Predictive value of autoimmune markers in assisted reproductive technologies
To improve the effectiveness of assisted reproductive technologies (ART), it is important to investigate possible risk factors, including autoantibodies of different specificity, associated with ART failure. Aim. To investigate autoantibody profiles and their value as predictors of ART outcomes in infertile women. Materials and methods. The study included 65 infertile patients before ovarian stimulation in ART and 48 fertile women. Using an enzyme-linked immunosorbent assay (ELISA), they were tested for serum antiphospholipid antibodies (aPL), antibodies to human chorionic gonadotropin, progesterone (aPG), and estradiol (aE). Results. Among infertile patients, 15.4% had aPLs, and 47.7–56.9% had antibodies (M, G) to hormones (P≤0.0001). The implantation rate in patients seropositive for IgM aPG and aE was lower (7.1%; 6.25%) than in seronegative (37.3%; 38.8%) (P <0.05). Testing for these antibodies has shown high sensitivity and specificity with the AUC of 0.722 and 0.725 and the test accuracy of 71.4%. Conclusion. In infertile patients, serum autoantibodies to steroid hormones are risk factors for ART failure and may have a role in predicting outcomes.Menzhinskaya I.V., Kraevaya E.E., Kalinina E.A., Vanko L.V., Dolgushina N.V.
Keywords
Currently, the annual infertility rate is estimated as 9% worldwide, and 8 to 20 % of infertility cases are labeled as unexplained or idiopathic. These facts highlight the need for advances in reproductive medicine and the widespread use of new technologies to improve infertility treatment effectiveness, including assisted reproductive technologies (ART) [1]. According to the Russian Association of Human Reproduction, in 2016, Russian ART centers performed more than 120,000 ART cycles, i.e., 839 cycles per million population [2]. At the same time, the pregnancy rate in the in vitro fertilization (IVF) program reached 34.8% per cycle and 38.7% per embryo transfer cycle. In frozen-thawed embryo transfer programs, these figures were 38.5% and 40.0%, respectively.
Causes of ART failures associated with ovarian stimulation, the quality of the obtained embryos, embryo transfer technique into the uterine cavity can be corrected in subsequent ART programs [3]. However, approximately 30% of married couples receiving this treatment have repeated ART cycles' failures [4]. Fertilization failure can be caused by implantation losses, in particular, due to various endometrial disorders, impaired endometrial receptivity, and inadequate interaction between the embryo and uterine epithelium [5, 6].
Dysregulation of the immune system and increased autoantibody production are considered as possible reasons for unexplained infertility and ART failure. Moreover, repeat failed ART cycles are associated with the presence of autoantibodies of different specificities, such as including thyroid autoantibodies, antiphospholipid autoantibodies, antinuclear autoantibodies, antisperm, antiendometrial, antiovarial, antibodies to the pellucid zone, antitrophoblastic, and anti-smooth muscle autoantibodies [1, 7]. However, there are conflicting data regarding the effect of specific antibodies and the immune system's general activation on the preimplantation embryo, implantation process, or placenta formation.
Of particular note are reports on the association between antibodies to gonadotropic hormones and ovarian autoimmunity, endometriosis, and polycystic ovary syndrome, leading to infertility and ART failure [8, 9]. Anti-gonadotropin antibodies have been detected in women using human menopausal gonadotropins and human chorionic gonadotropin (hCG) for ovarian stimulation and ovulation induction. It has been assumed that anti-gonadotropin antibodies can modify the response to hormonal therapy and have an additional value in predicting ovarian stimulation outcomes [8].
Studies of hypersensitivity to steroidal sex hormones, which can have cutaneous, respiratory, and systemic manifestations, as well as the production of polyclonal antibodies to steroid hormones in women with reproductive dysfunctions, are of great scientific interest [10–13].
Immune tolerance to steroid hormones can be impaired when the level of endogenous hormones increases, for example, during pregnancy or in the luteal phase of the menstrual cycle and under the influence of exogenous exposure to hormonal drugs, contraceptives, or environmental estrogens [10]. Progesterone (PG) is widely used to support the luteal phase in natural and induced cycles and during pregnancy to prevent and treat complications such as threatened miscarriage and threatened preterm birth [14, 15].
