58; 95% CI 5.56–49.47), VV (OR=9.43; 95% CI 2.64–33.75), TAC (OR=5.61; 95% CI 2.22–14.17), CBPD (OR=5.18; 95% CI 2.07–12.99), and ABT (OR=3.96; 95% CI 1.49–10.50). Each subsequent birth in the medical history increases the probability of recurrence of anterior POP by 3.04 times (OR=3.04; 95% CI 1.51–6.10).
Thus, the recurrence prediction model for surgery using native tissues was as follows:
logit (p) = -12.39 + 1.38×ABT + 1.01×VVD + 1.72×TAC + 1.65×CBPD + 2.81×HVL + 2.24×VV + 0.08×Age + 1.11×Parity,
where p is the probability of recurrence, which can be calculated using the formula:
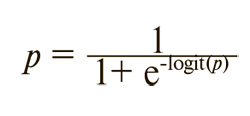
If the logit (p) value exceeded 0.8, the probability of recurrence approached 100%. Conversely, with a logit(p) value of 0 or lower, the probability of recurrence remained below 50%. ROC curve analysis demonstrated high sensitivity and specificity for the obtained model: area under the ROC curve (AUC) = 0.903, standard error = 0.028, 95% CI = 0.855–0.938.
In general, the most significant predictors of POP recurrence in patients with mesh implants were, in descending order: VV, CBPD, and VVD. These uCTD manifestations were also significant in group 2, with the additional presence of HVL, TAC, and ABT.
Surgical treatment of anterior POP in women with uCTD using mesh implants is an effective method, provided that strict patient selection is based on the assessment of uCTD severity. The developed patient selection algorithm enables optimization of the approach for surgical treatment of anterior POP, minimizing the likelihood of disease recurrence, and reducing the risk of postoperative complications.
Conclusion
The frequency of clinical manifestations of uCTD in patients with anterior POP was studied and models for predicting the recurrence of surgical treatment using mesh or native implants were proposed.
All patients with anterior POP had several uCTD manifestations; clinical manifestations of moderate and severe degrees were predominant among 328 (71%) patients. A correlation was revealed between these clinical manifestations of uCTD and a probability of >95% for the presence of paired symptoms of uCTD.
The presence of uCTD affects the likelihood of POP recurrence after surgical correction. Based on clinical and phenotypic data, a high-quality model for predicting the recurrence of anterior POP in women was developed, which can personalize the choice of surgical correction method for POP.
According to the modeling results, three manifestations of uCTD were identified as universal for the development of anterior POP recurrence: VVD (from the group of minor manifestations of uCTD), CBPD (from major signs), and VV (from severe manifestations of uCTD). If, along with these, three additional manifestations of uCTD (ABT, TAC, and HVL) are present, it is advisable to choose a mesh implant immediately.
Using a mesh implant in the presence of clinical manifestations of severe uCTD is associated with a lower risk of recurrence within one year (1.7%) and in the remote postoperative period (4.1%). Additionally, age was important for the choice of implant; each additional birth in group 2 increased the probability of POP recurrence by 1.51 times.
References
- Краснопольская И.В. Дисфункция тазового дна у женщин: Клиника, диагностика, принципы лечения. Акушерство и гинекология. 2018; 2: 82-6. [Krasnopolskaya I.V. Pelvic floor dysfunction in women: clinical presentation, diagnosis, and principles of treatment. Obstetrics and Gynecology. 2018; (2): 82-6. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.2.82-86.
- American College of Obstetricians and Gynecologists and the American Urogynecologic Society; INTERIM UPDATE: This Practice Bulletin is updated as highlighted to reflect the US Food and Drug Administration order to stop the sale of transvaginal synthetic mesh products for the repair of pelvic organ prolapse. Pelvic Organ Prolapse. Female Pelvic Med. Reconstr. Surg. 2019; 25(6): 397-408. https://dx.doi.org/10.1097/SPV.0000000000000794.
- Смольнова Т.Ю., Чупрынин В.Д. Пролапс гениталий: взгляд на проблему. Акушерство и гинекология. 2018; 10: 33-40. [Smolnova T.Yu., Chuprynin V.D. Genital prolapse: a look at the problem. Obstetrics and Gynecology. 2018; (10): 33-40. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.10.33-40.
- Tunn R., Baeßler K., Knüpfer S., Hampel C. Urinary incontinence and pelvic organ prolapse in women. Prevention and treatment. Dtsch. Arztebl. Int. 2023; 120: 71-9. https://dx.doi.org/10.3238/arztebl.m2022.0406.
