Placental dysfunction in HIV-infected pregnant women
Voevodin S.M., Shemanayeva Т.V., Shchegolev A.I., Parkhomenko Yu.G.
Aim. To investigate the clinical features of the course and outcomes of pregnancy and placental morphology in HIV-infected pregnant women. Material and methods. This study is a retrospective analysis of 29 pregnant women. The study group comprised 14 pregnant women with antenatal human immunodeficiency virus infection. Fifteen women with a physiological course of pregnancy made up a control group. The mean age of patients in the study and control group was 28.0 ± 2.6 and 21.1±2.3 years, respectively. The analysis included gynecological history, the course of pregnancy and childbirth, and perinatal outcomes of newborns. The morphological study of placenta included macroscopic and histological examinations, and immunohistochemical studies using antibodies targeting CCR5 receptors. Results. Pregnant women in the study group had a gynecological history of sexually transmitted infections. The most frequent complications of the second and third trimesters of pregnancy were anemia (78.6%), the threatened preterm birth (35.7%), and preeclampsia (28.6%). Complications of labor were premature rupture of membranes (35.7%) and uncoordinated uterine activity (14.3%). Placental morphology showed signs of inflammation and hypoxia. Immunohistochemical studies identified a higher expression of CCR5 in chorionic villi. Conclusion. The findings indicate increased levels of CCR5 expression and the development of chronic placental insufficiency in HIV-infected pregnant women, which indicates the need for dynamic monitoring of this group of patients.
Keywords
Viral infection plays an important role in the development of pathological conditions in pregnant women, fetuses, and newborns [1, 2]. The problem of viral infections in perinatal medicine has increased owing to societal changes that have resulted in an increased risk of infections that occur during pregnancy, especially the human immunodeficiency virus infection (HIV) [3, 4].
Previous studies [5, 6] have suggested that viral infections can lead to both fetal abnormalities and pregnancy loss, and neonatal complications.
The diagnosis of HIV infection is based on the detection of a CCR5-tropic strain of HIV-1. In most cases, mainly in the late stages of the disease, strains tropic to CXCR4 (X4-tropic) or simultaneously to CCR5 and CXCR4 (R5X4-tropic) are also identified [7, 8]. The main problem with chronic viral infections (including HIV infection) is the development of fetoplacental pathological lesions, which, unfortunately, have not yet been extensively studied [9, 10].
This study aimed to investigate the clinical features of the course and outcomes of pregnancy and placental morphology in HIV-infected pregnant women.
Material and methods
The present study represents a retrospective analysis of data from 29 pregnant women. The study group comprised 14 pregnant women with antenatal human immunodeficiency virus infection. Fifteen women with a physiological course of pregnancy made up a control group. The mean age of patients in the study and control group was 28.0 ± 2.6 and 21.1±2.3 years, respectively.
The analysis included gynecological history, the course of pregnancy and childbirth, and perinatal outcomes of newborns. Fetal growth retardation syndrome was diagnosed when an estimated fetal weight was below the 10th percentile for gestational age as determined by an ultrasound.
The form and extent of the fetal growth retardation syndrome were determined based on the guidelines of the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) [11, 12].
The morphological study of placenta included macroscopic and histological examinations by generally accepted recommendations [13, 14]. For the microscopic examination, tissue sections were taken from the paracentral zone of the placenta and fixed in a 10% solution of neutral formalin. Histological examination was carried out on paraffin sections stained with hematoxylin and eosin. Immunohistochemical study was performed on 3 μm thick tissue sections from the paraffin block using the BenchMark-XT automated staining system (Ventana Medical Systems, Roche) and rabbit monoclonal antibody to CCR5 (clone E164) in 1:100 dilution by Epitomics. The marker expression was quantitatively evaluated in the syncytiotrophoblast and endothelium of placental villous capillaries using the Nikon Eclipse 80i microscope with NIS-Elements 3.2 imaging software. Statistical analysis of quantitative data was carried out using Statistica 8.0 software.