Progesterone hypersensitivity in women has been reported after progestin-only oral contraceptives, infertility treatment, and ART programs [12, 16]. Hypersensitivity to steroidal sex hormones can be detected by skin testing in premenstrual syndrome, recurrent miscarriage. It is associated with adverse pregnancy outcomes, early reproductive loss, and subsequent secondary infertility [10, 17, 18]. The use of desensitization therapy effectively relieves the symptoms of premenstrual syndrome, promotes pregnancy onset in ART, and increases the chances of a successful pregnancy outcome in patients with recurrent spontaneous miscarriage [17, 19, 20].
Polyclonal antibodies directed against steroidal sex hormones and gonadotropins were found using enzyme-linked immunosorbent assay (ELISA) in women with premenstrual syndrome, infertility, and recurrent miscarriage [13]. In women with infertility and failed IVF attempts, a high detection rate of antibodies to hormones is shown in blood serum and follicular fluid [21]. Antibodies directed against hormones are considered a possible risk factor for IVF failure.
Given all of the above, the present study aimed to investigate autoantibody profiles and their value as predictors of ART outcomes in infertile women.
Materials and methods
The study included 65 women undergoing infertility treatment with ART at the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia (group 1). The mean age of patients was 33.5 (3.9) years.
The inclusion criteria were normal karyotype of husbands, age of women 18–40, body mass index 18–29.9 kg/m2, and normal ovarian response to ovarian stimulation. Exclusion criteria were contraindications to ART, pathospermia in men, use of donor gametes, surrogacy, reduced ovarian reserve, inadequate response of the ovaries to stimulation, and ART complications.
The women were examined according to the Order of the Ministry of Health of Russia dated 30.08.2012 No. 107n “On the Procedure for Using Assisted Reproductive Technologies, Contraindications and Restrictions on Their Use”. In ART programs, ovarian stimulation was performed using the recombinant follicle-stimulating hormone, a combination of follicle-stimulating and luteinizing hormone, gonadotropin-releasing hormone antagonists, and hCG. The transvaginal ovarian puncture was performed 36 hours after hCG administration to trigger ovulation.
According to sperm analysis, 26 (40%), 24 (36.9%), and 15 (23.1%) partners had normozoospermia, teratozoospermia, and asthenozoospermia, respectively. In 58 (89.2%) couples, oocyte fertilization was carried out by intracytoplasmic sperm injection into the oocyte due to the husband's subfertile sperm and low fertilization rate in the previous IVF. Only 7 (10.8%) couples underwent IVF for oocyte fertilization.
On day five after transvaginal puncture, the assessment of the embryos was made according to the Gardner grading system [22] followed by the transfer of one good or excellent quality blastocyst of into the uterine cavity. The post-transfer period was maintained using a standardized protocol. Pregnancy onset was diagnosed by β-human chorionic gonadotropin (β-hCG) level on day 14 after the embryo transfer and ultrasonography on day 21.
The comparison group (group 2) included healthy fertile women (n=48, mean age 32.2 (3.8) years) who had not previously received hormonal drugs and contraceptives.
The spectrum of serum antiphospholipid antibodies (aPL), anti-laminin-1 antibodies, and antibodies against hCG (ahCG), PG (aPG), estradiol (aE) were analyzed using ELISA. Antibody testing was performed before ovarian stimulation. Antibodies of classes M and G directed against cardiolipin (aCL), β2-glycoprotein-I (aβ2GPI), phosphatidylserine (aPS), phosphatidylethanolamine (aPE), annexin V (aANVAs), phosphatidylserine/prothrombin complex (aGPS-PT), and laminin-1 were determined using quantitative enzyme immunoassays (ORGENTEC Diagnostika and IBL International GmbH, Germany). Antibodies against hormones of M and G classes were determined according to the previously described modifications of ELISA, in which hCG conjugates of progesterone 3-(O-carboxy-methyl) oxime-BSA and β-estradiol 6-(O-carboxy-methyl) oxime were used BSA (Sigma-Aldrich, United States) [23, 24]. Optical density (OD) was measured on a MULTISKAN EX photometer (Thermo Electron (Shanghai) Instrument Co., China).
Statistical analysis
Statistical analysis was performed using Microsoft Office Excel 2010 and MedCalc (v.12) statistical software. The distribution of continuous variables was tested for normality using the Kolmogorov-Smirnov test and the Shapiro–Wilk W-test. Quantitative variables showing normal distribution were expressed as means (M) and standard deviation (SD) and presented as M (SD) ); otherwise, the median (Me) with a 95% confidence interval (95% CI) and the quartiles Q1 and Q3 were reported. Categorical variables were compared with the χ2 test, continuous variables with the Mann– Whitney U-test. Correlation analysis was conducted by calculating Spearman's rank correlation coefficients. The diagnostic accuracy of antibody testing was analyzed based on the receiver-operating characteristic (ROC) curve construction. Differences were considered statistically significant at P <0.05.