- Радзинский В.Е., Ханзадян М.Л., Демура Т.А. Матриксные металлопротеиназы и их ингибиторы в патогенезе пролапса тазовых органов.Доктор.Ру. 2014; S1: 7-10. [Radzinsky V.E., Khanzadyan M.L., Demura T.A. Matrix metalloproteinases and their inhibitors in the pathogenesis of pelvic organ prolapse. Doctor.Ru. 2014; S1: 7-10. (in Russian)].
- Lim V.F., Khoo J.K., Wong V., Moore K.H. Recent studies of genetic dysfunction in pelvic organ prolapse: the role of collagen defects. Aust. N. Z. J. Obstet. Gynaecol. 2014; 54(3): 198-205. https://dx.doi.org/10.1111/ajo.12169.
- Васин Р.В., Филимонов В.Б., Мнихович М.В., Каприн А.Д., Костин А.А., Васина И.В. Морфологическая структура и иммуногистохимический анализ стенок влагалища у женщин с пролапсом гениталий. Урология. 2019; 6: 12-20. [Vasin R.V., Filimonov V.B., Mnikhovich M.V., Kaprin A.D., Kostin A.A., Vasina I.V. Morphologic structure and immunohistochemical analysis of vaginal wall in women with pelvic organ prolapsed. Urologiia. 2019; (6): 12-20. (in Russian)]. https://dx.doi.org/10.18565/urology.2019.6.12-20.
- Смольнова Т.Ю., Савельев С.В., Буянова С.Н., Титченко Л.И., Гришин В.Л., Яковлева Н.И. Фенотипический симптомокомплекс дисплазии соединительной ткани у женщин. Клиническая медицина. 2003; 81(8): 42-7. [Smolnova T.Yu., Buyanova S.N., Savelyev S.V., Titchenko L.I., Grishin V.L., Yakovleva N.I. The phenotypical symptom complex of connective tissue dysplasia in females. Clinical Medicine. 2003; 81(8): 42-7. (in Russian)].
- Трунченко Н.В., Макаров К.Ю., Киселева Т.В., Крашенинникова О.О., Айдагулова С.В., Маринкин И.О. Особенности хронического эндометрита у пациенток репродуктивного возраста с недифференцированной дисплазией соединительной ткани. Сибирский научный медицинский журнал. 2017; 37(1): 99-104. [Trunchenko N.V., Makarov K.Yu., Kiseleva T.V., Krasheninnikova O.O., Aidagulova S.V., Marinkin I.O. Chronic endometritis peculiarities in reproductive-age women with undifferentiated connective tissue dysplasia. Siberian Scientific Medical Journal. 2017; 37(1): 99-104. (in Russian)].
- Clark B.A., Sekhon A. Nephroptosis in a young woman with joint laxity. Nat. Rev. Nephrol. 2009; 5(12): 722-5. https://dx.doi.org/10.1038/nrneph.2009.169.
- Coolen A.W.M., Bui B.N., Dietz V., Wang R., van Montfoort A.P.A., Mol B.W.J. et al. The treatment of post-hysterectomy vaginal vault prolapse: a systematic review and meta-analysis. Int. Urogynecol. J. 2017; 28(12): 1767-83. https://dx.doi.org/10.1007/s00192-017-3493-2.
- Fritel X., de Tayrac R., de Keizer J., Campagne-Loiseau S., Cosson M., Ferry P. et al. Serious complications and recurrences after pelvic organ prolapse surgery for 2309 women in the VIGI-MESH registry. BJOG. 2022; 129(4): 656-63. https://dx.doi.org/10.1111/1471-0528.16892.
- Jha S., Cutner A., Moran P. The UK National Prolapse Survey: 10 years on. Int. Urogynecol. J. 2018; 29(6): 795-801. https://dx.doi.org/10.1007/s00192-017-3476-3.
- Dällenbach P., Jungo Nancoz C., Eperon I., Dubuisson J.B., Boulvain M. Incidence and risk factors for reoperation of surgically treated pelvic organ prolapse. Int. Urogynecol. J. 2012; 23(1): 35-41. https://dx.doi.org/10.1007/s00192-011-1483-3.
- Bodner-Adler B., Bodner K., Carlin G., Kimberger O., Marschalek J., Koelbl H. et al. Clinical risk factors for recurrence of pelvic organ prolapse after primary native tissue prolapse repair. Wien Klin. Wochenschr. 2022; 134(1-2): 73-5. https://dx.doi.org/10.1007/s00508-021-01861-8.
- Maher C., Feiner B., Baessler K., Christmann-Schmid C., Haya N., Brown J. Surgery for women with anterior compartment prolapse. Cochrane Database Syst. Rev. 2016; 11(11): CD004014. https://dx.doi.org/10.1002/14651858.CD004014.pub6.