Results and discussion
In the study group, 12 (85.7%) of women had a gynecological history of sexually transmitted infections (ureaplasma, papillomavirus, chlamydia, herpetic, and cytomegalovirus infection), pelvic inflammatory diseases, menstrual irregularities and uterine cervical conditions. No patients in the control group had a history of these diseases/conditions. In the study group, 3 (21.4%) of pregnant women were primigravida, and 11 (78.6%) of women had a history of pregnancy termination before 12 weeks’ gestation. The women in the control group had no history of early or late pregnancy termination. Ten (66.7%) and 5 (33.3%) of them were primigravida and reported a history of full-term delivery, respectively. Four (28.6%) pregnant women in the study group had a co-infection of HIV and chronic viral hepatitis C in an inactive state.
The vast majority of women in the study group registered late for an antenatal care program: 8 (57.1%) after 20 weeks and 4 (28.6%) from 12 to 20 weeks’ gestation.
In the second trimester of pregnancy, the most frequent complications in the study group were anemia and threatened preterm birth. Thus, mild anemia, moderate preeclampsia, threatened preterm birth, acute respiratory disease (ARI), and exacerbation of cystitis were diagnosed in 10 (71.4%), 2 (14.3%), 3 (21.4%), 4 (28.6%), and in 1 (7.1%) women, respectively. In the control group, 2 (13.3%) pregnant women had mild anemia the third trimester.
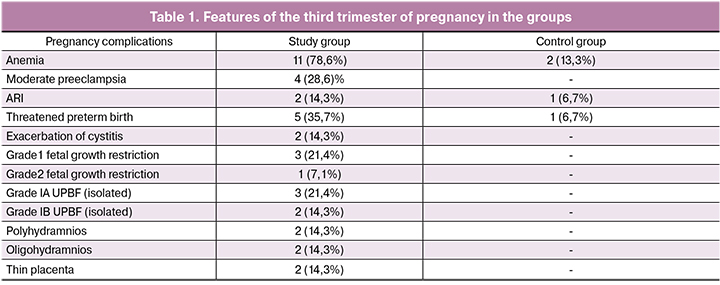
Among women in the study group, placental dysfunction manifested by changes in the amniotic fluid volume: moderate oligohydramnios and moderate polyhydramnios were observed in 3 (21.4) and one (7.1%) women, respectively. Isolated impairment of uteroplacental blood flow (UPBF), isolated impairment of blood flow in the umbilical cord, and thin placenta in the present gestation were observed in 2 (14.3%), 1 (7.1%), and 14.3% of women, respectively. The pregnant women in the control group did not have these signs of placental dysfunction. The main complications in the third trimester, both in the study and control group, were threatened preterm birth, anemia, and ARI (Table 1).
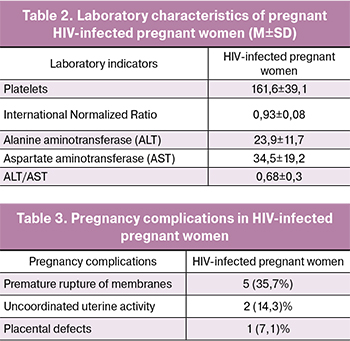 Laboratory characteristics of pregnant HIV-infected pregnant women are presented in table 2.
Laboratory characteristics of pregnant HIV-infected pregnant women are presented in table 2.
Five (35.7%) pregnant women with HIV infection experienced premature rupture of membranes. Labor complications observed among women in the study group are presented in Table. 3. There were no labor complications in the control group.
The mean newborn birth weight was 2900 ± 158.1 g in the study group and 3450 ± 115.2 g in the control group. All newborns of the control group were born with the first- and fifth-minute Apgar scores of 9/9 points. Infants born to HIV-infected mothers had prematurity, morphofunctional immaturity, neonatal hypotrophy, and hypoxic-ischaemic injury to the central nervous system. Perinatal outcomes and neonatal complications in newborns of the study group are presented in Table. 4.
To prevent vertical transmission of the infection, all the women in the study group used various anti-retroviral medications (Kaletra, Epivir, Combivir, Retrovir). These drugs did not have a direct effect on the process of liver fibrosis.