Results and discussion
In group 1, 38 (58.5%) and 27 women (41.3%) had secondary and primary infertility, respectively (P=0.05); the mean infertility duration of was 6.0±3.5 years. Thirty-seven (56.9%) women were multipara. There were high rates of past ectopic pregnancies (26.2%) and spontaneous miscarriages (33.8%); however, only 15.4% of pregnancies ended in full-term deliveries. More than half of the patients (56.9%) had previous IVF attempts (from 1 to 8); 43.1% of women had no history of ART treatment. In 24.6% of cases, pregnancy occurred in the IVF cycle.
Among gynecological pathology, the most prevalent were infectious and inflammatory pelvic diseases, including chronic salpingo-oophoritis (52.3%), chronic endometritis (21.5%), and endometrial polyps (29.2%). Some patients had genital endometriosis (20%), adenomyosis (10.8%), uterine fibroids (12.3%), and PCOS (16.9%), as well as a history of previous surgeries (tubectomy (24.6%), myomectomy (7.7%) or endometriotic ovarian cyst resection (13.8%)).
Among somatic comorbidities there were chronic gastrointestinal and urinary diseases [12 (18.5%) and 13 (20%) women, respectively], lower limb varicose veins [17 (26.2%)], allergic diseases [9 (13.8%)], upper respiratory tract diseases [9 (13.8%)], endocrine disorders [7 (10.8%)], a history of thromboembolic complications, mainly in the form of lower limb vein thrombosis [in 3 (4.6%)].
 Ten (15.4%) patients with infertility were found to have antiphospholipid antibodies (aPLs). aPEs were detected in 7 (10.8%) patients, with IgM aPE in 5 (7.7%) and IgG aPE in 2 (3.1%) patients. Other aPLs such as IgG aβ2-GP-1, IgM aCL, and IgG aPS/PT were found in one patient each. Antibodies against PE were found significantly more often than aPL of different specificity (P=0.03). No IgG antibodies to laminin-1 were detected in the patients.
Ten (15.4%) patients with infertility were found to have antiphospholipid antibodies (aPLs). aPEs were detected in 7 (10.8%) patients, with IgM aPE in 5 (7.7%) and IgG aPE in 2 (3.1%) patients. Other aPLs such as IgG aβ2-GP-1, IgM aCL, and IgG aPS/PT were found in one patient each. Antibodies against PE were found significantly more often than aPL of different specificity (P=0.03). No IgG antibodies to laminin-1 were detected in the patients.
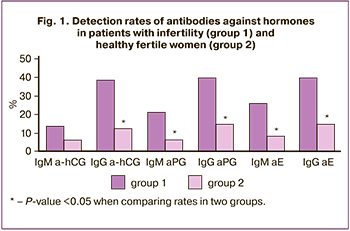
In contrast to aPL, there was a high detection rate of antibodies against hormones among infertile patients. ahCG, aPG, and aE were detected in 31 (47.7%) 33 (50.8%), and 37 (56.9%) patients, respectively, which was significantly more often than aPL (P <0.001). In infertile patients, IgG antibodies directed against hormones, IgM-aPG and aE were detected more often than in healthy fertile women (Fig. 1), while IgG-a-hCG and aPG were detected more often than IgM (P=0.001; P=0.004).
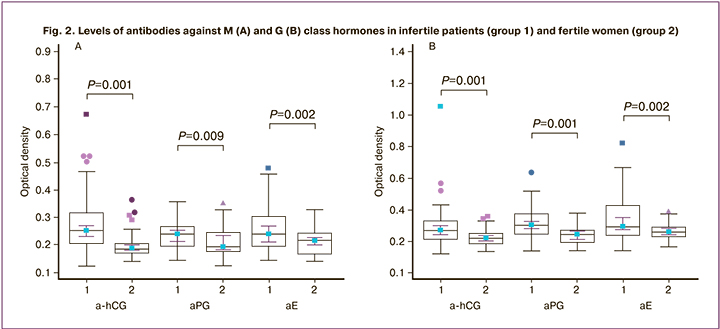
The levels of antibodies to hormones of classes M and G were higher in patients with infertility compared with fertile women (Fig. 2). Figure 2 shows median antibody levels, 95% CI, 25th and 75th percentiles, minimum and maximum antibody levels in OD units, P values when comparing the two groups.
