Одной из важных задач вспомогательных репродуктивных технологий (ВРТ) является повышение вероятности наступления беременности при селективном переносе одного эмбриона [1]. По данным J. Gunbi и соавт. [2], только треть циклов ВРТ приводит к развитию беременности и около четверти из них – к рождению ребенка. Наступление беременности во многом зависит от трех составляющих: рецептивности эндометрия, функционально полноценного эмбриона, способного к имплантации, и доставки эмбриона к зоне имплантации [3].
Таким образом, особенно важной становится задача выбора эмбриона для переноса. Для обоснованного выбора эмбриона необходимо выявление и изучение предикторов эффективности его дальнейшего развития in vitro и, после переноса, in vivo.
В первые 5–6 суток преимплантационного развития эмбрион человека находится внутри блестящей оболочки (англ. – zona pellucida, ZP) [4]. Доказано, что ZP играет важную роль во время оплодотворения и преимплантационного развития эмбриона [5]. Выход эмбриона из ZP обозначается термином хетчинг [6]. Хетчинг обусловлен механическими периодическими сокращениями и набуханиями бластоцисты, в генезе которых важную роль играют лизирующие факторы, выделяемые бластоцистой и эндометрием [7, 8].
По данным, полученным in vitro, до 75% всех морфологически нормальных бластоцист человека не способны к самостоятельному выходу из ZP [9]. Отсутствие спонтанного хетчинга может являться одной из важных причин привычной неудачи имплантации [10], а определение факторов риска позволит определить показания для проведения процедуры вспомогательного хетчинга [11]. Несмотря на большое число исследований, проведенных на животных моделях, а также широкое распространение методов ВРТ, биомеханические и молекулярные механизмы хетчинга до сих пор неизвестны в полной мере и требуют дальнейшего изучения.
Цель исследования: изучить роль клеточных, генетических, клинико-лабораторных и ятрогенных факторов в эффективности спонтанного хетчинга бластоцист человека в программах ВРТ.
Материал и методы исследования
Для проведения исследования были изучены 83 бластоцисты. Отбор бластоцист осуществлялся в отделении вспомогательных технологий в лечении бесплодия среди супружеских пар, обратившихся по поводу проведения программы ВРТ и донировавших бластоцисты для научных исследований. Критериями включения в исследование были нормальный кариотип супружеских пар, донировавших эмбрионы и подписавших информированное согласие на участие в исследовании. Критериями исключения были: остановка эмбриона в развитии до 5-х суток культивирования, дегенеративные изменения в бластоцистах и проведение вспомогательного хетчинга исследуемых эмбрионов.
Полученные ооциты отмывали от фолликулярной жидкости и крови и инкубировали в культуральной среде в течение 2–3 часов, после чего производили денудирование ооцитов. Оценку степени зрелости, качества ооцитов и эмбрионов, толщины блестящей оболочки, выявление дисморфизмов ооцитов и мониторинг хетчинга проводили при помощи световой микроскопии (Nikon TE 300, общее увеличение х400). Оценку успеха самостоятельного хетчинга проводили через 144–146 часов после оплодотворения [12]. Анализ эякулята проводили согласно рекомендациям ВОЗ 2010 года [13].
Исследование экспрессии мРНК генов катепсина L2 (CTSL2), активатора транскрипции GATA3 и хорионического гонадотропина (CGB) осуществляли методом полимеразной цепной реакции в реальном времени с предварительной обратной транскрипцией (ООО «НПО ДНК-Технология», Россия).
Статистическую обработку данных выполняли при помощи пакета программ Statistica V10 (США) с применением χ2-теста для оценки частотных показателей, тестов Манна–Уитни и Крускала–Уаллиса для сравнения непараметрических данных, t-теста и ANOVA для сравнения параметрических данных. Мерой ассоциации для сравнения бинарных данных стали относительный риск (ОР) с 95% доверительным интервалом (95% ДИ). Различия считали статистически значимыми при уровне достоверности р<0,05.
Исследование было одобрено комиссией по этике ФГБУ Научный центр акушерства, гинекологии и перинатологии им. академика В.И. Кулакова Минздрава России.
