Синдром множественных эндокринных неоплазий 1-го типа (МЭН-1) – заболевание с аутосомно-доминантным типом наследования, обусловленное герминальными мутациями в гене-супрессоре опухолевого роста MEN1. Распространенность МЭН-1 не превышает 2–3 случая на 100 000 человек и относится к орфанным заболеваниям; встречается с одинаковой частотой как у мужчин, так и у женщин [1]. «Классическая триада» заболевания характеризуется сочетанием опухолей околощитовидных желез (ОЩЖ, 95%), передней доли гипофиза (15–55%) и островкового аппарата поджелудочной железы (30–80%), но может проявляться опухолями надпочечников, нейроэндокринными образованиями тимуса, легких и желудочно-кишечного тракта (ЖКТ), липомами, менингиомами и другими [2–4]. Ожидаемая продолжительность жизни пациентов с МЭН-1 ниже по сравнению с общей популяцией и составляет около 55 лет. Наиболее часто причиной смерти становятся дуоденопанкреатические нейроэндокринные опухоли, карциноиды тимуса и бронхов [5, 6].
Пенетрантность первичного гиперпаратиреоза (ПГПТ) при МЭН-1 высока и с возрастом достигает 90–100% [7]. Дебют, как правило, приходится на период между 20 и 25 годами [8, 9]. Поражение ОЩЖ чаще носит множественный характер, что обуславливает высокий риск рецидива ПГПТ. Среди аденом гипофиза преобладают пролактиномы (65%) и соматотропиномы (25%); средний возраст манифестации – 38 лет [2]. Гормонально-неактивные нейроэндокринные опухоли поджелудочной железы относятся к наиболее распространенным энтеропанкреатическим образованиям в структуре МЭН-1 (50–80% в возрасте до 50 лет) и имеют худший прогноз, по сравнению с гормонально-активными образованиями [4].
Несмотря на особенности соматического статуса, пациенты с МЭН-1 ставят вопросы о возможности продолжения рода, что вызывает немало дискуссий в медицинском сообществе. Для пациентов репродуктивного возраста, имеющих в своем фенотипе опухоли гипофиза, на первый план выходит восстановление фертильности. Манифестация ПГПТ, особенно в развернутой клинической форме с выраженным повышением паратгормона и кальция крови диктует необходимость проведения паратиреоидэктомии в адекватном объеме до наступления беременности. Опухоли гастропанкреатической области несут в себе высокие риски прогрессирования во время беременности. Отдельного внимания заслуживает вопрос наследования генетической патологии от матери или отца.
В настоящее время не разработано клинических рекомендаций по данной проблеме, однако применяются общие принципы ведения той или иной патологии, входящей в симптомокомплекс заболевания, что требует организации междисциплинарной команды для эффективного контроля исходов, как для матери, так и для плода. Количество публикаций, посвященных сложностям и специфике ведения пациенток с МЭН-1, в зарубежной литературе ограничено [10–13], а российские работы по данной проблеме вовсе отсутствуют. Мы представляем два наблюдения успешного ведения беременности у пациенток с МЭН-1, верифицированным до зачатия, с благоприятным исходом для матери и ребенка.
Описание клинических наблюдений
Клиническое наблюдение № 1
Пациентка Л., которую с 25 лет беспокоили приступы почечных колик, к врачам с этими жалобами не обращалась. В возрасте 26 лет при обследовании по поводу вторичной аменореи диагностирована соматопролактинома, активная стадия акромегалии (пролактин – 3158 меМЕ/мл (59–619), инсулиноподобный фактор роста 1(ИФР1) – 409 нг/мл (117–329), соматотропный гормон (СТГ)≥1 нг/мл в ходе перорального глюкозотолерантного теста). По данным магнитно-резонансной томографии (МРТ) головного мозга визуализирована макроаденома гипофиза размерами 7×12×10 мм с супраселлярным ростом без признаков сдавления хиазмы. При дальнейшем лабораторно-инструментальном обследовании верифицирован ПГПТ, образование эктопированной ОЩЖ справа и кпереди от брахиоцефального ствола. При мультиспиральной компьютерной томографии (МСКТ) выявлены множественные образования поджелудочной железы: в перешейке — размерами 12,5×12,5×12,5 мм; в хвосте – размерами 4,3×4,3×3 мм; объемное образование левого надпочечника. Данных за гормональную активность образований поджелудочной железы и инциденталомы надпочечника получено не было. Клинический фенотип МЭН-1 у пациентки молодого возраста был подтвержден генетическим анализом MEN1 (гетерозиготная мутация с. 1231dupG p.A411GfsX47).