Antibodies to hormones were detected in women with both primary and secondary infertility, including ahCG in 14 (51.9%) and 17 (44.7%), aPG in 14 (51.9%) and 19 ( 50%), aE in 16 (59.3%) and 21 (55.3%) patients, respectively. At the same time, the detection rate of antibodies to hormones and their mean levels in women with primary and secondary infertility did not differ (P>0.05).
The rates and levels of ahCG in women with previous ART attempts and the first ART cycle did not differ significantly. In women with the first ART attempt, the median levels of IgG antibodies to PG (0.317 [0.168; 0.636] OD units) and E (0.424 [0.226; 0.884] OD units) were higher than in women with repeat ART attempts (respectively, 0.280 [0.140; 0.438] OD units; 0.334 [0.200; 0.614] OD units) (P=0.04; P=0.02), while the median levels of IgM antibodies did not differ in these women.
There was a strong direct correlation between the levels of IgM antibodies against PG and E in infertile patients (r=0.76) and a direct correlation of moderate strength between the levels of IgG antibodies (r=0.63) (P<0.001). No significant correlations were found between the levels of ahCG and steroid hormones.
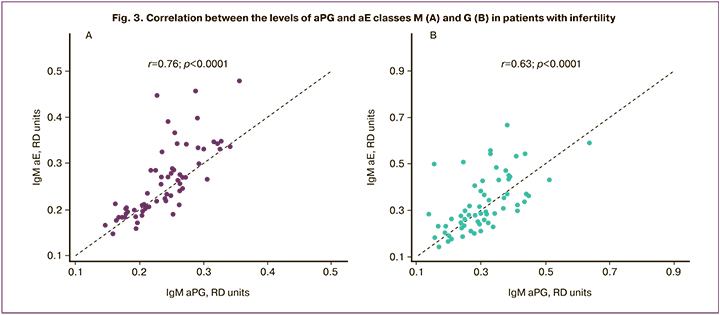
The results are consistent with our earlier data on the high detection rates of antibodies against steroid and gonadotropic hormones in infertile women in IVF programs [21]. It is assumed that small-sized PG molecules and estrogens, after binding to proteins of tissues and blood plasma, acquire full-fledged antigens' properties and can trigger Th2 lymphocyte production, which regulates antibody synthesis and allergic reactions [13].
Increased production of antibodies against hormones is associated with hormonal contraceptives and hormonal therapy to stimulate ovulation and support the luteal phase. High doses of gonadotropins that are used to stimulate ovaries and induce ovulation, and non-physiological concentrations of E and PG in the luteal phase of the induced cycle, 5–10 times higher than their level in the spontaneous menstrual cycle, can stimulate the production of antibodies to hormones [8]. It is assumed that the excess of hormones above the critical level can stimulate autoimmune reactions. At the same time, estrogens can exert a stimulating effect on the immune system and enhance the humoral immune response [25].
The formation of antibodies in women can be facilitated by previous medical abortions and spontaneous reproductive losses, which are accompanied by abrupt changes in hormonal and immune states. It is assumed that an increased immune response to PG can disrupt the physiological function of the hormone and, as a result, lead to disturbances in the luteal phase, implantation, and the physiological course of pregnancy. This hypothesis is supported by data on the high risk of dysmenorrhea and endometrial hypoplasia in women with recurrent miscarriage and elevated aPG levels [26].
Patients with infertility were found to have a wide range of risk factors for the formation of antibodies to hormones, which, in addition to the use of hormonal contraceptives and hormonal drugs, can include pelvic infectious and inflammatory diseases, endometriosis and polycystic ovary syndrome, pelvic surgical interventions, as well as ectopic pregnancies, spontaneous miscarriages, medical abortions, allergic and autoimmune diseases, frequent infectious diseases, and the carriage of viral infections.
Twenty (30.8%) patients who underwent ART achieved pregnancy. In women seropositive for IgM-aPG and aE [1 (7.1%) and 1 (6.3%)] pregnancy rates were significantly lower than in seronegative women [19 (37.3%) and 19 (38.8%)] (P=0.03; P=0.02). Patients with ART failure had higher levels of aPG and aE class M compared with women who achieved pregnancy (P<0.05) (Fig. 4). These results are consistent with previous data on the high detection rates of antibodies against hormones in women with a history of IVF failure and a high incidence of adverse outcomes in seropositive women's IVF cycle [21].
