POLYMORPHIC LOCI OF THE LHCGR GENE ARE ASSOCIATED WITH THE DEVELOPMENT OF UTERINE LEIOMYOMA
Objective. To investigate the associations of the LHCGR polymorphism rs4374421, rs7579411, rs6729809, and rs4953616 with the development of uterine leiomyoma.Ponomarenko I.V., Polonikov A.V., Churnosov M.I.
Subjects and methods. The investigation enrolled 1265 women: 569 patients with uterine leiomyoma and 696 individuals in the control group. Four polymorphic loci (rs4374421, rs7579411, rs6729809, and rs4953616) of the LHCGR gene were genotyped. The associations of LHCGR gene polymorphism with the development of uterine leiomyomas, its regulatory potential, and impact on gene expression were studied.
Results. The polymorphic loci rs4374421 and rs7579411 of the LHCGR gene were found to be associated with the development of uterine leiomyoma. The risk factors for the disease were the C/C genotype of rs7579411 (OR = 1.35) and TS haplotype of the polymorphic loci rs4374421-rs7579411 (OR = 1.21). The T allele (dominant model: OR=0.74) and the C/T genotype (OR = 0.80) rs7579411, as well as T/C genotype of rs4374421 (OR=0.78) are of protective value for the development of uterine leiomyoma. These polymorphic loci have a significant regulatory potential (are located in the region of histones that label promoters and enhancers in the culture cells, precursors of neurons and mesenchymal cells, etc., in the region of regulatory DNA motifs), and the polymorphism rs7579411 is associated with the expression level of the STON1-GTF2A1L gene in the thyroid gland.
Conclusion. The LHCGR polymorphisms rs4374421 and rs7579411 are associated with the development of uterine leiomyoma.
Keywords
Uterine leiomyoma, or uterine fibroids, is a benign monoclonal tumor of the uterine smooth muscle cells (myometrium) [1]. The prevalence of uterine leiomyoma varies from 30 to 77% among women of reproductive age [1–3]. Estimates suggest that 5-10% of all cases of infertility are associated with uterine leiomyoma [4]. In the Russian Federation, 50–70% of hysterectomies are performed for uterine fibroids [1, 3]; in the United States, uterine leiomyomas remain the primary indication for hysterectomy and account for 200 000 hysterectomies annually [5]. At the same time, in the United States, uterine fibroids are estimated to cost $5.9 to $34.4 billion a year [6]. Fibroid tumors are estrogen-dependent, and female sex hormones play an important role in fibroid growth and phenotypic manifestation of cytogenetic, epigenetic and molecular genetic disorders in the uterine smooth muscle cells [1, 2, 7, 8].
According to the generally accepted hypothesis, the development and growth of uterine leiomyoma is a result of a hyperestrogenic state - increased production of estrogens and/or increased sensitivity of the myometrium to estrogens, including their local production; luteal phase deficiency, progesterone deficiency, chronic anovulation and increased production of gonadotropic hormones [3]. One of the key roles in the functioning of the female hypothalamic–pituitary–gonadal system is played by the luteinizing hormone, which realizes its biological effects through interaction with specific receptors (LHCGR) [9].
This study investigated the association of the luteinizing hormone receptor (LHCGR) gene with the development of uterine leiomyoma. According to the GeneCards®: The Human Gene Database (http://www.genecards.org/), the LHCGR gene encodes the receptor for both luteinizing hormone and chorionic gonadotropin. Luteinizing hormone, interacting with its receptors, initiates ovulation and stimulates corpus luteum to secrete progesterone and estrogen that are involved in the pathogenesis of uterine leiomyoma [1, 2, 3, 10].
The purpose of the study is to investigate the associations of polymorphisms rs4374421, rs7579411, rs6729809, and rs4953616 of the LHCGR gene with the development of uterine leiomyoma.