Принимая во внимание смешанную секрецию СТГ и пролактина аденомой гипофиза, назначен агонист дофамина (каберголин) с постепенным увеличением дозы до 2,5 мг в неделю, с достижением нормализации уровня пролактина и ИФР1/СТГ, восстановлением менструального цикла. По месту жительства проведено удаление правой нижней ОЩЖ, без достижения ремиссии ПГПТ. По результатам гистологического исследования операционного материала верифицирована гиперплазия ОЩЖ.
В возрасте 27 лет пациентка начала планировать беременность и настаивала на естественном зачатии и родах. Мультидисциплинарной командой врачей разработана поэтапная концепция прегравидарной подготовки. В первую очередь, в ФГБУ «НМИЦ эндокринологии» Минздрава России выполнена тотальная паратиреоидэктомия с ревизией мест типичного расположения ОЩЖ, удалением эктопированного образования правой нижней железы (2,5 см) вместе с рогами тимуса. Учитывая измененную структуру всех желез, аутотрансплантация части ОЩЖ в мягкие ткани не проводилась. В послеоперационном периоде развился транзиторный гипопаратиреоз, потребовавший краткосрочного приема активного метаболита витамина D – альфакальцидола (1 мкг в сутки). Спустя месяц после операции препарат был отменен с сохранением стойкой нормокальциемии. Принимая во внимание недостаточность 25(ОН)D в сыворотке крови и планируемую беременность, назначен колекальциферол.
Учитывая стойкоесохранение нормопролактинемии и целевых значений СТГ/ИФР1, а также уменьшение размеров аденомы гипофиза до 6×7×6 мм, от трансназальной аденомэктомии было решено воздержаться. Таким образом, по результатам проведенных мероприятий был достигнут благоприятный фон для зачатия.
В январе 2018 г. наступила желанная беременность, при установлении факта которой каберголин был отменен. Беременность протекала физиологично, патологических изменений плода не отмечалось. Во II триместре пациентка отметила появление выраженных головных болей, в связи с чем проведена компьютерная периметрия и МРТ головного мозга без контрастного усиления; отрицательной динамики размеров аденомы не зафиксировано. 17.09.2018 в исходе срочных родов естественным путем родился живой мальчик (8 баллов по шкале Апгар) весом 3650 г, ростом 52 см. Учитывая возраст, молекулярно-генетическое исследование MEN1 у ребенка пока не проведено.
Спустя 3 месяца на фоне самостоятельного прекращения лактации определялось повторное повышение уровней пролактина, ИФР1 и СТГ. По данным МРТ головного мозга выявлена умеренная отрицательная динамика размеров соматопролактиномы (максимально до 11 мм). Назначена комбинированная терапия каберголином и аналогами соматостатина и рекомендован динамический контроль с последующим решением вопроса о плановом радикальном лечении.