Morphological assessment of placental specimens taken from the patients of the study group revealed signs of inflammation and hypoxia. Thus, signs of chorioamnionitis were found in 3 (21.4%) women. Chronic vasculitis and funisitis were diagnosed in 2 (14.3%) and 3 (21.4%) women, respectively. Eleven (78.6%) pregnant women were found to have acute diffuse basal deciduitis (Fig. 1).
 In 8 (57.1%) women of the study group, the maturation of the placental villous tree matched gestational age, while in 2 (14.3%) and 4 (28.6%) it was retarded, and accelerated, respectively. Signs of chronic utero-placental hypoxia in the form of numerous small terminal villi with multiple syncytial nodules were detected in 6 (42.9%) patients. In 2 (14.3%) cases there were large and small villous tree infarcts and numerous accumulations of avascular villi, and 3 (21.4%) patients had an expansion and multiple thrombi of intervillous space. In the control group, in 12 (80%) women, the maturation of the placental villous tree matched the gestational age, and in 3 (20%) it was accelerated.
In 8 (57.1%) women of the study group, the maturation of the placental villous tree matched gestational age, while in 2 (14.3%) and 4 (28.6%) it was retarded, and accelerated, respectively. Signs of chronic utero-placental hypoxia in the form of numerous small terminal villi with multiple syncytial nodules were detected in 6 (42.9%) patients. In 2 (14.3%) cases there were large and small villous tree infarcts and numerous accumulations of avascular villi, and 3 (21.4%) patients had an expansion and multiple thrombi of intervillous space. In the control group, in 12 (80%) women, the maturation of the placental villous tree matched the gestational age, and in 3 (20%) it was accelerated.
It is known that HIV viruses require coreceptors, in addition to CD4, to infect target cells. The C-C chemokine receptor type 5 (CCR5) serves as the major co-receptor for HIV-1 infection. Chemokine receptors belong to the G protein-coupled receptor family. It is the interaction of HIV with the CD4 receptor and co-receptor that underlies the penetration of the virus into the cell and results in the development of the disease [10, 15]. In turn, homozygous carriers of the CCR5-Δ32 mutation are resistant to HIV-1 infection [16].
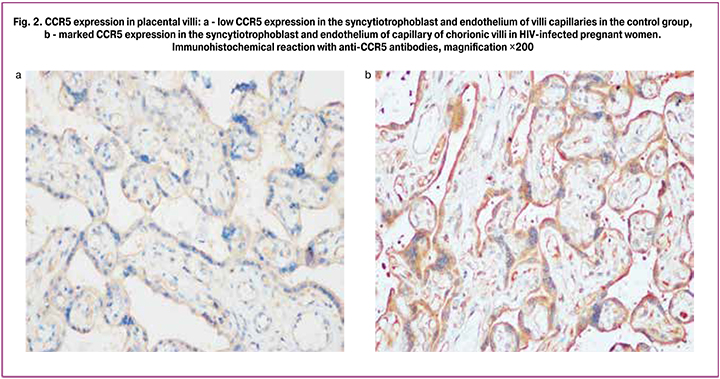
 Immunohistochemical analysis showed that the syncytiotrophoblast, stromal cells and endothelial cells of the villi capillaries had a positive reaction with CCR5 antibodies (Fig. 2). The levels of placental expression of CCR5 in the HIV-infected women were significantly higher than in the control group (p <0.05): 2.07 and 1.93 times higher in syncytiotrophoblast and endothelial cells of the villi capillaries, respectively.
Immunohistochemical analysis showed that the syncytiotrophoblast, stromal cells and endothelial cells of the villi capillaries had a positive reaction with CCR5 antibodies (Fig. 2). The levels of placental expression of CCR5 in the HIV-infected women were significantly higher than in the control group (p <0.05): 2.07 and 1.93 times higher in syncytiotrophoblast and endothelial cells of the villi capillaries, respectively.
Our findings on characteristic features of placental injury are consistent with the literature on nonspecific involute-dystrophic changes and circulatory disorders in placental specimens from HIV-infected pregnant women with the development of chronic placental insufficiency of varying severity [17, 18]. As is known, there is a direct correlation between the risk of HIV transmission and the occurrence of chorioamnionitis, placental insufficiency, placental abruption, and uterine bleeding [19].
