Prediction and prevention of preeclampsia in the city Center for family planning and reproduction
Objective. To analyze the prediction and prevention of preeclampsia using prenatal screening performed in the City Center for Family Planning and Reproduction.Shalina R.I., Kasum-zade N.K., Konoplyannikov A.G., Latyshkevich O.A., Shekhovtsov D.B.
Materials and methods. A total of 2374 case histories were analyzed. The study revealed 209 women at high risk for preeclampsia with likelihood of its developing 1:100 and higher. Additionally, 102 patients with a low risk for preeclampsia and 18 pregnant women who had severe preeclampsia at different gestation periods were identified.
Results. The incidence of preeclampsia was 10.0%; it was 11.8% in the absence of preventive measures, and it was 9.7% in their presence (p=0.72). In case of hemodynamic disorders in the uterine arteries, the incidence of preeclampsia was 12.5%, in the absence of disorders it was 9.1%; the incidence was 6.7% with changes in PAPPA-A and 7.1% with changes in PLGF.
Conclusion. The criteria proposed in the program are not absolute and finalized. For the prevention of preeclampsia, it is more appropriate to use a preventive complex including general hygiene measures, diet, treatment of extragenital pathology, and administration of medications depending on the detected changes.
Keywords
Nowadays, hypertensive complications during pregnancy, in particular preeclampsia (PE), represent the fourth leading cause of maternal mortality [1]. Every year 50,000 pregnant women die from PE and its complications worldwide. In Russia, PE and eclampsia account for 12.4% of cases of maternal deaths (2017). In this regard, it is important to identify patients at risk for PE and provide them with preventive measures.
In the Russian Federation, numerous methods for predicting PE have been proposed by the obstetricians over the past 20–30 years. Both clinical data (case history, presence of extragenital diseases, etc.) and laboratory data related primarily to the peculiarities of blood circulation were used [1–3].
The clinical risk factors for PE are presented in the latest recommendations “Hypertensive Disorders during Pregnancy, Childbirth and Postpartum Period. Preeclampsia. Eclampsia” (2016).
They are also presented in the recommendations of the National Institute for Health and Care Excellence (NICE, 2019) [4] and the American College of Obstetricians and Gynecologists (ACOG, 2019) [5]. The presence of one of the risk factors was considered in the NICE recommendations; and risk factors were divided into predictors of moderate and high risk in the ACOG recommendations.
The use of clinical risk factors for predicting PE does not require financial expenses and is easy to use. However, only when the risk factors being considered, the frequency of false positive results can reach 64.2% [5]. Moreover, their introduction into clinical practice did not reduce the frequency of severe forms of PE and its role in maternal mortality.
In order to identify more precisely the risk of developing PE, the Fetal Medicine Foundation (FMF) under the guidance of Kypros Nicolaides has developed a scheme for prenatal screening based on a set of clinical data, indicators of average blood pressure, pulsation index (PI) in the uterine arteries (UA), the level of pregnancy-associated plasma protein A (PAPP-A) and placental growth factor (PLGF) in maternal blood. This scheme was tested on 60,000 pregnant women [6].
Nowadays, risk factors of PE can be determined at 11-14 weeks’ gestation during the 1st prenatal screening. This time, early-onset (up to 34 weeks) and late-onset (up to 37 weeks) PE can be predicted. The risk of PE is calculated automatically, according to the Astraia program and taking into account the data on race, smoking, methods of conception, patient’s age, chronic arterial hypertension (CAH), type I and II diabetes mellitus, antiphospholipid syndrome and hereditary thrombophilia, a history of preterm birth ≥ 24 weeks, a history of PE of the patient’s mother, body mass index, blood pressure (BP), biochemistry indicators (PAPP-A, PLGF), PI in the UA.
The principles of PE prevention were developed on the basis of the identified risk factors for PE at 11–14 weeks’ gestation. [6]. For this purpose, a randomized placebo-controlled study, ASPRE (aspirin for evidence-based preeclampsia prevention trial), was conducted. The study included more than 30,000 pregnant women in the UK, Belgium, Italy, Spain, Greece, and Israel. The Russian Federation did not participate in the study [7].
The induction of interleukin (IL)-3 can be considered as one of the possible mechanisms of action of acetylsalicylic acid. IL-3 is an important humoral factor of placental growth and development. In addition, there is evidence of the effect of acetylsalicylic acid on the processes of apoptosis in the trophoblast, which may determine the effect of these drugs for the prevention of pregnancy complications associated with impaired cytotrophoblast migration [8]. At different stages, researchers recommended different doses of acetylsalicylic acid, namely from 50 to 162 mg per day [6]. According to the results of the study, taking acetylsalicylic acid at a dose of 150 mg per day in the evening (before going to bed), during the period from 12–16 weeks’ gestation till 36 weeks’ gestation is the most effective.