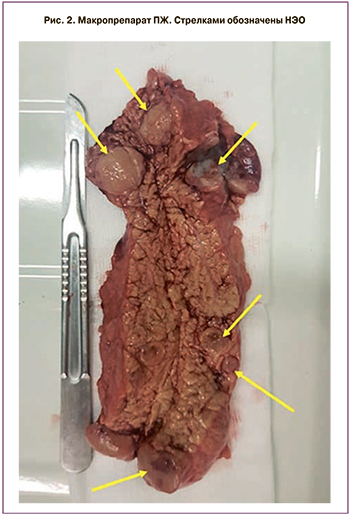 Во время последней госпитализации в ФГБУ «НМИЦ эндокринологии» Минздрава России по данным МСКТ поджелудочной железы зафиксирована отрицательная динамика образований в виде увеличения их количества и размеров (рис. 1). Уровни маркеров нейроэндокринных опухолей (хромогранина А, гастрина, глюкагона, панкреатического полипептида, серотонина в крови, метилированных катехоламинов в моче) были в пределах референсных значений, за исключением умеренного повышения 5-оксииндолуксусной кислоты в суточной моче до 60 мкмоль/сутки (в норме до 53). В ФГБУ «НМИЦ онкологии» им. Н.Н. Блохина Минздрава России выполнена пункционная биопсия доступных образований, по результатам которой обнаружены клетки, подозрительные в отношении нейроэндокринных опухолей. 22 мая 2019 г. проведено хирургическое лечение в объеме дистальной субтотальной резекции поджелудочной железы с сохранением селезенки. При интраоперационном УЗИ в теле и хвосте поджелудочной железы дополнительно выявлены еще три опухолевых образования до 5 мм в диаметре (рис. 2). Гистологический анализ послеоперационного материала подтвердил диагноз нейроэндокринной опухоли солидно-трабекулярного и альвеолярного строения (Grade 2).
Во время последней госпитализации в ФГБУ «НМИЦ эндокринологии» Минздрава России по данным МСКТ поджелудочной железы зафиксирована отрицательная динамика образований в виде увеличения их количества и размеров (рис. 1). Уровни маркеров нейроэндокринных опухолей (хромогранина А, гастрина, глюкагона, панкреатического полипептида, серотонина в крови, метилированных катехоламинов в моче) были в пределах референсных значений, за исключением умеренного повышения 5-оксииндолуксусной кислоты в суточной моче до 60 мкмоль/сутки (в норме до 53). В ФГБУ «НМИЦ онкологии» им. Н.Н. Блохина Минздрава России выполнена пункционная биопсия доступных образований, по результатам которой обнаружены клетки, подозрительные в отношении нейроэндокринных опухолей. 22 мая 2019 г. проведено хирургическое лечение в объеме дистальной субтотальной резекции поджелудочной железы с сохранением селезенки. При интраоперационном УЗИ в теле и хвосте поджелудочной железы дополнительно выявлены еще три опухолевых образования до 5 мм в диаметре (рис. 2). Гистологический анализ послеоперационного материала подтвердил диагноз нейроэндокринной опухоли солидно-трабекулярного и альвеолярного строения (Grade 2).
Несмотря на отягощенный анамнез, пациентка высказала желание о второй беременности.
Клиническое наблюдение № 2
Пациентка К., с 18 лет отмечала рецидивирующее течение нефролитиаза, по поводу которого неоднократно проводились дистанционные литотрипсии. Спустя год диагностирован ПГПТ. Учитывая визуализацию одного объемного образования левой нижней ОЩЖ, проведена селективная паратиреоидэктомия с достижением ремиссии заболевания.
Через 3 года после хирургического лечения развился рецидив ПГПТ: паратгормон – 77,1 пг/мл (15–65), альбумин-скорректированный кальций – 2,68 ммоль/л (2,15–2,55), по данным УЗИ и МСКТ визуализировано объемное образование правой верхней ОЩЖ. В возрасте 25 лет пациентка перенесла повторное хирургическое лечение ПГПТ с ревизией мест типичного расположения ОЩЖ и удалением двух измененных желез с нормализацией показателей кальция и паратгормона. Гистологическое исследование операционного материала выявило гиперплазию из главных и оксифильных клеток ОЩЖ.