In the histological study of placental specimens, A.N. Rymashevsky et al. [20] reported a marked collagenization of stem villus stroma, vascular wall sclerosis with the proliferation of endothelial cells, and the presence of areas of calcification and fibrinoid deposits.
It is noteworthy that HIV-infected puerperal women had an increased content of type IV collagen in the vessel walls of the stem villi and vasculo-syncytial membranes of terminal chorionic villi [18], and the presence of numerous so-called afunctional sites [20].
According to S. Bustamante et al. [21], the positive expression of the CCR5 and CXCR4 receptors is characteristic of the mature trophoblast cells, stroma and endothelium of the villi of the mature placenta. Moreover, in a larger number of trophoblast cells, CCR5 and CXCR4 were predominantly localized in the intracellular compartment rather than on the membrane [22].
At the same time, according to the literature [23], Kashchenko-Goughbauer cells, which are placental macrophages, have lower CD4 and CCR5 expression then macrophages in other organs. In A.V. Kolobov [24] opinion, a similar metabolic feature of placental macrophages underlies their congenital immunity to HIV infection.
A higher content of CCR5 in placental structures has been found to increase the risk of developing HIV infection [25]. Among HIV-infected mothers, a higher viral load was observed in women with a high level of placental expression of CCR5 [26].
According to our studies, HIV-infected puerperal women have a higher level of immunohistochemical expression of CCR5 in placental tissue. Moreover, a more pronounced difference with the control group is characteristic of the villi syncytiotrophoblast cells, which is due to their localization. Syncytiotrophoblast is the first barrier of the placental villi that comes in contact with the circulating maternal blood. In this connection, in our opinion, an important mechanism of antenatal vertical mother-to-child transmission of HIV is not only high expression of CCR5 but also damage to the syncytiotrophoblast, facilitating the virus to enter through the placenta into the fetal blood circulation.
Another important link in the pathogenesis of impaired maturation of chorionic villi and chronic placental insufficiency in HIV-infected pregnant women is the imbalance between pro-angiogenic and anti-angiogenic factors in placental structures. D.A. Niauri et al. [27] reported that analysis of the placental tissue of HIV-infected pregnant women demonstrated a predominant expression of anti-angiogenic factors, in particular, TGF-β1. At the same time, the imbalance between the factors contributing to and inhibiting angiogenesis is considered now as a leading mechanism of the pathogenesis of preeclampsia [28, 29].
It should also be added that HIV infection, which contributes to the formation of placental insufficiency also causes fetal growth restriction and postnatal maladaptation [30].
Conclusion
Therefore, HIV-infected women were found to have increased expression of CCR5 receptors in the chorion villus and varying degrees of placental injury underlying chronic placental insufficiency. Clinical complications of pregnancy and childbirth included anemia (78.6%), placental insufficiency (42.9%), threatened preterm birth (35.7%), mild preeclampsia (28.6%), uncoordinated uterine activity (14, 3%), indicating that prenatal care of pregnancies complicated by HIV infection should include dynamic monitoring of this category of pregnant women.
All infants who were tested positive for HIV had no ultrasound markers of intrauterine infection. This circumstance warrants the need for integrated expert clinical care and counseling involving an infectious disease specialist. The early registration for antenatal care (before 12 weeks gestation), timely administration of antiviral therapy depending on the viral load and the stage of HIV infection, and the ultrasonic control of markers of intrauterine infection and placental insufficiency reduce the risk of complications. Late initiation of antiretroviral therapy because of late registration for antenatal care that was observed among the majority of the women in the study group was a risk factor for HIV infection in the fetus and newborn.