Increasing the dose to 150–162 mg per day is due to less resistance of patients to high doses of acetylsalicylic acid [9–11].
Since 2011, Russia has launched a prenatal screening system: the calculation of the risk for developing PE before 34 and 37 weeks of pregnancy was carried out at 11–14 weeks’ gestation, along with traditional ultrasound and combined screening for chromosomal abnormalities in the fetus at the prenatal screening department according to the Astraia program. There are few known studies based on the results of the screening. The data presented by M.V. Medvedev and co-authors (2018) showed positive results in reducing the frequency of PE and fetal growth retardation when implementing the Astraia program in clinical practice in Moscow [12].
The aim of the study is to analyze the prediction and prevention of PE using prenatal screening performed in the City Center for Family Planning and Reproduction.
Materials and Methods
This was a retrospective analysis of the histories of 2374 pregnant patients who were examined and screened prenatally in 2017–2019 in the City Center for Family Planning and Reproduction, Moscow, Russia.
Taking into account the probability of developing PE calculated by the Astraia program, patients were identified as high and low risk groups for this disease. The threshold value for inclusion in a high-risk group for PE was considered as the probability ≤1:100. The study identified 209 (8.8%) patients at high-risk group including 21 (10.0%) women who subsequently developed PE.
Additionally, 102 patients with a low risk for pre-eclampsia and 18 pregnant women who had severe pre-eclampsia at different gestation periods were identified.
Of the 209 pregnant women in the risk group, 175 (83.7%) patients took preventive measures: 92 (52.6%) women took acetylsalicylic acid, among which 12 (13%) women developed PE: moderate – 8 (66.7%), severe – 4 (33.3%).
All patients who took acetylsalicylic acid signed an informed consent to take it at a dose of 150 mg from 12 to 36 weeks in accordance with Order No. 572n.
Dipyridamole1 (75 mg) was given to 55 patients (31.4%), one of them (1.8%) had moderate PE.
Taking into account hereditary thrombophilia and hypercoagulation, 28 women (16%) were administered enoxaparin sodium (0.4 mg/day) simultaneously with acetylsalicylic acid at doses of 100 mg per day (n=16) or dipyridamole at a dose of 75 mg per day (n=12). Moderate PE developed in 4 (14.3%) patients.
There were 34 pregnant women (16.3%) who refused to take medications, among them 4 patients (11.8%) had symptoms of the disease; all the women subsequently developed moderate PE.
The patients were examined in the antenatal clinic in accordance with Order No. 572n.
The biochemical parameters (PAPP-A, PLGF) were determined using DELFIA Xpress analyzer (Perkin Elmer). Ultrasound tests and determining PI in the UA were performed on the Voluson E8 (GE) ultrasound devices according to the rules of FMF2.
Statistical processing of materials was performed using the SPSS Statistics 25 and Excel software package.
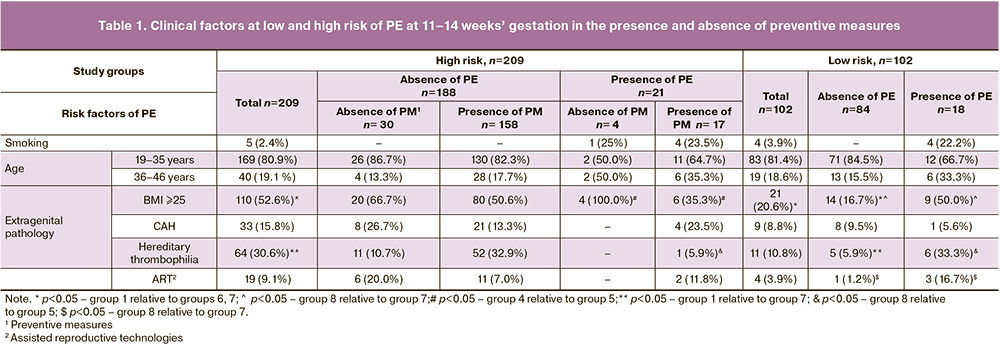
The differences between the groups were analyzed using the Pearson Chi-square test, which was used to compare two relative indicators that characterize the frequency of a certain category with two values (clinical factors are presented in Table 1).