- da Silveira S.D.R.B., Auge A.P., Jarmy-Dibella Z.I., Margarido P.F., Carramao S., Alves Rodrigues C. et al. A multicenter, randomized trial comparing pelvic organ prolapse surgical treatment with native tissue and synthetic mesh: a 5-year follow-up study. Neurourol. Urodyn. 2020; 39(3): 1002-11. https://dx.doi.org/10.1002/nau.24323.
- Carroll L., Sullivan C.O., Doody C., Perrotta C., Fullen B.M. Pelvic organ prolapse: women's experiences of accessing care & recommendations for improvement. BMC Women's Health. 2023; 23(1): 672. https://dx.doi.org/10.1186/s12905-023-02832-z.
- Siff L.N., Barber M.D., Zyczynski H.M., Rardin C.R., Jakus-Waldman S., Rahn D.D. et al.; NICHD Pelvic Floor Disorders Network. Immediate postoperative pelvic organ prolapse quantification measures and 2-year risk of prolapse recurrence. Obstet. Gynecol. 2020; 136(4): 792-801. https://dx.doi.org/10.1097/AOG.0000000000004043.
Received 19.08.2024
Accepted 28.11.2024
About the Authors
Igor’ O. Marinkin, Dr. Med. Sci., Professor, Head of the Obstetrics and Gynecology Department, Rector, Novosibirsk State Medical University, Ministry of Health of Russia, Krasny Ave., 52, Novosibirsk, 630091, Russia, +7(383)222-32-04, rector@ngmu.ru, https://orcid.org/0000-0002-9409-4823Fedor A. Rakitin, Gynecologist, Senior Researcher, Research Institute of Clinical and Experimental Lymрhology – Branch of the Institute of Cytology and Genetics, Siberian Branch of Russian Academy of Sciences, Ac. Lavrentyev str., 10, Novosibirsk, 630060, Russia, +7(961)875-99-08, rakitinfedorr@mail.ru,
https://orcid.org/0000-0002-5927-6883
Alexander V. Volchek, Gynecologist, Senior Researcher, Novosibirsk State Medical University, Ministry of Health of Russia, 52, Krasny Ave., Novosibirsk, 630091, Russia, +7(913)003-17-22, alexander@volcheck.ru, https://orcid.org/0000-0003-4458-1188
Mikhail Yu. Soluyanov, PhD, Researcher at the Laboratory of Operative Lymphology and Lymphodetoxication, Research Institute of Clinical and Experimental Lymрhology – Branch of the Institute of Cytology and Genetics, Siberian Branch of Russian Academy of Sciences, Ac. Lavrentyev str., 10, Novosibirsk, 630060, Russia, +7(913)952-65-29, msoluyanov@mail.ru, https://orcid.org/0000-0003-2635-9161
Vitaliy M. Kuleshov, Dr. Med. Sci., Professor, Obstetrics and Gynecology Department, Novosibirsk State Medical University, Ministry of Health of Russia,
52, Krasny Ave., Novosibirsk, 630091, Russia, +7(913)9127346, kuleshov_vm@mail.ru, https://orcid.org/0000-0003-3304-1581
Konstantin Yu. Makarov, Dr. Med. Sci., Professor, Obstetrics and Gynecology Department, Novosibirsk State Medical University, Ministry of Health of Russia,
52, Krasny Ave., Novosibirsk, 630091, Russia, +7(913)909-11-22, fdpngma@mail.ru, https://orcid.org/0000-0003-3574-6382
Tatyana M. Sokolova, Dr. Med. Sci., Professor, Obstetrics and Gynecology Department, Novosibirsk State Medical University, Ministry of Health of Russia,
52, Krasny Ave., Novosibirsk, 630091, Russia, +7(913)984-52-58, tatyana3965@mail.ru, https://orcid.org/0000-0003-3435-3536
Vadim V. Nimaev, Dr. Med. Sci., Head of the Laboratory of Operative Lymphology and Lymphodetoxication, Research Institute of Clinical and Experimental Lymрhology – Branch of the Institute of Cytology and Genetics, Siberian Branch of Russian Academy of Sciences, Ac. Lavrentyev str., 10, Novosibirsk, 630060, Russia, +7(913)930-24-87, nimaevvv@yandex.ru, https://orcid.org/0000-0002-9889-3729
Svetlana V. Aidagulova, Dr. Bio. Sci., Professor, Head of the Laboratory of Cellular Biology and Fundamental Basis of Reproduction, Central Research Laboratory,
Novosibirsk State Medical University, Ministry of Health of Russia, 52, Krasny Ave., Novosibirsk, 630091, Russia, +7(913)9092251, asvetvlad@yandex.ru,
https://orcid.org/0000-0001-7124-1969
Corresponding author: Svetlana V. Aidagulova, asvetvlad@yandex.ru