Materials and methods
The study comprised 1265 women, including 569 patients with uterine fibroids and 696 healthy subjects. All patients with uterine fibroids (n = 569, 100%) underwent hysterectomy with subsequent morphological verification of the diagnosis at the Department of Gynecology of the Perinatal Center, the St. Ioasaph Belgorod Regional Hospital. The control group included women who did not have clinical and ultrasound signs of benign proliferative diseases of the female reproductive system. The control group was selected from women undergoing periodic health examination at the Perinatal Center of the St. Ioasaph Belgorod Regional Hospital. Participants of both study groups were of Russian descent with residence in the Central Chernozem region of Russia and were not related to each other. The sampling was carried out from 2008 to 2013. The mean age of women in the study group (43.22 ± 8.35 years) and the control group (42.49 ± 7.55 years) was comparable (p> 0.05, Mann – Whitney U test). The study was conducted under the supervision of the ethics committee of the Medical Institute of the Belgorod State National Research University. Written informed consent was obtained from all women enrolled in the study.
All study participants were genotyped for four polymorphic loci rs4374421, rs7579411, rs6729809, and rs4953616 of the LHCGR gene. These polymorphic loci were chosen for the study due to their significant regulatory potential and the effect on gene expression (according to the HaploReg database (v.4.1.) (http://compbio.mit.edu/HaploReg).
The material for the study was whole venous blood. Blood samples (8–9 ml) were drawn from the antecubital vein. Genomic DNA was extracted using the standard phenol-chloroform extraction method. The analysis of the studied loci was performed by polymerase chain reaction of DNA synthesis using oligonucleotide primers and probes.
Concordance of genotype frequencies with Hardy–Weinberg equilibrium was tested using c2 test. Comparison of the frequencies of alleles and genotypes between patients and controls was tested by contingency tables 2×2 and χ2 test with Yates’ correction for continuity. The analysis was performed using STATISTICA for Windows 6.0. Logistic regression using additive, dominant, and recessive genetic models was performed to analyze the association between polymorphisms and the development of uterine leiomyoma. Associations between haplotypes of the studied polymorphic loci and the development of the disease were analyzed using logistic regression. A correction for multiple comparisons was performed using an adaptive permutation test (pperm). The pperm level <0.05 was considered statistically significant. The calculations were carried out in PLINK v. 2.050 software (http://zzz.bwh.harvard.edu/plink/).
An odds ratio (OR) and its 95% confidence interval (95% CI) was used as a measure of association between polymorphic loci and the disease. OR> 1 indicates that the polymorphic variant was a risk factor for the disease; OR <1 indicates that polymorphic variant is a protective factor against the development of the disease; there were no associations with OR = 1.
The regulatory potential of polymorphic loci was identified using the HaploReg v4.1 online software (http://archive.broadinstitute.org/mammals/haploreg/haploreg.php) [11].
The association of the polymorphic locus (reference and alternative alleles) with the affinity of the DNA motif to transcription factors was evaluated by the difference between the log-odds (LOD) scores of the alternative (alt) and reference (ref) alleles (ref) [12]. The negative value of this parameter indicates an increase in the affinity of the motif by the reference allele; on the contrary, a positive value demonstrates the association of the alternative allele with an increase in the affinity of the analyzed DNA motif.
The effect of the polymorphism on gene expression (cis-eQTL) was studied using data from the Genotype-Tissue Expression (GTEx) project (http://www.gtexportal.org/). The analysis included data with p <8×10-5, pFDR≤0.05. The direction of the association of allelic polymorphism variants with the level of gene transcription was estimated by the linear regression coefficient (β), which describes the change in the normalized index of gene expression by one polymorphic (alternative) genetic variant [13].
Results and discussion
The observed and expected frequencies of polymorphisms rs4374421, rs7579411, rs6729809, rs4953616 of the LHCGR gene both in the group of patients with uterine fibroids and control subjects were in concordance with Hardy-Weinberg equilibrium (p>0.05).
The results of a comparative analysis of the allele and genotype frequencies of polymorphic loci of the LHCGR gene in patients with uterine myoma and the control group are presented in the table. Polymorphisms rs4374421 and rs7579411 were found to be associated with the development of uterine leiomyoma.