The Fisher criterion was used for analyzing fourfold tables in case if values of the expected phenomenon are less than 5, which could be a restriction for applying the Pearson χ2 criterion. The data were considered statistically significant at the level of p<0.05.
The Mann-Whitney U test was used to compare nonparametric features.
To describe quantitative data (the distribution of all presented quantitative data was different from normal), median indicators were used with quartiles in the format (Me; [Q1;Q3]). The risk for developing PE, calculated by the Astraia program, is given in the format 1:n, which means that PE can develop in one patient out of n people.
ROC analysis was used for determining PI in the UA and PAPP-A in the calculations in order to show the dependence of the proportion of correctly classified positive examples on the proportion of incorrectly classified negative examples. The area under the ROC curve is marked as AUC.
Results and Discussion
The study revealed 209 (8.8%) women at high risk for preeclampsia out of 2374 patients who were examined from 2017 to 2019, the probability of developing PE varied from 1:100 to 1:4.
The analysis of the data (Table 2) showed that only 2 (1.0%) patients had a risk of early-onset PE, while 128 (61.2%) women had a risk of late-onset PE. There were 79 patients (37.8%) at risk for both early-onset and late-onset disease.
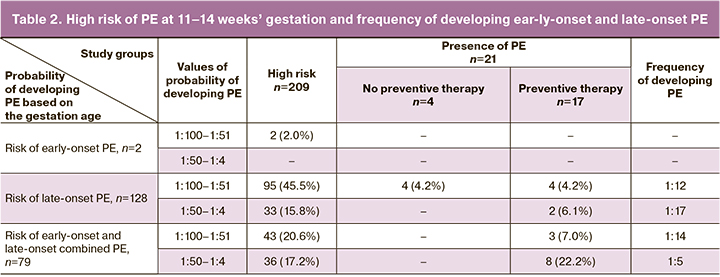
The risk of an early form of the disease with a probability of 1:100–1:51 was observed in two women, however, no one developed PE.
The risk of a late form of the disease with a probability of 1:100–1:51 was revealed in 95 patients (45.5%); PE was observed in eight patients (8.4%), and one woman developed early-onset PE of a severe degree. A high probability of predicting (1:50– 1:4) late-onset PE was observed in 33 women (15.8%). Two patients among them had a late form of the disease.
The probability of developing PE was 1:100–1:51 in 43 women (20.6%) among the patients with a combined risk of early-onset and late-onset PE; after 37 weeks, PE developed in three of them. Risks of developing PE more than 1:50 were observed in 36 patients (17.2%), two of them subsequently developed earlyonset PE, and six women had late-onset PE.
The analysis of the prognosis revealed that the frequency of developing PE in each group ranged from 1:17 to 1:5.
In general, 17 women (81.0%) had moderate PE and 4 women (19.0%) had severe PE.
The results of the study indicated that the frequency of PE in the group of patients at risk was 10.0% (n=21). If preventive measures were not taken, moderate PE was observed in 4 women, its frequency was 11.8%; when preventive measures were taken, the disease occurred in 17 patients (9.7%), including 13 women with moderate PE and 4 women with severe PE, p=0.72. The frequency of PE in general population reaches 5–12%.
The characteristic of clinical indicators that determine the presence or absence of the risk of PE at 11–14 weeks’ gestation and its subsequent development in the absence of preventive measures is of great interest (Table 1).
All the examined patients were of Caucasian race. None of them had type I or II diabetes mellitus and preterm births ≥ 24 weeks; the next of kin did not have a history of PE.
Among high-risk patients, five women (2.4%) were smokers, and all of them developed PE. Among patients with PE with a low risk of its development, every fourth patient smoked.
The data in the literature show that the age of 36-40 years and older is one of the risk factors for PE both in our country and abroad (ACOG-2019). According to the U.S. Preventive Services Task Force (USPSTF, 2013), it is recommended to consider women at the age of ≥35 years and with at least one additional clinical risk factor as a high-risk patient for this disease [5].
When analyzing the data of patients (in groups of high and low risk of the disease, with the presence and absence of PE), it was found that patients aged 36-46 years, both in general risk group and in the absence of risk, were observed in 19.1% and 18.6% of cases, respectively. At the same time, patients with PE were older than 36 years in 33.3-38% of cases, regardless of the presence or absence of risk of disease at 11-14 weeks. There was no statistically significant difference in the age of patients who agreed to preventive therapy and those who refused it (Table 1).