Результаты исследования
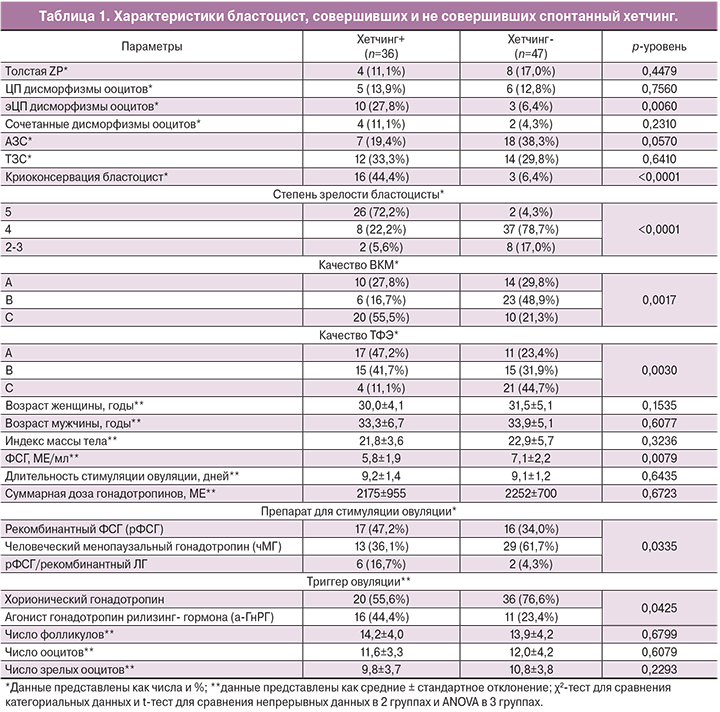
Результаты исследования представлены в табл. 1. 36 бластоцист (43,4%) совершили спонтанный хетчинг и составили группу наблюдения (группа 1). 47 бластоцист (56,6%), не совершивших спонтанный хетчинг, составили группу сравнения (группа 2).
Состояние ZP не влияло на успех хетчинга. Доля бластоцист с утолщением или какими-либо дефектами блестящей оболочки составила 14,4% (n=12) и была сопоставимой в группах сравнения.
Большая часть ооцитов, из которых были получены эмбрионы, не имела дисморфизмов. Доля цитоплазматических (ЦП) дисморфизмов составила 13,2% (n=11), экстрацитоплазматических (эЦП) – 15,7% (n=13), сочетанных – 7,2% (n=6). ЦП дисморфизмы ооцитов не оказывали влияния на успех хетчинга. При этом наличие эЦП дисморфизмов в виде патологии перивителлинового пространства (расширение или наличие в нем дебриса) увеличивало эффективность хетчинга в 4,3 раза (ОР=4,3; 95% ДИ=1,3;14,7).
При оценке влияния свойств сперматозоидов на способность бластоцист к хетчингу было выявлено, что тератозооспермия (ТЗС) не влияла на успех спонтанного хетчинга, тогда как при наличии астенозооспермии (АЗС) эффективность хетчинга бластоцист снижалась в 1,9 раза (ОР=1,9; 95% ДИ=0,9; 4,2), что было погранично значимо. Это может косвенно свидетельствовать о вовлечении в процесс хетчинга не только материнских, но и отцовских генетических факторов, что соответствует данным о времени активации эмбрионального генома [4].

22,8% включенных в исследование эмбрионов (n=19) были подвергнуты криоконсервации, которая улучшала эффективность хетчинга бластоцист в 6,9 раза (ОР=6,9; 95% ДИ=2,2; 22,1), что, по-видимому, объясняется лучшим качеством бластоцист, подвергнутых криоконсервации, а также может быть связано с механическо-физическим воздействием процедуры криоконсервации на блестящую оболочку.
При оценке качества бластоцист на пятые сутки культивирования по классификации Гарднера, было выявлено, что качество бластоцист влияет на их способность к хетчингу.
Большая часть бластоцист была 4-й степени зрелости (n=45, 54,2%). При этом бластоцист 5-й и 6-й степени зрелости было значимо больше в группе 1, а бластоцист 2-й и 3-й степени зрелости – в группе 2 (р<0,0001) (рисунок А).