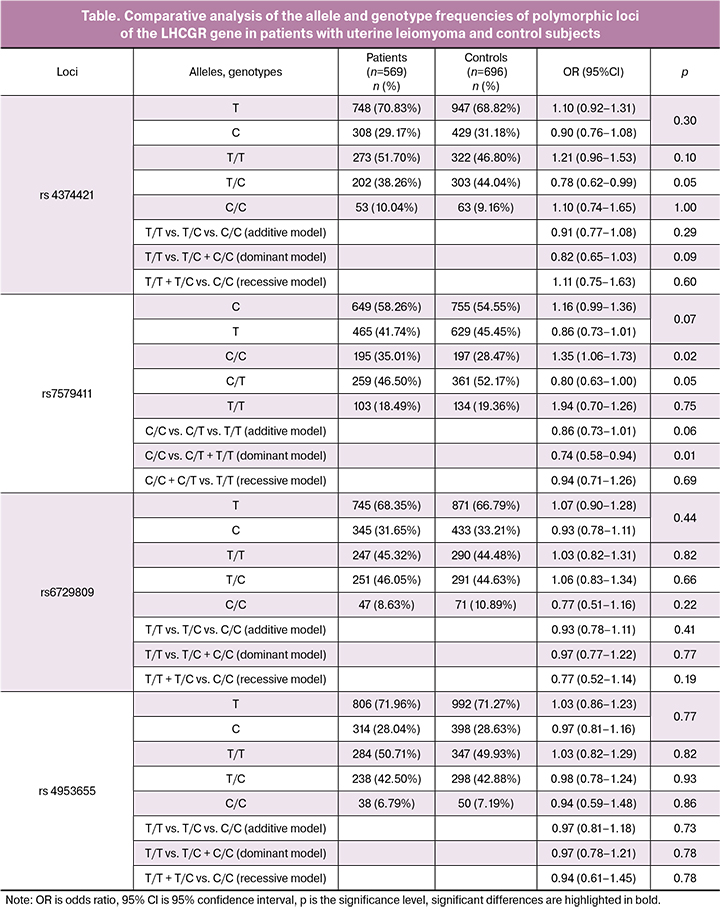
Among patients with uterine leiomyoma, the frequency of T/C rs4374421 genotype was 1.15 times lower than in the control group (p = 0.05). This genotype is a protective factor against the development of the disease (OR = 0.78 95% CI 0.62–0.99).
The frequency of the C/C rs7579411 genotype in the group of patients with uterine myoma was 1.23 times higher, and the frequency of the C/T genotype of this locus was 1.12 times lower than among women in the control group (p = 0.02 and p = 0.05, respectively). The dominant model of allele interaction showed an association between T rs7579411 allele and the development of uterine fibroids (p = 0.01, pperm = 0.02). Thus, the C/C rs7579411 genotype of the LHCGR gene is a risk factor for the development of uterine fibroids (OR = 1.35 95% CI 1.06–1.73), while the allele T (OR = 0.74–95% CI 0.58– 0, 94) and the C/T genotype (OR = 0.80 95% CI 0.63–1.00) of this polymorphic locus are protective against the development of the disease.
Patients with uterine leiomyoma were more likely (58.85%) to have the haplotype of TC rs4374421-rs7579411 polymorphic loci (these polymorphic loci are located at a distance of 3kb and are linked to each other, the force of adhesion is r2 = 0.57, LD = -0.98) than women in the control group (54.85%, p = 0.04, pperm = 0.05).
Thus, the TC haplotype of the polymorphic rs4374421-rs7579411 loci of the LHCGR gene is a risk factor for the development of uterine fibroids (OR = 1.21).