References
- Kulakov V.I., Ordzhonikidze N.V., Tyutyunnik V.L. Placental insufficiency and infection. Moscow; 2004. 494p. (in Russian)
- Serov V.N. Features of infection in obstetrics, gynecology and Perinatology. Russian Medical Journal. 2006; 14(1): 2-5. (in Russian)
- Kulakov V.I., Baranov I.I. HIV: prevention of mother-to-child transmission Moscow: VEDI; 2003. 168 p. (in Russian)
- Sukhikh G.T., Baranov I.I. Reproductive health and HIV-infection, Moscow, Tver: Triada-X; 2009. 344 p. (in Russian)
- Mazus A.I., Olshansky A.Ya., Voevodin S.M., Makarov I.O., Shemanaeva T.V., Muravei A.Yu. Modern features of pregnancy management tactics against the background of chronic hepatitis in pregnant women with HIV infection. Vrach-aspirant. 2013; 57(2.1): 191-6. (in Russian)
- Makarov I.O., Shemanaeva T.V., Voevodin S.M., Muravei A.Y. Role of endothelial dysfunction in the development of obstetric complications in HIV-infected pregnant women. Vrach-aspirant. 2012; 54(5.1): 168-76. (in Russian)
- Doms R.W., Peipert S.C. Unwelcomed guests with master keys: How HIV uses chemokine receptors for cellular entry. Virology. 1997; 235: 179-90.
- Kristiansen T.B., Knudsen T.B., Eugen-Olsen J. Chemokine receptors and their crucial role in human immunodeficiency virus infection: Major breakthrough in HIV research. Scand. J. Immunol. 1998; 48: 339-46.
- Michael N.L., Moore J.P. HIV-1 entry inhibitors: evading the issue. Nat. Med. 1999; 5(7): 740-2.
- Al-Husaini A.M. Role of placenta in the vertical transmission of human immunodeficiency virus. J. Perinatol. 2009; 29(5): 331-6.
- Salomon L.J., Alfirevic Z., Berghella V., Bilardo C., Hernandez-Andrade E., Johnsen S.L. et al.; ISUOG Clinical Standards Committee. Practice guidelines for performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet. Gynecol. 2011; 37(1): 116-26.
- Clinical Standards Committee. ISUOG Practice Guidelines: use of Doppler ultrasonography in obstetrics. Ultrasound Obstet. Gynecol. 2013; 41: 233-9.
- Milovanov A.P. Pathology of the mother-placenta-fetus system. Moscow: Medicine; 1999. 448p. (in Russian)
- Shchegolev A.I. Current morphological classification of damages to the placenta. Akusherstvo i ginekologiya/Obstetrics and Gynecology. 2016; (4): 16-23. (in Russian) http://dx.doi.org/10.18565/aig.2016.4.16-23
- David F.J., Autran B., Tran H.C., Menu E., Raphael M., Debre P. et al. Human trophoblast cells express CD4 and are permissive for productive infection with HIV-1. Clin. Exp. Immunol. 1992; 88: 10-6.
- Samson M., Libert F., Doranz B.J., Rucker J., Liesnard C., Farber C.M. et al. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996; 382(6593): 722-7.
- Peretyatko L.P., Vasilyeva M.E., Kruglova L.V. Morphological changes in the placenta in HIV-infected women who received antiretroviral therapy with zidovudine before birth. Arhiv patologii. 2010; 72 (Appendix: Proceedings of the IV Congress of the Russian Society of Child Pathologists): 58-62. (in Russian)
- Burakovsky E.S. Immunomorphological peculiarities of placenta in HIV infection. Patologiya. 2011; 1: 22–25. (in Russian)
- Novikova O.M., Shvets E.M. Risk factors and features of pregnancy, childbirth and the state of the newborn in HIV-infected women. Mat’ i ditya v Kuzbasse. 2017; 3: 16-20. (in Russian)
- Rymashevsky A.N., Opruzhenkov A.V., Terekhina L.A., Kovaleva E.A. Сlinical and morphological features of HIV-associated pregnancy. Vestnik RUDN. Seriya: Medicina. 2011; 4: 103-8. (in Russian)
- Bustamante S., García Y., Garrido H., Bethencourt S., Tovar R., Ponce L. et al. CXCR-4 AND CCR-5 expression in normal term human placenta. Invest. Clin. 2005; 46(1): 25-35.
