High-risk human papillomavirus persistence and other molecular genetic predictors for cervical intraepithelial neoplasias
Objective. To analyze the significance of high-risk human papillomavirus (hrHPV) persistence, viral load, and mRNA expression of the MKI67, CDKN2A, PGR, and BCL2 genes in predicting the development of squamous intraepithelial lesions (SIL).Sycheva E.G., Nazarova N.M., Burmenskaya O.V., Prilepskaya V.N., Trofimov D.Yu., Sukhikh G.T.
Subjects and methods. The investigation included 85 women (mean age, 34±11 years) with NILM cytology and positive hrHPV. A comprehensive clinical and laboratory examination involved HPV typing with viral load assessment; real-time PCR assay of the mRNA expression of human genes; liquid cytology; extended colposcopy once every 12 months during 2-year follow-up, and cervical biopsy (if indicated).
Results. There were 2 groups: 1a) 52 (61%) patients with transient papillomavirus infection and a persistent negative response, 1b) 12 (14%) with transient infection and an unstable negative response; 2) 21 (25%) with persistent hrHPV. There was a worse colposcopic pattern in 12 (57%) patients in the persistent hrHPV group. There was persistence of HPV types (16, 31, 39, 52, 18, and 68). Histologically, SIL was verified for 2 years only in Group II: LSIL in 10 (47%) and HSIL in 1 (5%); 6 (55%) of them had changes in the mRNA expression of the MKI67, CDKN2A, PGR and BCL2 genes.
Conclusion. Persistent hrHPV with changes in the mRNA expression of the MKI67, CDKN2A, PGR, and BCL2 genes and in the colposcopic pattern are considered as markers for SIL progression.
Keywords
Human papillomavirus (HPV) is considered to be the most frequent sexually transmitted infection. HPV contamination is mainly typical for women aged under 25. Long-term persistence of one and the same high-risk HPV type (hrHPV) is believed to be etiologic factor of cervical intraepithelial neoplasia (CIN) development. American Society for Colposcopy and Cervical Pathology (ASCCP) proposed to use the term “small” forms of cervix uteri epithelial damage in order to define patients with HPV types 16 or 18 and hrHPV persistence when the patients’ cytology results are normal [1].
At the moment, scientific society is aware of 201 HPV types, 25 of which have oncogenic potential [2]. HPV are responsible for a wide range of diseases, from benign to invasive tumors. International Agency for Research on Cancer (IARC) identifies 12 hrHPV types (HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58 and 59) (group 1), associated with cervical cancer. These 12 HPV types trace their origin to one and the same evolutionary prototype, which also includes Alphapapillomavirus groups: А5, А6, А7, А9. Thirteen extra HPV types were classified as probably high-risk viruses, namely group 2B (HPV 26, 69, 82, 30, 53, 66, 70, 85, 97, 67) on the basis of their genetic affinity with group 1, and also HPV 34, 73 (А11), excluding HPV 68, which was included in a high-risk group IIa. Viruses of groups 1 and 2Aa cause 96 % of cervical cancer, while viruses of group 2B produce only 2.6% of cervical cancer [3].
Etiological factor of cervical cancer development is the hrHPV contamination of cervix uteri epithelium basal cells. There are two stages of HPV: 1) transient stage (reproductive and episomal infection), when virus exists autonomously; 2) integrative stage, when DNA virus genome integrates into the virus contamination of cells. Virus elimination happens in 70% of cases during the first year of contamination and in 91% of cases during 24 months [4]. According to the research data, transient infection can be divided into several variants: one with a persistent negative response which is also known as a negative HPV-test within a 12-month interval; the other with an unstable negative response which means HPV detection in a 12-month period or its elimination in 24 months [5]. In accordance with research findings, HPV was not detected in 50% of women in a 6-month period and in 75% of women by the end of the first year of contamination.