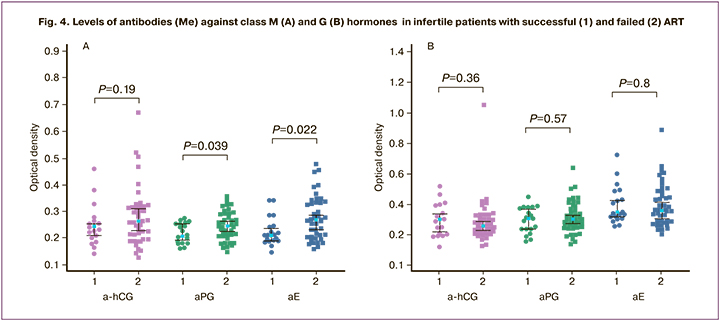
Earlier, women who were seropositive for a-hCG were shown to have a low pregnancy rate in IVF programs, comparable to that observed in patients with aPL or antitrophoblastic antibodies, and the need to determine a-hCG before IVF [27]. Recently, a report was published on the effectiveness of combination therapy, including membrane plasmapheresis, glucocorticoids, and intravenous immunoglobulins in patients with repeated IVF failures and seropositive for a-hCG [28].
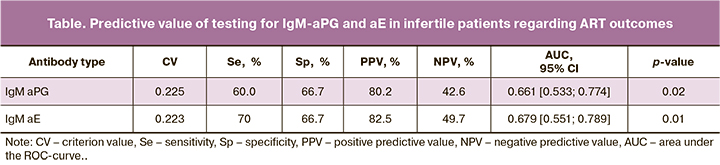
According to the ROC analysis, testing for IgM-aPG and aE had the highest sensitivity, specificity, area under the curve (AUC), and positive predictive value (table); the tests' accuracy was 67.7% and 69.2%, respectively.
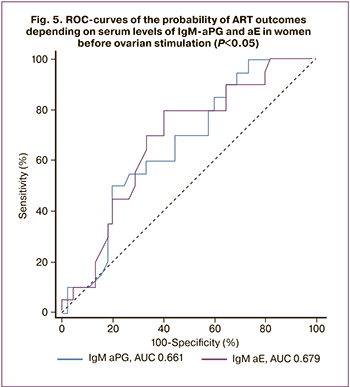 The constructed ROC curves made it possible to differentiate women by ART outcomes based on M class antibodies' levels against PG and E before ovarian stimulation (Fig. 5). The model quality was average. It should be noted that in the subgroup of patients with the first ART attempt, the AUC for IgM-aPG and aE were 0.722 [0.522; 0.874] and 0.725 [0.525; 0.875] (P=0.03; P=0.03), and the quality of the model was good. The accuracy of simultaneous testing for these antibodies reached 71.4%. IgM-aPG and aE are risk factors for ART failure in infertile women, and testing for them can be used to predict ART outcomes, including in women with the first attempt.
The constructed ROC curves made it possible to differentiate women by ART outcomes based on M class antibodies' levels against PG and E before ovarian stimulation (Fig. 5). The model quality was average. It should be noted that in the subgroup of patients with the first ART attempt, the AUC for IgM-aPG and aE were 0.722 [0.522; 0.874] and 0.725 [0.525; 0.875] (P=0.03; P=0.03), and the quality of the model was good. The accuracy of simultaneous testing for these antibodies reached 71.4%. IgM-aPG and aE are risk factors for ART failure in infertile women, and testing for them can be used to predict ART outcomes, including in women with the first attempt.
The presence of antibodies directed against class M hormones can be a manifestation of women's sensitization to these hormones, which is often detected in patients with infertility and recurrent miscarriage using skin tests. At the same time, although most skin reactions are mediated by immunoglobulins E, the formation of polyclonal antibodies of different isotypes is assumed, as in the case of most autoimmune conditions [13]. It has been noted that desensitization reduces clinical manifestations, improves ART outcomes and pregnancy course [17, 20]. Desensitization protocols with intravaginal, intramuscular, and oral administration of progestogens have been described [19].
PG production triggers morphological and physiological changes in the endometrium, providing endometrial receptivity and an environment appropriate for embryo implantation, which begins with blastocyst reposition and fixation [29]. It is important to note that steroid hormones contribute to endometrium susceptibility during the window of implantation. PG plays a crucial role in this process, blocking the proliferative effect of estrogen on uterine epithelial cells and inducing genes that ensure the normal response of the refractory endometrium and embryo attachment. PG provides endometrial decidualization for normal embryo implantation, promotes the invasion of extravillous trophoblast into the decidual tissue by inhibiting its apoptosis. It acts as a trophoblast invasion regulator, controlling the activity of matrix metalloproteinases [30].