According to the numerous data of the Russian and foreign studies (NICE, ACOG), extragenital pathology is an important risk factor for PE. The data presented in Table 2 show that among various types of extragenital pathology the impairment of fat metabolism (BMI > 25 kg/m2) is an essential factor for both inclusion in the high-risk group of the disease and in the presence of PE. Regardless of the presence or absence of PE, the number of patients with BMI ≥25 at high risk group is twice higher as at low risk group (p<0.001). Among pregnant women with PE and a low risk of its development, the impairment of fat metabolism was observed in half of the examined women; while it was revealed only in 16.7% of women with a low risk or absence of PE (p=0.003). It is noteworthy that the impairment of fat metabolism in patients with PE who refused to take preventive measures (100%) was detected more often than in patients with PE (35.3%) (p=0.02).
The patients suffering from CAH were also included in a high risk group for PE [12].
The incidence of CAH in the high-risk group (15.8%) was 3–4 times higher than in other pregnant women (4–5%).
In high-risk patients, there was no significant difference in frequency of CAH in patients with subsequent development of PE (19.0%) and in the absence of the disease (15.4%) (p=0.67). Relatively low frequency of CAH was observed in patients with a low risk of the disease, both in the presence of PE (5.6%) and in its absence (9.5%).
The frequency of CAH did not differ in patients who agreed and refused to take preventive measures.
In the high-risk group, the frequency of hereditary thrombophilia was 5 times higher than in the low-risk group with the absence of PE (p<0.001). It should be noted that high-risk patients who agreed to take preventive measures showed a higher frequency of hereditary thrombophilia, it was 32.9%. It is possible that the use of low-molecular-weight heparin contributed to the fact that PE developed only in one woman (5.9%). This is confirmed by the fact that this pathology was 7 times more common (6–33.3%) in patients with low risk and the presence of PE than in the group of women with high risk (p=0.021). Apparently, the lack of preventive measures in this situation contributed to the progression of hemodynamic disorders and the development of PE.
Pregnancy of patients from a high-risk group which occurred due to the use of assisted reproductive technologies was twice as common as in patients from a low-risk group (Table 1).
Thus, the analysis of clinical risk factors indicated that their diversity leads to both overdiagnosis and underestimation of the possibility of developing the disease. Data analysis showed that predicting PE at 11–14 weeks’ gestation based on the patient’s case history and extragenital pathology is a difficult task and less effective without additional studies.
According to the NICE algorithm [4] based only on the case history and the frequency of extragenital diseases, less than 40% of PE is predicted; according to the ACOG algorithm, about 67% can be predicted. The development of PE is more likely to be predicted using instrumental examination methods and laboratory tests.
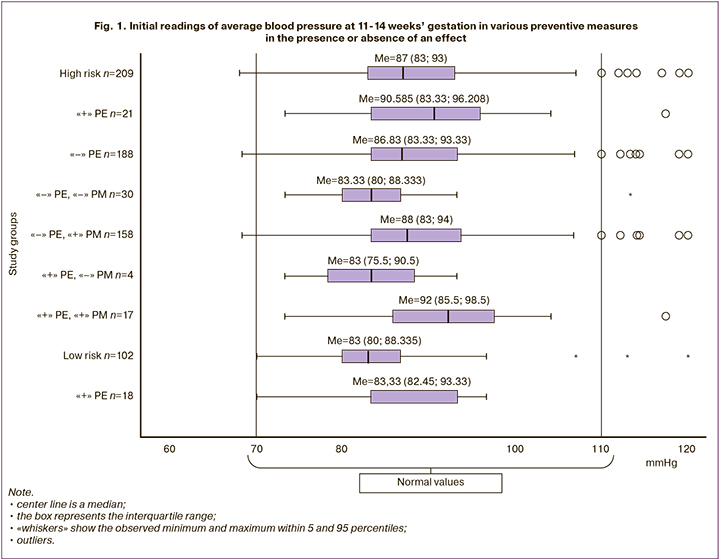
The readings of systolic blood pressure in patients who were included in the high-risk group (Me=87 [83;93]) did not differ from those at low risk (Me=83 [80;88]), regardless of the outcome, the presence or absence of preventive measures (p=0.91) (Fig. 1). In an individual analysis, it was noted that pathological readings of systolic blood pressure (>110 mmHg)3 were only in nine patients (4.3%); one (11.1%) of them developed moderate PE. The sensitivity and specificity of this indicator for predicting PE were 55.0% and 60.4%, respectively. It can be assumed that the obtained readings of systolic blood pressure are related to the fact that all patients with arterial hypertension received antihypertensive therapy, after which the blood pressure readings were normal.
