Anti-inflammatory activity of serum cytokines (IL-4, IL-10, IL-13) and the natural IL-1β receptor antagonist (IL-1Ra) in women with uterine myoma
Aim. To investigate serum levels of anti-inflammatory cytokines (interleukins (IL) -4, IL-10, IL-13) and a natural IL-1β receptor antagonist (IL-1Ra) in women with uterine myoma using multiplex analysis. Material and methods. The study comprised 36 women with uterine myoma aged 23 to 54 years. All women underwent general clinical and standard diagnostic examination, hemostatic testing, and cytokine profiling (Bio-Plex 200). Results. The women with uterine myoma had significantly decreased serum levels of IL-4 (p <0.000001) and IL-1Ra (p = 0.00002). Serum levels of the two other cytokines, IL-10 and IL-13, were within normal range. Conclusion. Low concentrations of factors inhibiting tissue inflammation and angiogenesis can produce an unfavorable effect on the proliferation and differentiation of uterine tissues. Low levels of the anti-inflammatory component of a cytokine network can also be one of the factors contributing to the development of chronic infectious and inflammatory processes in women with uterine myoma.Konenkov V.I., Koroleva E.G., Orlov N.B., Prokof’ev V.F., Shevchenko A.V., Novikov A.M., Dergacheva T.I., Ostanin A.A.
Keywords
A uterine myoma (fibroid, leiomyoma) is the most common benign tumor occurring in women of reproductive age with the prevalence increasing with age [1, 2]. Although in most patients the disease remains asymptomatic, clinical manifestations of fibroids include menstrual disorders and reproductive dysfunction [3]. During pregnancy, intramural fibroids can cause miscarriages [4], and large fibroids may impair fetal development and myometrial contractility [5, 6]. Until recently, there was no consensus on the causes of uterine fibroids. Several factors are believed to contribute to the development of uterine fibroids: sex hormone disorders; chronic inflammatory diseases the female genital tract (chronic salpingo-oophoritis, sexually transmitted infections); abortions, intrauterine contraceptives; endocrine (thyroid, adrenal, etc.) disorders. Also, there is evidence for a genetic predisposition to uterine fibroids, and family history of the disease is considered to be a risk factor for the condition. “Family forms” of uterine fibroids have been reported in 5-10% of patients. However, no specific mechanisms of such predisposition have been described until recently [7]. Besides, higher numbers of inflammatory cells such as macrophages and mast cells were found inside and close to fibroids as compared to the more distant myometrium [8].
It can be assumed that such excessive proliferation of inflammatory, smooth muscle and connective tissue cellular elements that are observed in uterine fibroids is a consequence of the loss of control over the normal processes of cell division and differentiation, which are largely controlled by the system of growth and cytokine regulatory factors. The integral expression of the proinflammatory, proangiogenic and sclerogenic capacity of these regulatory factors is compensated by a family of cytokines producing anti-inflammatory and other effects suppressing functional cell activity.
This study was aimed to investigate the total serum concentration of the most active representatives of this family of cytokines. Its distinctive feature of the study was the use of multiplex analysis. With this technology, multiple relevant inflammatory cytokines could be detected in a single run in one sample using xMAP technology licensed by Luminex. The analysis involves the use of two fluorescent dyes at distinct ratios leading to more than 100 different assays in one sample. This report presents the results of the determination of such major factors with pronounced anti-inflammatory activity, as interleukin (IL) -4, IL-10, IL-13, and a naturally occurring interleukin- IL-1β receptor antagonist (IL-1Ra).
Material and methods
The study comprised 36 women with uterine myoma aged 23 to 54 years (mean 41.13 ± 6.68), who underwent laparoscopic myomectomy. Among them, 35.89% of patients were overweight and obese (body mass index> 24.99). Twenty patients had menstrual disorders in the form of polymenorrhea. Twenty-eight participants were parous women. Twenty-five patients had a history of induced termination of pregnancy. Eight women experienced spontaneous miscarriages. Five patients were nulliparas. Presenting symptoms included pulling lower abdominal pain (n=28) and bleeding (n=23). Fibroid size, as measured by ultrasound ranged from 5 to 180 mm (mean 106.5 ± 41.05). The nodules were located in the anterior and posterior walls of the uterus in 19 and seven patients, respectively; two women had atypical fibroid locations (retroperitoneal, paracervical), and ten patients had combined locations. Histological finding after preoperative hysteroscopy revealed endometrial polyps and simple endometrial hyperplasia 7 and 6 patients, respectively.