The results of the analysis using a HaploReg (v4.1) online software showed that rs4374421 polymorphism of the LHCGR gene is located in the region of histone H3K4me1, which marks enhancers (in cell culture, precursors of neurons and mesenchymal cells, epithelial cells of the mammary glands, peripheral blood cells, etc.), and histone H3K4me3, which marks promoters (in cell culture, nerve tissue precursors, etc.), in the region of regulatory DNA motifs that are binding sites for two transcription factors (NRSF_disc3 and Sin3Ak-20_disc6).
At the same time, the T allele, which is part of the “risky” haplotype, increases affinity for the Sin3Ak-20_disc6 transcription factor (the difference between LOD scores of the T (alt) and C (ref) alleles is 11.1) and reduces the affinity of the NRSF_disc3 transcription factor (the difference between LOD scores of alleles T (alt) and C (ref) is 7.8). The polymorphic locus rs7579411 of the LHCGR gene is located in the histone H3K4me1 region, which marks enhancers in cell culture, neural tissue precursors and mesenchymal cells, primary osteoblasts, etc.).
Thus, polymorphic loci rs4374421 and rs7579411 of the LHCGR gene have significant epigenetic effects, which may constitute a biological basis for their involvement in the development of uterine fibroids. According to the literature, luteinizing hormone plays a key role in the functioning of the female hypothalamus-pituitary-ovarian system [9] and realizes its biological effects through specific receptors (LHCGR). Biological mechanisms associated with the action of luteinizing hormone in the body (the synthesis of androgens and estrogens in the follicles, initiation of ovulation, luteinization of granulose cells of the ovulated follicle with the formation of the corpus luteum, synthesis of progesterone and other steroids by the corpus luteum cells [9]) play an important role in the pathogenesis of the uterine leiomyoma [1, 2, 3, 10]. Of note are studies suggesting that the risk for uterine fibroids in women may be increased with higher levels of luteinizing hormone [14, 15].
The results of the analysis using a Web-based GTEx portal containing data on gene expression in 48 human organs and tissues in silico indicated a link between the polymorphism rs7579411 of the LHCGR gene and the level of expression of the STON1-GTF2A1L gene in the thyroid gland (linear regression coefficient for the T allele is β = -0, 21, p = 2.6 × 10-5, FDR≤0.05). Thus, the T allele polymorphism rs7579411 (which is protective against uterine leiomyoma) is associated with reduced expression of the gene STON1-GTF2A1L, and accordingly, the C allele of this polymorphic locus, which is a part of the “risky” haplotype, confers increased transcription of the STON1-GTF2A1L gene in the thyroid gland.
The data presented in the online database GeneCards: The Human Gene Database (http://www.genecards.org/) show that the joint transcription of the adjacent STON1 and GTF2A1L genes results in the STON1-GTF2A1L transcript, which determines the synthesis of the “united” protein, including the main elements of proteins encoded by the STON1 (stonin 1) and GTF2A1L (general transcription factor IIA subunit 1 like) genes. At the same time, there may be several alternative splicing options resulting in protein products of unknown significance. It should be noted that the protein product of the gene GTF2A1L (general transcription factor IIA subunit 1 like) is part of the main transcription factor TFIIA, which plays an important role in the regulation of gene expression. The protein encoded by the STON1 gene (stonin 1) is one of the components of the cell endocytosis system and participates in the processes of local cell adhesion and mobility (http://www.genecards.org/). It should be noted that, by our data, the polymorphism rs7579411 of the LHCGR gene is associated with the level of the STON1-GTF2A1L gene expression in the thyroid gland. According to literature data, iodothyronine hormones are produced in the thyroid gland and have pronounced multiple metabolic effects: regulation of growth processes, development and differentiation of tissues and organs (especially the central nervous system) - growth hormone synergists, stimulation of protein synthesis and increase in basal metabolism, regulation of insulin synthesis and carbohydrate metabolism, lipolytic effect and activation of cholesterol synthesis (is a precursor of sex hormones), etc. [16]. These biomedical effects may have important etiopathogenetic significance in the development of uterine leiomyoma [1, 3]. There are literature data on the association of polymorphic loci localized in the region of the LHCGR, STON1-GTF2A1L genes with the formation of polycystic ovary syndrome in the Chinese population [17, 18] and increased expression of these genes in samples of subcutaneous adipose tissue in patients with polycystic ovaries [19]. Previous studies have identified associations between polymorphic loci located in the region of the LHCGR, STON1-GTF2A1L genes and polycystic ovary syndrome in the Chinese population [17, 18] and increased expression of these genes in samples of subcutaneous adipose tissue in patients with polycystic ovaries [19]. B.J. Davis et al. [20] have reported differences in the levels of the STON1-GTF2A1L transcript expression in uterine fibroids in “older” black and white women (aged 35 and older). We believe that we have been the first to link the LHCGR gene polymorphism with the development of uterine leiomyoma.