Внутренняя клеточная масса (ВКМ) категории А в равном числе случаев была выявлена в обеих группах. Тогда как ВКМ категории В было больше в группе 2 по сравнению с группой 1 (48,9 и 16,7% соответственно), а ВКМ категории С – больше в группе 1 по сравнению с группой 2 (55,5 и 21,3% соответственно) (р=0,0017) (рисунок Б).
Трофэктодермальный слой (ТФЭ) в равном числе случаев был оценен на категории A, B или C. При этом ТФЭ категории А была выявлена у 47,2% бластоцист группы 1 и лишь у 23,4% бластоцист группы 2, тогда как ТФЭ категории С была выявлена у 44,7% бластоцист группы 2 и у 11,1% бластоцист в группе 1 (р=0,0030) (рисунок В).
При оценке клинико-анамнестических факторов пациентов, донировавших бластоцисты, не было выявлено значимых отличий. Отмечался более низкий уровень фолликулостимулирующего гормона (ФСГ) в группе 1 (р=0,0079).
При изучении влияния особенностей протокола стимуляции суперовуляции на эффективность спонтанного хетчинга, было отмечено, что длительность стимуляции овуляции и доза вводимых гонадотропинов не различались в группах сравнения. Однако у бластоцист, совершивших хетчинг, чаще использовалась стимуляция с помощью рФСГ/рЛГ, а у бластоцист, не совершивших хетчинг – с помощью чМГ (р=0,0325). При применении в качестве триггера овуляции хорионического гонадотропина риск неудачи спонтанного хетчинга был значимо выше, чем при применении а-ГнРГ (р=0,0425).
Параметры фолликулогенеза и оогенеза: число фолликулов, общее число ооцитов, а также число зрелых и незрелых ооцитов, не отличалось в группах сравнения.
При анализе экспрессии мРНК генов, ответственных за эффективность хетчинга, в сравниваемых группах было выявлено, что в группе бластоцист с эффективным хетчингом отмечалась более высокая экспрессия мРНК генов CTSL2, GATA3 и CGB (табл. 2).
Степень зрелости эмбрионов, качество ВКМ и качество ТФЭ слоя были связаны с экспрессией мРНК генов CTSL2 и GATA3, которая была выше у эмбрионов 5-й степени зрелости и эмбрионов класса А (табл. 3).

Обсуждение
Полученные в нашем исследовании данные о частоте спонтанного хетчинга (43,4%), согласуются с имеющимися в литературе указаниями, что до 50–75% бластоцист не способны самостоятельно покинуть блестящую оболочку [9].
Считается, что утолщение ZP негативно влияет на хетчинг, так как в этом случае для ее растворения требуется большее количество литических ферментов [7]. Однако в нашем исследовании не было выявлено увеличения частоты неудач хетчинга при утолщении ZP. Это позволяет предположить, что полноценный эмбрион высокого качества имеет достаточный запас адаптационных возможностей для своевременного выхода не только из нормальной, но и из утолщенной ZP.
Качество ооцитов оказывает непосредственное влияние на качество полученных эмбрионов, их способность к имплантации и исходы ВРТ [14–18]. Тем не менее, в литературе отсутствуют данные, однозначно подтверждающие влияние дисморфизмов ооцитов на способность бластоцист, из которых они были получены, к хетчингу. В нашем исследовании мы также не наблюдали подобного влияния ЦП дисморфизмов ооцитов, однако наличие эЦП дисморфизмов увеличивало эффективность спонтанного хетчинга в 4,3 раза. эЦП дисморфизмы были представлены в виде расширения перивителлинового пространства и наличия в нем дебриса. По данным литературы, на развитие эЦП дисморфизмов значимое влияние оказывает применение а-ГнРГ в качестве триггера овуляции [19]. В нашем исследовании также отмечалась связь между применением а-ГнРГ в качестве триггера овуляции и спонтанным хетчингом. Возможно, данные дефекты эЦП структур механически облегчают разрыв ZP и вылупление бластоцисты, а влияние а-ГнРГ на эффективность спонтанного хетчинга реализуется за счет развития эЦП дисморфизмов.
Мы также отметили тенденцию к снижению эффективности хетчинга при патозооспермии в виде АЗС, однако данные были погранично значимы. В литературе отсутствует информация о влиянии патологии сперматозоидов на эффективность спонтанного хетчинга.