Before cytokine analysis, once-frozen serum samples were completely thawed at room temperature and centrifuged at 10 000 rpm for 10 min at 4 C to remove the sediment. Cytokine concentrations were determined using the Bio-Plex Pro Human Cytokine 27-Plex Immunoassay by flow-through fluorometry on a Bio-Plex 200 two-beam laser analyzer, manufactured by Bio-Rad (USA). After creating a calibration curve according to validated standards, serum concentrations of IL-1β, IL-1Ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p70 IL-13, IL-15, IL-17, Basic FGF, Eotaxin, G-CSF, GM-CSF, IFN-γ, IP-10, MCP-1 (MCAF), MIP-1α, MIP-1β, PDGF-BB, RANTES, TNF-α, and VEGF were determined. The data were processed using the Bio-Plex Manager Software version 4.1. The concentration of cytokines was expressed in pg/ml.
Statistical analysis was performed with StatSoft Statistica 10.0 and IBM SPSS Statistics 23 (USA). Normal distribution of quantitative variables was tested by the Shapiro-Wilk and Kolmogorov-Smirnov test with Lilliefors correction. Parametric Student’s t-test was used to compare independent samples showing normal distribution and homogeneity of variance; also nonparametric Mann Whitney test was used to compare samples with non-normal distribution. Correlation analysis was conducted by calculating Spearman’s rank correlation coefficients. Data were expressed as a mean ± standard error of the mean (M ± m) and median (Me). The critical level of significance when testing a statistical hypothesis was considered at p <0.05.
Results
Primary analyses of the quantitative data showed a significant dispersion of virtually all variables related to serum levels of the studied regulatory factors. This conclusion is confirmed by significant differences in minimum – maximum ranges, means and medians of serum levels of all cytokines (Table 1).
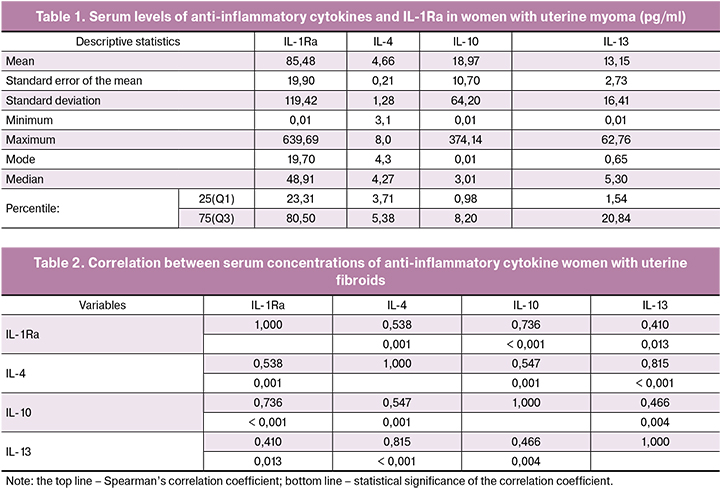
To compare our findings with the levels of anti-inflammatory cytokines in healthy women, we used normative values from a large sample of healthy Caucasian women, obtained by a laboratory that cooperates with the 27-Plex Immunoassay manufacturer (Bio-Rad) [9]. The authors examined the correlation between serum cytokine concentration and sex, age, ethnicity of the participants, time points of sampling, and even different types of plates. The results were analyzed using the Bio-Plex Manager software version 6.2 (Bio-Rad) and were considered suitable as normative values for healthy Caucasian women. Even though 25 of 27 cytokines (except MCP-1 and TNF-α) had no differences in concentrations between men and women, in the comparative analysis we used data from 94 healthy Caucasian women.
Our findings showed that women with uterine fibroids had significantly decreased serum concentrations of such a key anti-inflammatory cytokine as IL-4. IL-4 has many biological functions including the switching B-cells synthesis by IgG1, IgG4, and IgE, activating macrophages, affecting the actions of the epithelial and smooth muscle cell, and suppressing IL-1β, IL-6, TNF-α, IL-8 synthesis. This regulatory protein, along with IL-15 and IL-13, belongs to the family of cytokines produced by Th2 and ILC2s cells expressing the transcription factor GATA3 [10, 11]. The interleukin-4 receptor also binds to IL-13, which can contribute to many overlapping functions of this cytokine and IL-13. Their coordinated actions affect the processes of collagen deposition and tissue fibrosis, which have a role in the development of uterine myoma [12]. IL-13 converts macrophages to an M2 phenotype, which lacks the inflammatory activity of M1 cells [13]. Women with uterine myoma had significantly lower serum levels of IL-4 (4.66 ± 0.77 pg/ml) compared to healthy Caucasian women (36.0 ± 2.27 pg/ml) (p < 0.000001). Besides, serum levels of IL-13 in the compared groups of women were virtually identical (13.15 ± 2.73 and 14.1 ± 1.85 pg/ml), respectively (p = 0.757). When we used the same method to investigate serum concentrations of IL-13 in healthy women, we observed similar findings (15.01 ± 1.98pg/ml).