Conclusion
The findings of this study suggest the significant role of the polymorphic loci rs4374421 and rs7579411 of the LHCGR gene in the development of uterine leiomyoma. The risk factors for the development of the disease are the genotype C/C rs7579411 (OR = 1.35) and the TC haplotype based on polymorphisms rs4374421 and -rs7579411 (OR = 1.21). The T allele (OR = 0.74, dominant model) and the genotype C/T rs7579411 (OR = 0.80), as well as the genotype T/C rs4374421 (OR = 0.78) have a protective effect against the development of uterine leiomyoma. These polymorphic loci have significant regulatory potential (located in the histone region, marking promoters and enhancers in cell culture, neuron, and mesenchymal cell precursors, etc., and in the region of regulatory DNA motifs). The rs7579411 polymorphism is associated with the level of STON1-GTF2A1L gene expression in the thyroid gland.
References
1. Адамян Л.В., ред. Миома матки: диагностика, лечение и реабилитация. Клинические рекомендации по ведению больных. М.: ФГБУ «Научный Центр акушерства, гинекологии и перинатологии им. В.И. Кулакова» Минздрава России; 2015. 100с. [Adamyan L.V., ed. Uterine fibroids: diagnosis, treatment and rehabilitation. Clinical guidelines for the management of patients. Moscow: FGBU “Scientific Center for Obstetrics, Gynecology and Perinatology named after V.I. Kulakov, Ministry of Health of Russia; 2015. 100p. (in Russian)]
2. McWilliams M.M., Chennathukuzhi V.M. Recent advances in uterine fibroid etiology. Semin. Reprod. Med. 2017;35(2):181-9.
3. Киселев В.И., Сидорова И.С., Унанян А.Л., Муйжнек Е.Л. Гиперпластические процессы органов женской репродуктивной системы: теория и практика. М.: МЕДПРАКТИКА-М; 2010. 468с. [Kiselev V.I., Sidorova I.S., Unanyan A.L., Muizhnek E.L. Hyperplastic processes of the organs of the female reproductive system: theory and practice. Moscow: MEDPRAKTIKA-M; 2010. 468p. (in Russian)]
4. Carranza-Mamane B., Havelock J., Hemmings R. The management of uterine fibroids in women with otherwise unexplained infertility. J. Obstet. Gynaecol. Can. 2015; 37(3): 277-85.
5. Segars J.H., Parrott E.C., Nagel J.D., Guo X.C., Gao X., Birnbaum L.S. et al. Proceedings from the Third National Institutes of Health International Congress on Advances in Uterine Leiomyoma Research: comprehensive review, conference summary and future recommendations. Hum. Reprod. Update. 2014; 20(3): 309-33.
6. Cardozo E.R., Clark A.D., Banks N.K., Henne M.B., Stegmann B.J., Segars J.H. The estimated annual cost of uterine leiomyomata in the United States. Am. J. Obstet. Gynecol. 2012; 206(3): 211. e1-211. e9.
7. Moravek M.B., Yin P., Ono M., Coon J.S., Dyson M.T., Navarro A. et al. Ovarian steroids, stem cells and uterine leiomyoma: therapeutic implications. Hum. Reprod. Update. 2015; 21(1): 1-12.