Принимая во внимание множественное поражение ОЩЖ, рецидив ПГПТ и молодой возраст пациентки, заподозрен МЭН-1, в связи с чем проводился скрининг других компонентов синдрома. В 2009 г. впервые выявлена микропролактинома (пролактин – 1100 мЕд/л (90–540)); назначен каберголин в дозе 0,25 мг в неделю c положительной динамикой показателей (пролактин 378 мЕд/л (90-540)). Данных за другие нарушения функции аденогипофиза не получено. Уровни хромогранина А, гастрина, глюкагона не выходили за пределы референсного диапазона, однако отмечалось повышение серотонина крови и 5-оксииндолуксусной кислоты в суточной моче до 349,4 (30–200) и 75 мкмоль/сут (до 53) соответственно. При этом клинические проявления карциноидного синдрома отсутствовали. В 2011 г. по результатам МРТ брюшной полости визуализированы множественные образования поджелудочной железы: в перешейке – размерами 6 мм, в хвосте – 4 образования размерами от 6 до 19 мм. У пациентки отсутствовали абсолютные показания к хирургическому лечению, поэтому было выбрано динамическое наблюдение. По данным МРТ и лабораторных маркеров нейроэндокринных образований в динамике значимых изменений не выявлено.
Спустя 4 года после повторной паратиреоидэктомии вновь диагностирован рецидив ПГПТ (паратгормон – 78,3 пг/мл (15-65), альбумин-скорректированный кальций 2,63 ммоль/л (2,10–2,55)). Учитывая предшествующие операции в области шеи, умеренное повышение кальция и паратгормона, была выбрана консервативная тактика ведения ПГПТ. По результатам молекулярно-генетического анализа подтверждена гетерозиготная мутация MEN1: c.628_631delACAG p.Ser210fsTer222.
В марте 2016 г. наступила желанная беременность, в связи с чем произведена отмена агонистов дофамина. На фоне диетотерапии и расширенного питьевого режима в течение всей беременности сохранялась мягкая гиперкальциемия; таким образом, хирургического лечения не потребовалось. По результатам регулярной компьютерной периметрии нарушение полей зрения не зафиксировано. Скрининговые УЗИ патологических изменений со стороны плода не выявили. В декабре 2016 г. произошли своевременные оперативные роды путем кесарева сечения (КС). Родилась здоровая девочка (8 баллов по шкале Апгар) весом 3 070 г, длиной 51 см. По данным секвенирования фрагмента ДНК гена MEN1 ребенка патологических изменений не выявлено.
По результатам динамического обследования матери в 2019 г. сохраняется мягкое течение ПГПТ, нормопролактинемия, отсутствие увеличения размеров образований поджелудочной железы и значимых изменений маркеров нейроэндокринных образований. МРТ головного мозга с контрастным усилением продемонстрировала положительную динамику в виде исчезновения микропролактиномы с сохранением умеренной диффузной неоднородности гипофиза.
Обсуждение
Недавно опубликована работа, демонстрирующая отсутствие влияния МЭН-1 на фертильность. Исследование проведено среди жителей Австралийского острова Тасмании; одним из первых немногочисленных поселенцев был человек с МЭН-1-синдромом, передавшим мутацию последующим поколениям [14]. Небольшое население и невысокий уровень миграции позволили сохранить медицинские архивы в хорошем качестве. В результате анализа установлено, что по сравнению с родителями MEN1-, у родителей MEN1+ доля мертворожденных была сопоставима; при этом среднее количество детей и доля живорожденных на одного родителя была даже выше. Хотя репродуктивный потенциал был нарушен в подгруппе пациентов с патологией гипофиза [14].
ПГПТ не влияет на фертильность, но рассматривается в качестве неблагоприятного фактора на течение и исход беременности. Гиперкальциемический криз – наиболее опасное осложнение ПГПТ во время беременности; чаще развивается в раннем послеродовом периоде вследствие прекращения оттока материнского кальция через плаценту к плоду [15]. Учитывая повышенные риски осложнений, как со стороны матери, так и со стороны плода, рекомендовано хирургическое лечение ПГПТ до наступления беременности. Оптимальным сроком для выполнения паратиреоидэктомии во время беременности считается II триместр. Тем не менее, у пациенток с мягкой формой ПГПТ допускается консервативная тактика. Медикаментозная терапия ПГПТ во время беременности лимитирована [16–20].