Due to the importance of steroid hormones for implantation, it can be assumed that antibodies directed against these hormones can interfere with their physiological function and, as a result, lead to impaired endometrial receptivity and implantation. This hypothesis is supported by the lower pregnancy rate in seropositive women. It has been shown that the course of pregnancy in patients with recurrent miscarriage and elevated aPG levels is often complicated by chorionic hypoplasia and placental insufficiency [26]. According to V.M. Sidelnikova, the mechanism of miscarriage in the presence of aPG may be associated with the formation of an inferior first wave of trophoblast invasion, which is clinically manifested by chorionic hypoplasia at 5–6 weeks of gestation [14].
Further studies are needed to assess antibodies' pathogenetic role against hormones, optimize management of seropositive women before ART, and increase ART effectiveness by reducing autoimmune activity.
Conclusion
Infertile patients undergoing ART have a variety of autoantibodies, including antibodies directed against gonadotropic and steroid hormones. They also have a higher antibody detection rate than fertile women. At the same time, patients positive for IgM-aPG and aE have lower implantation and clinical pregnancy rates, and ART failures are associated with higher levels of these antibodies. Antibodies against steroid hormones are risk factors for adverse ART outcomes. Detection of an increased level of antibodies in women before ovarian stimulation is essential for predicting ART outcomes. It may help develop a management strategy, taking into account the autoimmune activity.
References
- Deroux A., Dumestre-Perard C., Dunand-Faure C., Bouillet L., Hoffmann P. Female infertility and serum auto-antibodies: a systematic review. Clin. Rev. Allergy Immunol. 2017; 53(1): 78-86. https://dx.doi.org/10.1007/s12016-016-8586-z.
- Корсак В.С., Смирнова А.А., Шурыгина О.В. ВРТ в России. Отчет за 2016 г. Проблемы репродукции. 2018; 24(6): 8-21. [Korsak V.S., Smirnova A.A., Shurygina O.V. ART in Russia. Report for 2016 Reproduction problems. 2018; 24(6): 8-21. (in Russian)].
- Вартанян Э.В., Мартышкина Е.Ю., Цатурова К.А. Роль сочетанной патологии в неудачных протоколах ЭКО. Акушерство, гинекология и репродукция. 2011; 5(4): 40-3. [Vartanyan E.V., Martyshkina E.Yu., Tsaturova K.A. The role of combined pathology in unsuccessful IVF protocols. Obstetrics, gynecology and reproduction. 2011; 5(4): 40-43. (in Russian)].
- Кривонос М.И., Зайнулина М.С., Чепанов С.В., Селютин А.В., Сельков С.А., Мысик О.Л. Клинико-иммунологические аспекты ведения женщин с неудачами ВРТ. Журнал акушерства и женских болезней. 2014; 63(5): 89-95. [Krivonos M.I., Zainulina M.S., Chepanov S.V., Selyutin A.V., Selkov S.A. Clinical and immunological aspects of management of women with ART failures. Journal of obstetrics and women's diseases. 2014; 63 (5): 89-95. (in Russian)].
- Simon A., Laufer N. Repeated implantation failure: clinical approach. Fertil. Steril. 2012; 97(5): 1039-43. https://dx.doi.org/10.1016/j.fertnstert.2012.03.010.
- Revel A. Defective endometrial receptivity. Fertil. Steril. 2012; 97(5): 1028-32. https://dx.doi.org/10.1016/j.fertnstert.2012.03.039.
- Chen X., Mo M.L., Huang C.Y., Diao L.H., Li G.G., Li Y.Y. et al. Association of serum autoantibodies with pregnancy outcome of patients undergoing first IVF/ICSI treatment: a prospective cohort study. J. Reprod. Immunol. 2017; 122: 14-20. https://dx.doi.org/10.1016/j.jri.2017.08.002.
- Haller-Kikkatalo K., Salumets A., Uibo R. Review on autoimmune reactions in female infertility: antibodies to follicle stimulating hormone. Clin. Dev. Immunol. 2012; 2012: 762541. https://dx.doi.org/10.1155/2012/762541.
- Менжинская И.В., Ванько Л.В. Ассоциация антител к гонадотропинам и женским половым гормонам с нарушениями репродуктивной функции. Акушерство и гинекология. 2017; 9: 20-7. https://dx.doi.org/ 10.18565/aig.2017.9.20-7. [Menzhinskaya I.V., Vanko L.V. Association of antibodies to gonadotropins and female sex hormones with reproductive disorders. Obstetrics and gynecology. 2017; 9: 20-27. (in Russian)].