As follows from Table 2, there was a strong direct correlation between serum concentrations of these two cytokines (Spearman’s correlation coefficient 0.815, p <0.0001).
Women with uterine fibroids had significantly reduced serum concentrations of the IL-1Ra, which binds to IL-1α and IL-1β receptors thus significantly decreasing their pro-inflammatory activity. The interleukin (IL)-1 family of cytokines comprises 11 members, including 7 pro-inflammatory agonists (IL-1α, IL-1β, IL-18, IL-33, IL-36α, IL-36β, IL-36γ) and 4 defined or putative antagonists (IL-1R antagonist (IL-1Ra), IL-36Ra, IL-37, and IL-38) exerting anti-inflammatory activities [14].
In our study, serum concentrations of IL-1Ra protein in women with uterine myoma and healthy women were 85.49 ± 19.83 pg/ml and 305.5 ± 45.76 pg/ml, respectively (p = 0, 00002). It can be assumed that insufficient production of this regulatory factor may result in an increase in the pro-inflammatory activity of IL-1β itself despite the fact that according to our data in women with uterine myoma its serum concentration (2.31 ± 0.42 pg/ml) was also lower than in healthy women (4.3 ± 0.59 pg/ml, p = 0.006).
Our findings seem to be ambiguous because patients with uterine fibroids had higher serum levels of IL-10 (18.98 ± 10.7 pg/ml) compared with healthy controls (2.70 ± 0.64 pg/ml) (p = 0.13). Due to strong anti-inflammatory properties, IL-10 plays a key role in limiting the body’s immune response to foreign antigens and is essential to counter hyperimmune reaction and maintain normal tissue homeostasis. IL-10 is produced by various cells, including T-helpers, monocytes, macrophages, dendritic cells, B cells, cytotoxic T cells, NK cells, neutrophils and eosinophils, epithelial cells and keratinocytes, and tumor cells.
The immunosuppressive activity of IL-10 is mediated by the IL-10 heterodimer receptor (IL-10R) consisting of two different subunits (IL-10R1 and IL-10R2). Although the complex of IL-10 receptors is expressed to varying degrees in many other types of cells, monocytes and macrophages appear to be the primary target of IL-10. Receptor ligation activates JAK/STAT signaling, leading to major changes in the expression profile of immunomodulating genes that serve to inhibit the release of pro-inflammatory mediators and simultaneously enhance the inhibitory function of these cells. Also, IL-10 can increase the overall anti-inflammatory effect by releasing such anti-inflammatory molecules as IL-1Ra, soluble TNF receptor, and interleukin-27 [15, 16].
Discussion
In summary, these results show that uterine fibroids are associated with in multidirectional changes in serum concentrations of anti-inflammatory cytokines, mainly with a significant decrease in the concentrations of IL-4 and the natural IL-1β receptor antagonist. The concentrations of the two other cytokines (IL-10 and IL-13) that exert a similar regulatory activity in inflammation are close to those in healthy women, although both of them have some upward and downward trends. The relationship between them is much weaker - the correlation coefficient is only 0.46 (p = 0.04).
The anti-inflammatory activity of IL-10 and other anti-inflammatory cytokines makes them promising as potential anti-inflammatory therapies in the management of diseases with pronounced inflammatory or hyperreactive components. However, considerable caution should be taken when implementing this strategy, as evidenced by the development of acute colitis in the presence of normal gut flora in IL-10-deficient mice [17]. At the same time, clinical observations revealed an increase in serum levels of IL-10 in women as a result of treating uterine fibroids [18].
Therapeutic applications of IL-1Ra seem to be in a more advanced stage of development. In an experiment, administration of IL-1Ra improved stroke outcomes in young and old comorbid rats. Besides, IL-1Ra not only increases the stem cell proliferation but also significantly increases the migration of neuroblasts and the number of newborn neurons after cerebral ischemia, indicating that systemic administration of IL-1Ra improves recovery and promotes neurogenesis after an experimental stroke, emphasizing once again the therapeutic potential of this drug [19]. On its basis, a biopharmaceutical drug Anakinra (Kineret), a recombinant version of IL-1Ra was developed, approved for clinical use and showed high efficacy in the treatment of rheumatoid arthritis and other systemic inflammatory diseases [20].