- Maldonado-Estrada J., Menu E, Roques P., Vaslin B., Dautry-Varsat A., Barre´-Sinoussi F., Chaouat G. Predominant intracellular expression of CXCR4 and CCR5 in purified primary trophoblast cells from first trimester and term human placentae. Am. J. Reprod. Immunol. 2003;50(4): 291-301.
- Melendez J., García V., Sánchez E., Delgado R., Torres G., Meléndez-Guerrero L.M. Is decreased HIV-1 infectivity of placental macrophages caused by high levels of beta-chemokines? Cell. Mol. Biol. (Noisy-le-grand). 2001; 47 Online Pub: OL51-9.
- Kolobov A.V. The place of retroviruses in perinatal pathology. Zhurnal infektologii. 2012; 4: 13-19. (in Russian)
- Behbahani H., Popek E., Garcia P., Andersson J., Spetz A.L., Landay A. et al. Up-regulation of CCR5 expression in the placenta is associated with human immunodeficiency virus-1 vertical transmission. Am. J. Pathol. 2000; 157(6): 1811-8.
- Joubert B.R., Franceschini N., Mwapasa V., North K.E., Meshnick S.R. Regulation of CCR5 expression in human placenta: insights from a study of mother-to-child transmission of HIV in Malawi. PLoS One. 2010; 5(2): e9212.
- Niauri D.A., Kolobov A.V., Tsinzerling V.A., Gzgzyan A.M., Dzhemlikhanova L.Kh., Kolobova O.L., Khubulava N.V. The placenta as the epidemic factor of vertical HIV transmission risk in conditions of comorbidity. VICh-infekciya i immunosupressii. 2016; 4: 7-16. (in Russian)
- Fan X., Rai A., Kambham N., Sung J.F., Singh N., Petitt M. et al. Endometrial VEGF induces placental sFLT1 and leads to pregnancy complications. J. Clin. Invest. 2014; 124(11): 4941-52.
- Pavlov K.A., Dubova Y.A., Shchegolev A.I. Fetoplacental angiogenesis during normal pregnancy: a role of placental growth factor and angiopoietins. Akusherstvo i ginekologiya/Obstetrics and Gynecology. 2010; 6: 10-15. (in Russian)
- Samarina A.V., Belyakov N.A. Implementation of approaches to reduce perinatal HIV transmission. VICh-infekciya i immunosupressii. 2014; 2: 7-24. (in Russian)
Received 08.04.2018
Accepted 20.04.2018
About the Authors
Voevodin Sergey Mikhailovich, MD, professor of the Department of Reproductive Medicine and Surgery MGMSU. I.A. Evdokimova.Address: 127473, Russia, Moscow, ul. Delegate, 20, building 1. Phone: 8 (916) 223-14-22. E-mail: voevod37@yandex.ru
Shemanayeva Tatyana Viktorovna, MD, professor of the Department of Obstetrics and Gynecology, the First Moscow State Medical University. THEM. Sechenov.
Address: 119991, Moscow, ul. Bolshaya Pirogovskaya, 2, p. 4. Phone: 8 (915) 308-77-94. E-mail: t.shemanaeva@rambler.ru
Schegolev Alexander Ivanovich, MD, Head of the Department. pathoanatomical department of FGBU NMIC academician V.I. Kulakov of the Ministry of Health of Russia. Address: 117997, Russia, Moscow, ul. Academician Oparin, 4. Phone: 8 (495) 438-28-92. E-mail: ashegolev@oparina4.ru
Parkhomenko Yuri Georgievich, MD, professor, head. pathologoanatomical department of the hospital of the Ministry of Health and Social Development No. 2 of the DZM. Address: Russia, Moscow, 8th st. Sokolina Mountain, 15. Telephone: 8 (495) 365-23-07. E-mail: paoikb2@mail.ru
For citations: Voevodin S.M., Shemanayeva Т.V., Shchegolev A.I., Parkhomenko Yu.G. Placental dysfunction in HIV-infected pregnant women. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; (9): 41-7. (in Russian)
https://dx.doi.org/10.18565/aig.2018.9.41-47