According to O’Gorman et al. [13], the FMF algorithm for predicting PE, including the additional study of plasma proteins and the data of Doppler assessment of the UA in the first trimester, detects >90% of early-onset PE and 75% of PE up to 37 weeks with approximately 10% of positive results.
Of particular importance is the study of the plasma proteome, which is essential in the pathogenesis of PE: PAPP-A, PLGF.
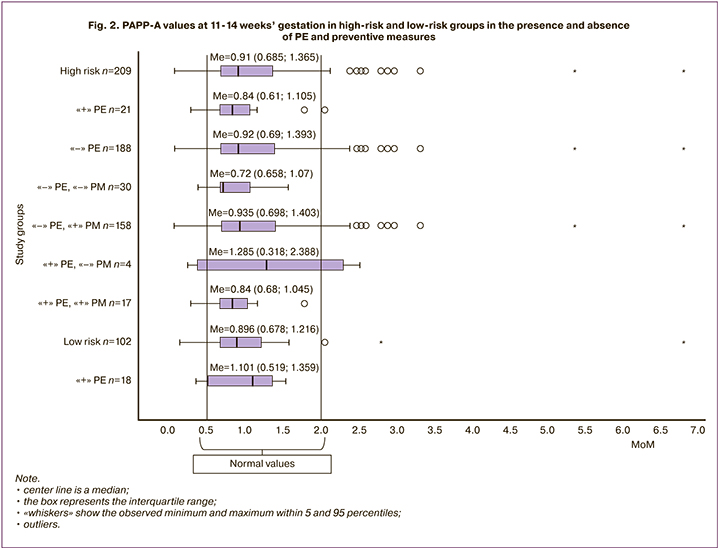
In our study, the values of PAPP-A (Me=0.910 [0.69;1.37]) in patients of a high-risk group did not differ from those at low risk (Me=0.896 [0.678;1.216]) (p=0.76). The individual analysis revealed that low values of РАРРА-А<0.5 MoM (multiples of median) at a high-risk group were only in 29 women (14.1%), at a low-risk group they were in 2 women (11.1%). PE developed in 2 patients (6.9%) at a high-risk group and in all women at a low-risk group (Fig. 2).
In order to determine the diagnostic significance of PAPP-A, a ROC analysis was performed (Fig. 3). The area under the curve was 0.430, which indicated a low prognostic significance of the indicator: sensitivity 67%, specificity 65%, which did not differ from the clinical data.
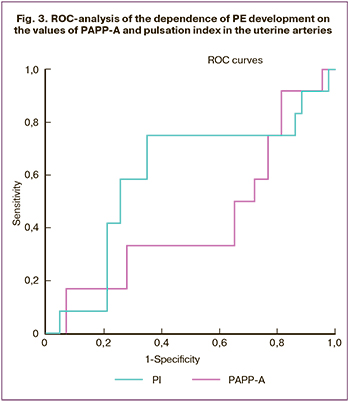 It should be noted that the earlier studies indicated a high significance of PAPP-A in predicting PE. A number of researchers [14] have shown a reduced level of PAPP-A at 11–14 weeks’ gestation in pregnant women who developed PE of varying severity in the third trimester. It was concluded that a decrease in the level of this protein at the preclinical stage of PE development can be used as a predictor of this disease.
It should be noted that the earlier studies indicated a high significance of PAPP-A in predicting PE. A number of researchers [14] have shown a reduced level of PAPP-A at 11–14 weeks’ gestation in pregnant women who developed PE of varying severity in the third trimester. It was concluded that a decrease in the level of this protein at the preclinical stage of PE development can be used as a predictor of this disease.
Later, there were more discreet conclusions about the significance of this protein for the prognosis of PE. According to K. Nicolaides [15], the detection rate of the disease was 68% if there was screening on the basis of clinical history, extragenital pathology, and PI in the UA; when PAPP-A was added to the algorithm, the indicator did not change.
The study of PLGF showed that in patients with high risk (Me=0.50 [0.3;0.72]), its values are 1.5 times lower, compared with those at low risk (Me=0.73 [0.572;0.972]) (p=0.81). The data obtained by us confirm the results of a study by M.V. Medvedev and co-authors [12] that low PLGF values contribute to the inclusion of patients in the high-risk group for the development of PE (Fig. 4). When analyzing the initial values of PLGF at 11-14 weeks’ gestation, no statistically significant differences were found in patients of a high-risk group in the presence of PE (Me=0.51 [0.375;0.830]) and absence of PE (IU=0.50 [0.298;0.720]) (p=0.67).