Our findings showed only a relative decrease in serum levels of IL-1Ra in women with uterine myoma, and the pathogenetic significance of this phenomenon remains to be determined. Besides, a recent study has described a rare genetic syndrome, deficiency of IL-1 receptor antagonist (DIRA), that presents with life-threatening systemic inflammation, aseptic multifocal osteomyelitis, and pustulosis [21].
Conclusion
This study has identified significant changes in serum concentrations of anti-inflammatory cytokines and IL-1Ra that can produce an unfavorable effect on the proliferation and differentiation of uterine tissues. These results emphasize the need for further studies aimed to determine the possible pathogenetic significance of these changes and establish potential therapeutic strategies for correcting the impaired function of the cytokine network.
References
1. Manyonda I., Sinthamoney E., Belli A.M. Controversies and challenges in the modern management of uterine fibroids. BJOG. 2004; 111(2): 95-102.
2. Stewart E.A. Uterine fibroids. Lancet. 2001; 357(9252): 293-8.
3. Zowall H., Cairns J.A., Brewer C., Lamping D.L., Gedroyc W.M., Regan L. Cost-effectiveness of magnetic resonance-guided focused ultrasound surgery for treatment of uterine fibroids. BJOG. 2008; 115(5): 653-62.
4. Van der Kooij S.M., Ankum W.M., Hehenkamp W.J. Review of nonsurgical/minimally invasive treatments for uterine fibroids. Curr. Opin. Obstet. Gynecol. 2012; 24(6): 368-75.
5. Rabinovici J., David M., Fukunishi H., Morita Y., Gostout B.S., Stewart E.A. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil. Steril. 2010; 93(1): 199-209.
6. Gavrilova-Jordan L.P., Rose C.H., Traynor K.D., Brost B.C., Gostout B.S. Successful term pregnancy following MR-guided focused ultrasound treatment of uterine leiomyoma. J. Perinatol. 2007; 27(1): 59-61.
7. Кузнецова М.В., Трофимов Д.Ю., Тихончук Е.Ю., Согоян Н.С., Адамян Л.В., Сухих Г.Т. Молекулярные механизмы патогенеза миомы матки: анализ мутаций гена MED12 в российской популяции. Акушерство и гинекология. 2016; 10: 85-90. [Kuznetsova M.V., Trofimov D.Yu.,Tikhonchuk E.Yu., Sogoyan N.S., Adamyan L.V., Sukhikh G.T. Molecular mechanisms of the pathogenesis of uterine myoma: Analysis of mutations in the MED 12 gene in the Russian population. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2016; (10): 85-90. (in Russian)] http://dx.doi.org/10.18565/aig.2016.10.85-90
8. Protic O., Toti P., Islam M.S., Occhini R., Giannubilo S.R., Catherino W.H. et al. Possible involvement of inflammatory/reparative processes in the development of uterine fibroids. Cell Tissue Res. 2016; 364(2): 415-27.
9. Biancotto A., Wank A., Perl S., Cook W., Olnes M.J., Dagur P.K. et al. Correction: Baseline levels and temporal stability of 27 multiplexed serum cytokine concentrations in healthy subjects. PLoS One. 2015; 10(7): e0132870.
10. Annunziato F., Romagnani C., Romagnani S. The 3 major types of innate and adaptive cell-mediated effector immunity. J. Allergy Clin. Immunol. 2015; 135(3): 626-35.
11. Björkström N.K., Kekäläinen E., Mjösberg J. Tissue-specific effector functions of innate lymphoid cells. Immunology. 2013; 139(4): 416-27.
12. Bamias G., Cominelli F. Role of Th2 immunity in intestinal inflammation. Curr. Opin. Gastroenterol. 2015; 31(6): 471-6.
13. Hall B.M. T cells: soldiers and spies - the surveillance and control of effector T cells by regulatory T cells. Clin. J. Am. Soc. Nephrol. 2015; 10(11): 2050-64.
14. Palomo J., Dietrich D., Martin P., Palmer G., Gabay C. The interleukin (IL)-1 cytokine family-Balance between agonists and antagonists in inflammatory diseases. Cytokine. 2015; 76(1): 25-37.
15. Iyer S.S., Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012; 32(1): 23-63.
16. Sabat R., Grütz G., Warszawska K., Kirsch S., Witte E., Wolk K. et al. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010; 21(5): 331-44.
17. O’Garra A., Barrat F.J., Castro A.G., Vicari A., Hawrylowicz C. Strategies for use of IL-10 or its antagonists in human disease. Immunol. Rev. 2008; 223: 114-31.