В первом клиническом наблюдении при планировании беременности нам удалось достичь ремиссии ПГПТ до зачатия и, таким образом, устранить потенциальное негативное влияние гиперкальциемии на мать и плод. Во втором наблюдении беременность наступила на фоне рецидива ПГПТ, но мягкое течение заболевания позволило ограничиться исключительно консервативными мероприятиями. Неонатальной гипокальциемии во втором случае отмечено не было.
Тактика ведения беременности у пациенток с аденомой гипофиза во многом зависит от типа секреции опухоли. В случае пролактином показана консервативная терапия агонистами дофамина для достижения наиболее благоприятного фона – нормализации уровня пролактина и уменьшения размеров опухоли <10 мм [1]. Несмотря на то что частота пролактином, резистентных к терапии агонистами дофамина, при МЭН-1 несколько выше, по сравнению со спорадической формой заболевания [4], в описанных нами случаях консервативная терапия была эффективна. Планирование беременности при акромегалии зависит от таких параметров, как степень гормональной активности соматотропиномы, размеров образования и наличия масс-эффектов [2]. Как правило, беременность не приводит к прогрессированию акромегалии, и само заболевание не сопровождается негативным влиянием на плод. Предполагается, что эстрогены формируют относительную резистентность тканей к гормону роста и опосредованно снижают уровень ИФР1 [3]. Однако определение уровней ИФР1 и пролактина во время беременности для оценки гормональной активности аденом нецелесообразно. В первом наблюдении на фоне приема каберголина наблюдалась ремиссия акромегалии и уменьшение объема аденомы, что позволило отсрочить проведение трансназальной аденомэктомии и, следовательно, снизить риски послеоперационного гипопитуитаризма.
Несмотря на то что у большинства пациенток не происходит увеличения объема образования гипофиза, риски компрессии хиазмы сохраняются. Показана регулярная оценка полей зрения с помощью компьютерной периметрии. МРТ (без контрастного усиления) проводится только при наличии клинических данных, свидетельствующих о росте опухоли [21, 22].
Информация о течении нейроэндокринных образований поджелудочной железы во время беременности ограничена. Предпочтительно произвести удаление гормон-секретирующей опухоли до зачатия. В представленных наблюдениях образования поджелудочной железы были гормонально-неактивными, абсолютных показаний к операции на этапе планирования беременности не отмечалось. Однако увеличение размеров опухолей поджелудочной железы в первом клиническом наблюдении стало показанием для планового хирургического лечения. Рост опухолей поджелудочной железы может быть спровоцирован физиологичным повышением уровня ИФР1 во время беременности.
Поскольку МЭН-1 имеет аутосомно-доминантное наследование и риск передачи мутации потомству составляет 50%, разрабатываются новые подходы в отношении прегравидарной подготовки. В частности, преимплантационная генетическая диагностика позволяет отобрать здоровые эмбрионы и повысить вероятность успешной беременности среди пациентов, являющихся носителями моногенных заболеваний. Недавно описано клиническое наблюдение успешной преимплантационной генетической диагностики с рождением здорового младенца у пары, в которой мужчина – носитель гетерозиготной мутации в гене MEN1 [23, 24]. Описанный вариант протокола не рационален для носителей мутаций MEN1 женского пола, поскольку стимуляция в рамках ЭКО может привести к прогрессированию опухолевых процессов. Тем не менее, остается возможность частичного применения данного подхода при суррогатном материнстве.
Заключение
Планирование и ведение беременности у пациенток с МЭН-1 осложняется сочетанием различных эндокринных и неэндокринных нарушений, способных усугублять течение друг друга и требующих серьезных лечебно-диагностических мероприятий. Основной терапевтической целью является корректное и последовательное управление МЭН-ассоциированными заболеваниями с анализом соотношения риска/пользы для выбранной тактики.