According to L. Poon, R. Napolitano et al. [16], numerical values of PI in the UA in the first trimester in the group of patients with early-onset and late-onset PE are higher than in patients with normal pregnancy (p<0.0001). Combined screening for PE, including maternal risk factors and Dopplerometry assessment in the UA, had a higher sensitivity (80%) for early-onset PE than for late-onset PE (45%), with a false-positive rate of 10% [16].
The results of our study showed that the values of PI (Me=1.346 [1.140;1.510]) in patients with a high risk of PE were 1.3 times higher than in patients with a low risk (Me=1.015 [0.828;1.205]) (p=0.68), which indicates the initial disorders of blood flow in the UA which can be associated with the development of PE (Fig. 5).
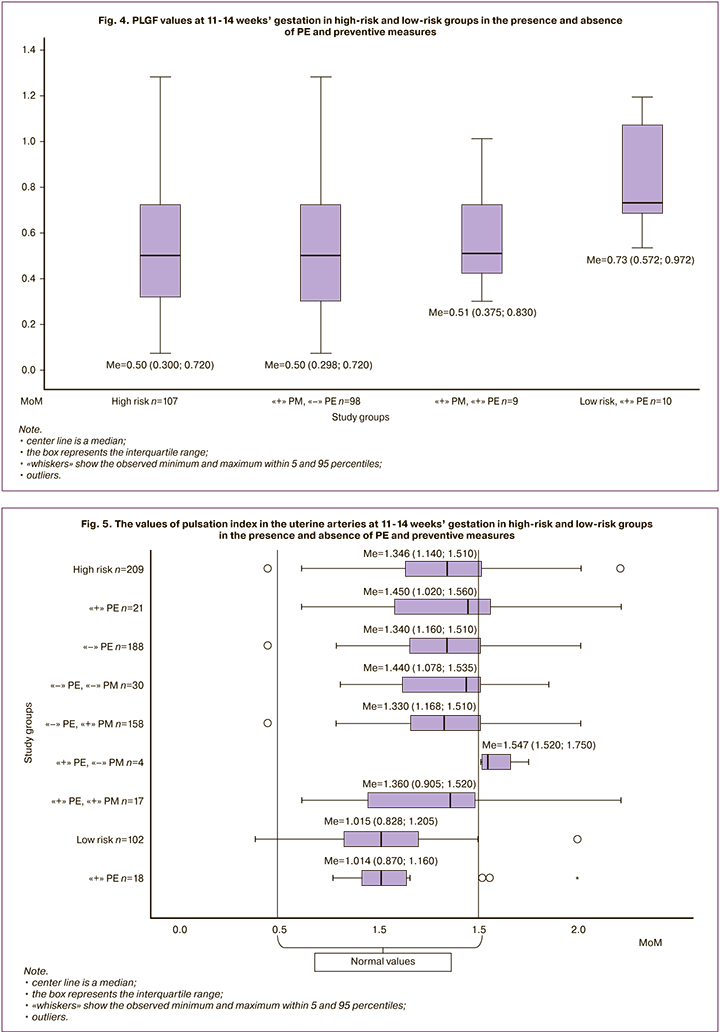
It can be assumed that low values of PI in the UA of patients with a low risk of PE and its development (Me=1.014 [0.870;1.160]) were the reason for their inclusion in the group of healthy pregnant women.
PI indicators (Me=1.450 [1.020;1.560]) in patients of the high-risk group with PE did not differ significantly from those in the patients without the disease (Me=1.344 [1.160;1.510]).
When analyzing PI, the significance of preventive measures should be noted. In the patients of a high-risk group with PE who refused to take preventive therapy, the initial values of PI were the highest (Me=1.547 [1.520;1.750]). It is possible that preventive measures in the patients with high PI values could prevent the development of PE.
When determining the diagnostic value of PI using ROC analysis, it was found (Fig. 3) that this indicator had an average prognostic significance (AUC=0.600, sensitivity 75%, specificity 65%).
Therefore, the study showed that the incidence of PE was 12.5% in case of hemodynamic disorders in the UA, which means that the disease developed in one patient out of eight; if such patients were absent, the incidence of the disease decreased by 1.4 times and amounted to 9.1% (1:11). The changes in plasma proteins allowed us to identify a risk group in which PE develops with a probability of 6.7% (1:15), that is, the values of PI in the UA were more prognostically significant than the indicators of protein markers.