- Itsekson A.M., Seidman D.S., Zolti M., Alesker M., Carp H.J. Steroid hormone hypersensitivity: clinical presentation and management. Fertil. Steril. 2011; 95(8): 2571-3. https://dx.doi.org/ 10.1016/j.fertnstert.2011.05.025.
- Untersmayr E., Jensen A.N., Walch K. Sex hormone allergy: clinical aspects, causes and therapeutic strategies - update and secondary publication. World Allergy Organ. J. 2017; 10(1): 45. https://dx.doi.org/10.1186/s40413-017-0176-x.
- Foer D., Buchheit K.M. Presentation and natural history of progestogen hypersensitivity. Ann. Allergy Asthma Immunol. 2019; 122(2): 156-9. https://dx.doi.org/10.1016/j.anai.2018.10.023.
- Roby R.R., Richardson R.H., Vojdani A. Hormone allergy. Am. J. Reprod. Immunol. 2006; 55(4): 307-13. https://dx.doi.org/10.1111/j.1600-0897.2006.00373.x.
- Сидельникова В.М. Подготовка и ведение беременности у женщин с привычным невынашиванием: методическое пособие и клинические протоколы. М.: МЕДпресс-информ; 2010. 224 с. [Sidelnikova V.M. Preparation and management of pregnancy in women with habitual miscarriage: a methodological guide and clinical protocols. M.: Medpress-inform; 2010. 224 p. (in Russian)].
- Ciampaglia W., Cognigni G.E. Clinical use of progesterone in infertility and assisted reproduction. Acta Obstet. Gynecol. Scand. 2015; 94(Suppl. 161): 17-27. https://dx.doi.org/10.1111/aogs.12770.
- Gupta A., Goenka D., Goenka M.L. Progesterone hypersensitivity: a challenge for luteal support. J. Hum. Reprod. Sci. 2018; 11(1): 79-81. https://dx.doi.org/10.4103/jhrs.JHRS_116_17.
- Itsekson A.M., Soriano D., Zolti M., Seidman D.S., Carp H.J.A. Intradermal sex hormone desensitization for relief of premenstrual symptoms may improve the obstetric outcome of women with recurrent pregnancy loss. Gynecol. Endocrinol. 2013; 29(2): 169-72. https://dx.doi.org/10.3109/09513590.2012.730582.
- Ellaithy M.I., Fathi H.M., Farres M.N., Taha M.S. Skin test reactivity to female sex hormones in women with primary unexplained recurrent pregnancy loss. J. Reprod. Immunol. 2013; 99(1-2): 17-23. https://dx.doi.org/10.1016/j.jri.2013.04.006.
- Foer D., Buchheit K.M., Gargiulo A.R., Lynch D.M., Castells M., Wickner P.G. Progestogen hypersensitivity in 24 cases: diagnosis, management, and proposed renaming and classification. J. Allergy Clin. Immunol. Pract. 2016; 4(4): 723-9. https://dx.doi.org/10.1016/j.jaip.2016.03.003.
- Prieto-Garcia A., Sloane D.E., Gargiulo A.R., Feldweg A.M., Castells M. Autoimmune progesterone dermatitis: clinical presentation and management with progesterone desensitization for successful in vitro fertilization. Fertil. Steril. 2011; 95(3): 1121. e9-3. https://dx.doi.org/10.1016/j.fertnstert.2010.10.038.
- Менжинская И.В., Безнощенко О.С., Сароян Т.Т., Корнеева И.Е., Ванько Л.В., Сухих Г.Т. Антитела к гормонам репродуктивной системы как возможный фактор риска неблагоприятного исхода в циклах экстракорпорального оплодотворения. Акушерство и гинекология. 2012; 2: 42-5. [Menzhinskaya I.V., Beznoshchenko O.S., Saroyan T.T., Korneeva I.E., Vanko L.V., Sukhikh G.T. Antibodies to hormones of the reproductive system as a possible risk factor for an unfavorable outcome in in vitro fertilization cycles. Obstetrics and gynecology. 2012; 2: 42-5. (in Russian)].
- Gardner D.K., Weissman A., Howles C.M., Shoham Z., eds. Textbook of assisted reproductive technologies: laboratory and clinical perspectives. 3rd ed. London: Informa Healthcare; 2009. 944p.