8. Sparic R., Mirkovic L., Malvasi A., Tinelli A. Epidemiology of uterine myomas: a review. Int. J. Fertil. Steril. 2016; 9(4): 424-35.
9. Plant T.M. 60 YEARS OF NEUROENDOCRINOLOGY: The hypothalamo-pituitary-gonadal axis. J. Endocrinol. 2015; 226(2): T41-54.
10. Wise L.A., Laughlin-Tommaso S.K. Epidemiology of uterine fibroids – from menarche to menopause. Clin. Obstet. Gynecol. 2016; 59(1): 2-24.
11. Ward L.D., Kellis M. HaploReg v4: systematic mining of putative causal variants, cell types, regulators and target genes for human complex traits and disease. Nucleic Acids Res. 2016; 44(D1): D877-81.
12. Ward L.D., Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012; 40(Database issue): 930-4.
13. The GTEx Consortium. Genetic effects on gene expression across human tissues. Nature. 2017; 550(7675): 204-13.
14. Baird D.D., Kesner J.S., Dunson D.B. Luteinizing hormone in premenopausal women may stimulate uterine leiomyomata development. J. Soc. Gynecol. Investig. 2006; 13(2): 130-5.
15. Пономаренко И.В., Чурносов М.И. Современные представления об этиопатогенезе и факторах риска лейомиомы матки. Акушерство и гинекология. 2018; 8: 27-32. [Ponomarenko I.V., Churnosov M.I. Current views on the etiopathogenesis and risk factors of uterine leiomyoma. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; (8): 27-32. (in Russian)] https://dx.doi.org/10.18565/aig.2018.8.27-32
16. Bassett J.H.D., Williams G.R. Role of thyroid hormones in skeletal development and bone maintenance. Endocr. Rev. 2016; 37(2): 135-87.
17. Chen Z.J., Zhao H., He L., Shi Y., Qin Y., Shi Y. et al. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat. Genet. 2011; 43(1): 55-9.
18. Shi Y., Zhao H., Shi Y., Cao Y., Yang D., Li Z. et al. Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat. Genet. 2012; 44(9): 1020-5.
19. Jones M.R., Brower M.A., Xu N., Cui J., Mengesha E., Chen Y.D. et al. Systems genetics reveals the functional context of PCOS loci and identifies genetic and molecular mechanisms of disease heterogeneity. PLoS Genet. 2015; 11(8): e1005455.
20. Davis B.J., Risinger J.I., Chandramouli G.V.R., Bushel P.R., Baird D.D., Peddada S.D. Gene expression in uterine leiomyoma from tumors likely to be growing (from Black Women over 35) and tumors likely to be non-growing (from White Women over 35). PLoS One. 2013; 8(6): e63909.
Received 21.02.2018
Accepted 02.03.2018
About the Authors
Ponomarenko, Irina V., MD, associate professor of the Department of Biomedical Disciplines of the Medical Institute, Belgorod State National Research University.308015, Russia, Belgorod, Pobedy str. 85. Tel.: +74722301383. E-mail: ponomarenko_i@bsu.edu.ru. https://orcid.org/0000-0002-5652-0166
Polonikov Alexey Valerevich, MD, Professor, Head of the Department of Biology, Medical Genetics and Ecology, Kursk State Medical University,
Ministry of Health of Russia. 305041, Russia, Kursk, K. Marx str. 3. Tel.: +74712588147. E-mail: polonikovav@kursksmu.net
Churnosov Mikhail Ivanovich, MD, Professor, Head of the Department of Biomedical Disciplines Medical Faculty, Belgorod State National Research University.
308015, Russia, Belgorod, Pobedy str. 85. Tel.: +74722301383. E-mail: churnosov@bsu.edu.ru. http://orcid.org/0000-0003- 1254-6134
For citations: Ponomarenko I.V., Polonikov A.V., Churnosov M.I. Polymorphic LHCGR gene loci associated with the development of uterine fibroids. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; (10): 86-91. (in Russian)
https://dx.doi.org/10.18565/aig.2018.10.86-91