Conclusion
Thus, the analysis of literature data and conducted research showed that the prediction and prevention of PE is important. According to our data, the criteria proposed for predicting PE are not absolute and have been finalized.
Due to the polymorphic pathogenesis and clinical course of PE, prediction methods should include clinical parameters and a set of indicators suggestive of the initial stages of disease development: changes in placenta formation (PAPP-A, PLGF, sFlt-1), the function of the uterine vascular system, primarily PI, which has a high specificity and sensitivity, both according to literature data and our research. In this case, PI characterizes not only the blood flow in the uterus, but also reflects the parameters of macrohemodynamics and, in particular, the vascular wall stiffness that determines blood circulation in the organ.
Despite numerous studies, methods of PE prevention have not been completely developed. It is more appropriate to use a preventive complex including general hygiene measures, diet, control of blood pressure, treatment of extragenital pathology, and administration of medications depending on the detected changes after additional research and optimization of diagnostic methods.
References
- Адамян Л.В., Артымук Н.В., Башмакова Н.В., Белокринницкая Т.У., Беломестнов С.Р., Братищев И.В., Вученович Ю.Д., Краснопольский В.И., Куликов А.В., Левит А.Л., Петрухин В.А., Пырегов А.В., Серов В.Н., Сидорова И.С., Филиппов О.С., Ходжаева З.С., Холин А.М., Шешко Е.Л., Шифман Е.М., Шмаков Р.Г. Гипертензивные расстройства во время беременноти, в родах и послеродовом периоде. Преэклампсия, эклампсия. Клинические рекомендации. М.; 2016. [Adamyan L.V., Artimuk N.V., Shifman E.M. Hypertensive disorders during pregnancy, health outcomes and postpartum period. Preeclampsia, eclampsia. Clinical recommendations. 2016. (in Russian)].
- Шалина Р.И., Коновалова О.В., Нормантович Т.О. Возможности прогнозирования и профилактики гестоза в I триместре беременности. Практическая медицина. 2010; 4: 38-43. [Shalina R.I., Konovalova O.V., Normantovich T.O. The possibilities of prediction and prevention of preeclampsia in the I trimester of pregnancy. Prakticheskaya meditsina / Practical Medicine, 2010; 4: 38-43. (in Russian)].
- Шалина Р.И., Коновалова О.В., Нормантович Т.О., Лебедев Е.В. Прогнозирование гестоза в первом триместре беременности: миф или реальность? Вопросы гинекологии, акушерства и перинатологии. 2010; 9(4): 82-7. [Shalina R.I., Konovalova O.V., Normantovich T.O., Lebedev E.V. Prognostication of gestosis in the first trimester of gestation: myth or reality? Vopr. ginekol. akus. perinatol. / Gynecology, Obstetrics and Perinatology. 2010; 9(4): 82-7. (in Russian)].
- National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management. NICE guideline. 25 June 2019. Available at: www.nice.org.uk/guidance/ng133.
- ACOG Practice Bulletin No. 202: Gestational hypertension and preeclampsia. Obstet. Gynecol. 2019; 133(1): e1-25. https//dx.doi.org/10.1097/AOG.0000000000003018.
- Poon L.C., Stratieva V., Piras S., Piri S., Nicolaides K.H. Hypertensive disorders in pregnancy: combined screening by uterine artery Doppler, blood pressure and serum PAPP-A at 11–13 weeks. Prenat. Diagn. 2010; 30(3): 216-23. https//dx.doi.org/10.1002/pd.2440.
- O'Gorman N., Wright D., Poon L.C., Rolnik D.L., Syngelaki A., de Al-varado M. et al. Multicenter screening for pre-eclampsia by maternal factors and biomarkers at 11-13 weeks' gestation: comparison with NICE guidelines and ACOG recommendations. Ultrasound Obstet. Gynecol. 2017; 49(6): 756-60. https//dx.doi.org/10.1002/uog.17455.
- Bose P., Black S., Kadyrov M., Weissenborn U., Neulen J., Regan L. et al. Heparin and aspirin attenuate placental apoptosis in vitro: implications for early pregnancy failure. Am. J. Obstet. Gynecol..2005; 192(1): 23-30.
- Navaratnam K., Alfirevic A., Jorgensen A, Alfirevic Z. Aspirin non-responsiveness in pregnant women at high-risk of pre-eclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018; 221: 144-50. https://dx.doi.org/10.1016/j.ejogrb.2017.12.052.