- Менжинская И.В., Кашенцева М.М., Ванько Л.В., Сухих Г.Т. Иммунохимические свойства аутоантител к хорионическому гонадотропину у женщин с невынашиванием беременности. Иммунология. 2015; 36(1): 30-5. [Menzhinskaya I.V., Kashentseva M.M., Vanko L.V., Sukhikh G.T. Immunochemical properties of autoantibodies to chorionic gonadotropin in women with miscarriage. Immunology. 2015; 36(1): 30-5. (in Russian)].
- Менжинская И.В., Гладкова К.А., Сидельникова В.М., Сухих Г.Т. Антипрогестероновые антитела в клинике привычной потери беременности. Иммунология. 2008; 29(1): 34-7. [Menzhinskaya I.V., Gladkova K.A., Sidelnikova V.M., Sukhikh G.T. Antiprogesterone antibodies in the clinic of recurrent pregnancy loss. Immunology. 2008; 29(1): 34-7. (in Russian)].
- Pennell L.M., Galligan C.L., Fish E.N. Sex affects immunity. J. Autoimmun. 2012; 38(2-3): J282-91. https://dx.doi.org/10.1016/j.jaut.2011.11.013.
- Гладкова К.А., Менжинская И.В., Сухих Г.Т., Сидельникова В.М. Роль сенсибилизации к прогестерону в клинике привычного невынашивания беременности. Проблемы репродукции. 2007; 13(6): 95-8. [Gladkova K.A., Menzhinskaya I.V., Sukhikh G.T., Sidelnikova V.M. The role of progesterone sensibilization in the clinic of habitual miscarriage. Reproduction problems. 2007; 13(6): 95-8. (in Russian)].
- Zou S.H., Yang Z.Z., Zhang P., Song D.P., Li B., Wu R.Y. et al. Autoimmune disorders affect the in vitro fertilization outcome in infertile women. Zhonghua Nan Ke Xue. 2008; 14(4): 343-6.
- Muller V., Ob’edkova K., Krikheli I., Kogan I., Fedorova I., Lesik E. et al. Successful pregnancy outcome in women with recurrent IVF failure and anti-hCG autoimmunity: a report of three cases. Case Reports Immunol. 2016; 2016: 4391537. https://dx.doi.org/10.1155/2016/4391537.
- Di Renzo G.C., Giardina I., Clerici G., Brillo E., Gerli S. Progesterone in normal and pathological pregnancy. Horm. Mol. Biol. Clin. Investig. 2016; 27(1): 35-48. https://dx.doi.org/10.1515/hmbci-2016-0038.
- Halasz M., Szekeres-Bartho J. The role of progesterone in implantation and trophoblast invasion. J. Reprod. Immunol. 2013; 97(1): 43-50. https://dx.doi.org/10.1016/j.jri.2012.10.011.
Received 02.10.2020
Accepted 22.10.2020
About the Authors
Irina V. Menzhinskaya, Dr. Med. Sci., Leading Researcher at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.Tel.: +7(495)438-11-83. E-mail: i_menzinskaya@oparina4.ru. 4, Oparina str., Moscow, 117997, Russia.
Elizaveta E. Kraevaya, Researcher at the Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Теl.: +7(985)294-03-18. E-mail: e_kraevaya@oparina4.ru. 4, Oparina str., Moscow, 117997, Russia.
Elena A. Kalinina, Dr. Med. Sci., Professor, Head of the Department of Assisted Technologies for the Treatment of Infertility, Scientific Secretary of the Dissertation Council, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Теl.: +7(499)131-86-49. E-mail: e_kalinina@oparina4.ru. 4, Oparina str., Moscow, 117997, Russia.
Ludmila V. Vanko, Dr. Med. Sci., Professor, Leading Researcher at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(495)438-11-83. E-mail: LVanko@oparina4.ru. 4, Oparina str., Moscow, 117997, Russia.
Nataliya V. Dolgushina, Dr. Med. Sci., Deputy Director - Head of the Department of Research Administration, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Теl.: +7(495)438-49-77. E-mail: n_dolgushina@oparina4.ru. 4, Oparina str., Moscow, 117997, Russia.
For citation: Menzhinskaya I.V., Kraevaya E.E., Kalinina E.A., Vanko L.V., Dolgushina N.V. Predictive value of autoimmune markers in assisted reproductive technologies.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 3: 130-137 (in Russian)
https://dx.doi.org/10.18565/aig.2021.3.130-137