18. Wang X., Qin J., Chen J., Wang L., Chen W., Tang L. The effect of high-intensity focused ultrasound treatment on immune function in patients with uterine fibroids. Int. J. Hyperthermia. 2013; 29(3): 225-33.
19. Pradillo J.M., Murray K.N., Coutts G.A., Moraga A., Oroz-Gonjar F., Boutin H. et al. Reparative effects of interleukin-1 receptor antagonist in young and aged/co-morbid rodents after cerebral ischemia. Brain Behav. Immun. 2017; 61: 117-26.
20. Fiocco U., Vezzù M., Cozzi L., Todesco S. IL-1Ra (recombinant human IL-1 receptor antagonist) in the treatment of rheumatoid arthritis: the efficac. Reumatismo. 2004; 56(1, Suppl. 1): 62-73.
21. Garg M., de Jesus A.A., Chapelle D., Dancey P., Herzog R., Rivas-Chacon R. et al. Rilonacept maintains long-term inflammatory remission in patients with deficiency of the IL-1 receptor antagonist. JCI Insight. 2017; 2(16). pii: 94838.
Received 11.12.2017
Accepted 22.12.2017
About the Authors
Konenkov Vladimir I., Dr.Med.Sci. Professor, Academician of the RAS, the Honored Scientist of the RF, Academic Administrator of RICEL — branch FRC ICG SB RAS, Head of the Laboratory of Clinical Immunogenetics. Address: 630060, Russia, Novosibirsk, Timakova St., 2. Tel.: 8 (383) 333-64-09. E-mail: vikonenkov@gmail.com.ORCID.org/0000-0001-7385-6270
Koroleva Elena G., Researcher at the Laboratory of Cell Technologies, RICEL - branch FRC ICG SB RAS .
Address: 630060, Russia, Novosibirsk, Timakova St., 2. Tel.: 8 (383) 335-93-32. E-mail: lymphology@niikel.ru
Orlov Nikolai B., Ph.D., Senior Researcher at the Laboratory of Clinical Immunogenetics, RICEL - branch FRC ICG SB RAS .
Address: 630060, Russia, Novosibirsk, Timakova St., 2. Tel.: 8 (383) 311-05-40. E-mail: nbo700@mail.ru. ORCID.org/0000-0002-3437-7151
Prokof’ev Viktor F., Ph.D., Leading Researcher at the Laboratory of Clinical Immunogenetics, RICEL - branch FRC ICG SB RAS.
Address: 630060, Russia, Novosibirsk, Timakova St., 2. Tel.: 8 (383) 311-05-40. E-mail: vf_prok@mail.ru. ORCID.org/0000-0001-7290-1631
Shevchenko Alla V., Dr.Bio.Sci., Leading Researcher at the Laboratory of Clinical Immunogenetics, RICEL - branch FRC ICG SB RAS .
Address: 630060, Russia, Novosibirsk, Timakova St., 2. Tel.: 8 (383) 311-05-40. E-mail: shalla64@mail.ru. ORCID.org/0000-0001-5898-950X
Novikov Aleksei M., Ph.D., Junior Researcher at the Laboratory of Clinical Immunogenetics, RICEL - branch FRC ICG SB RAS .
Address: 630060, Russia, Novosibirsk, Timakova St., 2. Tel.: 8 (383) 335-93-32. E-mail: novis.ngmu@gmail.com
Dergacheva Tat’yana I., Dr.Med.Sci., Professor, Leading Researcher at the Laboratory of Functional Morphology of the Lymphatic System,
RICEL — branch FRC ICG SB RAS . Address: 630060, Russia, Novosibirsk, Timakova St., 2. Tel.: 8 (383) 333-54-24. E-mail: dr-tanja@yandex.ru
Ostanin Aleksandr A., Dr.Med.Sci., Professor, Chief Researcher at the Laboratory of Cellular Immunotherapy, RIFCI.
Address: 630099, Russia, Novosibirsk, Yadrintsevskaya Str., 14. Tel.: 8 (383) 236-03-29. E-mail: ct_lab@mail
For citations: Konenkov V.I., Koroleva E.G., Orlov N.B., Prokof’ev V.F., Shevchenko A.V., Novikov A.M., Dergacheva T.I., Ostanin A.A. Anti-inflammatory activity of serum cytokines (IL-4, IL-10, IL-13) and the natural IL-1β receptor antagonist (IL-1Ra) in women with uterine myoma. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; (10): 80-5. (in Russian)
https://dx.doi.org/10.18565/aig.2018.10.80-85