- Wójtowicz A., Undas A., Huras H., Musiał J., Rytlewski K., Reroń A. et al. Aspirin resistance may be associated with adverse pregnancy outcomes. Neuroendocrinol. Lett. 2011; 32(3): 334-9 .
- Rolnik D.L., Wright D., Poon L.C., O'Gorman N., Syngelaki A., de Paco Matallana C. et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N. Engl. J. Med. 2017; 377(7): 613-22. https//dx.doi.org/10.1056/NEJMoa1704559.
- Медведев М.В., Алтынник Н.А., Князев П.В. Прогноз и предупреждение преэклампсии и замедления роста плода в 11–14 недель беременности: анализ 1001 наблюдения. Пренатальная диагностика. 2018; 17(3): 261-6. [Medvedev M.V., Altynnik N.A., Knyazev P.V. Prediction and prevention of preeclampsia and fetal growth restriction at 11-14 weeks of gestation: analysis of 1001 cases. Prenat. Diag. 2018; 17(3): 261-6. (in Russian)]. https//dx.doi.org/10.21516/2413-1458-2018-17-3-261-266.
- O'Gorman N., Wright D., Syngelaki A., Akolekar R., Wright A., Poon L.C. et al. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11-13 weeks gestation. Am. J. Obstet. Gynecol. 2016; 214(1): 103. e1-103. e12. https//dx.doi.org/10.1016/j.ajog.2015.08.034.
- Макацария А.Д., Бреннер Б., Бицадзе В.О., Акиньшина С.В. Системный венозный и артериальный тромбоэмболизм в акушерско-гинекологической практике. М.: МИА; 2016: 283-4. [Makatsaria A.D., Brenner B., Bitsadze V.O., Akinshina S.V. Systemic venous and arterial thromboembolism in obstetric and gynecological practice. 2016: 283-4. (in Russian)].
- First Trimester Prediction and Prevention of Preterm Preeclampsia. A transcript of Professor Kypros Nicolaides’s webcast, broadcast on April 24th 2018.
- Poon L.C.Y., Staboulidou I., Maiz N., Plasencia W. Hypertensive disorders in pregnancy: screening by uterine artery Doppler at 11-13 weeks. Ultrasound Obstet. Gynecol. 2009; 34(5): 142-8. https//dx.doi.org/10.1002/uog.7439.
Received 24.03.2020
Accepted 15.06.2020
About the Authors
Raisa I. Shalina, Doctor of Medical Sciences, professor, Professor of the Department of Obstetrics and Gynecology, Pediatric Faculty, N.I. Pirogov Russian National Research Medical University. Tel.: +7(495)718-34-72. E-mail: raisa.shalina@gmail.com. ORCID: 0000-0001-7121-1663.117209, Russia, Moscow, Sevastopol Ave., 24 “A”.
Nigyar K. Kasum-zade, obstetrician-gynecologist, postgraduate Department Obstetrics and Gynecology, Pediatric Faculty, N.I. Pirogov Russian National Research Medical University. Tel: +7(910)456-34-47. E-mail: nk260590@gmail.com. ORCID: 0000-0002-9607-9870.
117209, Russia, Moscow, Sevastopol Ave., 24 “A”.
Aleksandr G. Konoplyannikov, Doctor of Medical Sciences, Professor, Department of Obstetrics and Gynecology, N.I. Pirogov Russian National Research Medical University. Tel.:+7(499)723-04-20. E-mail: npo.med@gmail.com. ORCID: 0000-0001-9923-8833.
117997, Russia, Moscow, Ostrovityanova str., 1.
Oleg A. Latishkevich, Head physician of GBUZ “CPS” DZM, PhD, assistant of the Department of Obstetrics and Gynecology, Pediatric Faculty, N.I. Pirogov Russian National Research Medical University. Tel: +7(495)718-20-70. E-mail: latishkevich@mail.ru.
117209, Russia, Moscow, Sevastopol Ave., 24 “A”.
Dmitriy B. Shehovtsov, obstetrician-gynecologist of GBUZ “CPS” DZM. Tel: +7(963)979-04-26. E-mail: dr.dbs@yandex.ru.
117209, Russia, Moscow, Sevastopol Ave., 24 “A”.
For citation: Shalina R.I., Kasum-zade N.K., Konoplyannikov A.G., Latyshkevich O.A., Shekhovtsov D.B. Prediction and prevention of preeclampsia in the city Center for family planning and reproduction.
Akusherstvo i Ginekologiya / Obstetrics and gynecology. 2020; 7: 61-70 (in Russian).
https://dx.doi.org/10.18565/aig.2020.7.61-70