По полученным данным, высокая оценка качества бластоцист по всем трем параметрам шкалы Гарднера положительно влияет на хетчинг. Это может свидетельствовать о том, что хетчинг – это особый этап развития бластоцисты, характеризующийся хронологической и, в большей мере, хроногенетической детерминированностью, поскольку бластоцисты, не достигшие к 6-м суткам культивирования достаточного развития, не способны к хетчингу. С эволюционной точки зрения, это может быть механизмом предотвращения имплантации дефектного эмбриона с замедленным, или иными нарушениями развития.
Роль катепсинов в хетчинге в настоящее время изучена лишь на животных моделях [20]. Показана экспрессия генов катепсина класса L в клетках ТФЭ и ВКМ [21]; тем не менее, роль катепсинов в процессе хетчинга у человека окончательно не определена. Мы показали значимое увеличение экспрессии мРНК гена CTSL2 в бластоцистах, способных к спонтанному хетчингу, что позволяет предположить непосредственное участие катепсина L в хетчинге бластоцист человека. Также мы показали зависимость экспрессии мРНК гена CTSL2 и качества бластоцисты по классификации Гарднера. Таким образом, экспрессия гена CTSL2, то есть запуск одного из непосредственных механизмов хетчинга, детерминирована качеством бластоцисты и достаточной степенью её развития.
GATA3 – важнейший регулятор преимплантационного развития эмбриона человека [22, 23]. GATA3 экспрессируется в ТФЭ эмбриона и отвечает за регуляцию экспрессии гена Cdx2. Нокаут гена GATА3 приводит к выраженному снижению экспрессии Cdx2, что ведет к нарушению бластуляции эмбриона и, следовательно, невозможности формирования бластоцисты. В литературе имеются лишь косвенные данные, свидетельствующие об участии GATA3 в регуляции хетчинга бластоцист человека. Полученные в нашем исследовании данные свидетельствуют о влиянии GATA3 на хетчинг – уровень экспрессии мРНК гена GATA3 был значимо выше в бластоцистах, способных к спонтанному хетчингу, а также в бластоцистах высокого качества по Гарднеру.
Экспрессия мРНК гена CGB была также выше в группе бластоцист, способных к спонтанному хетчингу. В литературе отсутствуют данные, непосредственно подтверждающие участие хорионического гонадотропина в регуляции хетчинга бластоцисты человека. Учитывая более высокий уровень экспрессии гена CGB в группе бластоцист, способных к спонтанному хетчингу, можно предположить, что хорионический гонадотропин участвует в эпигенетической регуляции хетчинга. Как и для GATA3, очевидной стала взаимосвязь между высоким уровнем экспрессии гена CGB и хорошим качеством бластоцисты по классификации Гарднера.
Также были получены данные о связи спонтанного хетчинга с уровнем ФСГ и видом протокола стимуляция суперовуляции. Исходя из полученных данных, при использовании чМГ в протоколах стимуляции суперовуляции эффективность спонтанного хетчинга уменьшалась. Возможно, это происходит за счет воздействия на ZP, а в частности ее утолщения или изменения конфигурации [24], что может затруднять выход бластоцисты из блестящей оболочки в том случае, если у эмбриона имеется недостаточный запас адаптационных возможностей.
Предполагается, что базальный уровень ФСГ влияет на толщину и структуру ZP, а значит, является потенциальным предиктором спонтанного хетчинга [25].
Однако в нашей работе ФСГ был связан не с толщиной ZP, а с эффективностью спонтанного хетчинга, что может реализоваться за счет изменения структуры ZP. Кроме того, эти эффекты могут быть связаны с дифференцированным подходом к назначению препаратов для стимуляции суперовуляции.
Заключение
Таким образом, основное влияние на эффективность самостоятельного хетчинга бластоцист человека оказывает не качество блестящей оболочки и гамет, а качество самих бластоцист. Вероятно, бластоциста может моделировать свою дальнейшую судьбу с помощью собственных генетических факторов. Экспрессия мРНК генов CTSL2, GATA3 и CGB является более низкой у бластоцист низкого качества, что не позволяет им совершить спонтанный хетчинг и имплантироваться в эндометрий. Определенную роль в эффективности хетчинга играет выбор протокола стимуляции суперовуляции.