HPV oncogenic potential may be realized in case of high virus load, as well as of HPV integration into the host genome. It should also be caused by Е6/Е7 oncoproteins expression that change the cell cycle. Е6/Е7 interaction with p53 tumor suppressor protein and retinoblastoma protein (pRb) results in a decrease in apoptosis, pathologic proliferation, neoangiogenesis and tumor transformation activation. It is demonstrated that in all types of HPV groups 1, 2A and 2B, HPV-associated oncogenesis markers, such as mRNA E6/Е7, CDKN2А/p16 (up-regulation), ССND1, p53 and Rb (down-regulation) undergo analogous transformations [6]. Persistence means the existence of one and the same HPV type in a woman’s organism for the period of two and more years. During the contamination period the accumulation of somatic mutations occurs, which results in CIN development. The average duration of a contamination period in patients with CIN I is 586 days, in patients with koilocytotic atypia (according to cell smear) is 392.3 days [7]. According to the National Cancer Institute (NCI), CIN I progression to CIN III during a 24-month period occurs in 10% of women.
The aim of the study was to analyze the significance of high-risk human papillomavirus (hrHPV) persistence, viral load, and mRNA expression of the MKI67, CDKN2A, PGR, and BCL2 genes in predicting the development of squamous intraepithelial lesions (SIL).
Materials and Methods
The investigation included 85 women aged 18-45 (average age, 34±11 years), who applied to National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russia. The inclusion criteria were reproductive age, positive hrHPV, cytology negative for intraepithelial lesion or malignancy (NILM). A comprehensive clinical and laboratory examination involved HPV typing with viral load assessment, liquid cytology, extended colposcopy once every 12 months during a 2-year follow-up. If the persistence of one and the same hrHPV type was detected or colposcopy showed a process intensity decline, then the targeted cervical biopsy with endocervical curettage was conducted (if indicated).
The detection of 21 HPV types (6, 11, 16, 18, 26, 31, 33, 35, 39, 44(55), 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82) with viral load was carried out using the method of multiplex real-time PCR (DNA-Technology LLC, Russia, Product license № FSR 2010/08811). Endocervical sampling from the transitional epithelium was conducted using a special catheter. The sampling was placed into the 1.5 ml test-tube with sodium chloride (0.9%). Viral load assessment included a median as a measure of central tendency and an interquartile range as an integral criterion. The accuracy of intergroup differences was assessed by Mann-Whitney U-test.
In order to detect the risk of CIN development, the integral criterion of the MKI67, CDKN2A (р16), PGR and BCL2 mRNA genes expression was used together with real-time reverse transcription PCR [8, 9].
Risk index (RI) was assessed using the formula:
×100 (formula 1), where z is a regressive function:
Z = 0,8×ln [МKI67]/[PGR] + 1,6×ln [CDKN2A]/[BCL2] – 4 (formula 2), where [МKI67]/[PGR] is a correlation between mRNA expression of МKI67 and PGR mRNA genes, [CDKN2A]/[BCL2] is a correlation between mRNA expression of CDKN2A and BCL2 genes.
If RI was more than 57 points, then there was a high risk of CIN development [9].
Cell smear was assessed according to Bethesda System (TBS). Histological examination of the biopsy material was assessed using the WHO classification: low-grade dysplasia (CIN I, LSIL), high-grade dysplasia (CIN II–III, HSIL).
Extended colposcopy with its assessment was conducted in accordance with new colposcopy terminology system approved by the International Federation for Cervical Pathology and Colposcopy at the 14th World Congress in Rio de Janeiro in 2011.
Results and Discussion
The investigation included 85 women aged 18-45 (average age, 34±11 years). The most frequent HPV types were 16, 31, 51, 52, 39 and 18. HPV type 16 was detected in 25 women (29%), type 31 was in 17 women (20%), types 51 and 52 were in 12 women (14%), type 39 was in 11 women (13%), type 18 was in 7 women (9%) (Fig.1). According to the IARC classification, all these types comprise a high-risk oncogenic group. HPV types 33, 35, 45, 56, 58 and 68 occurred less than in 9% of cases. HPV type 6 was detected in 7 patients (9%).
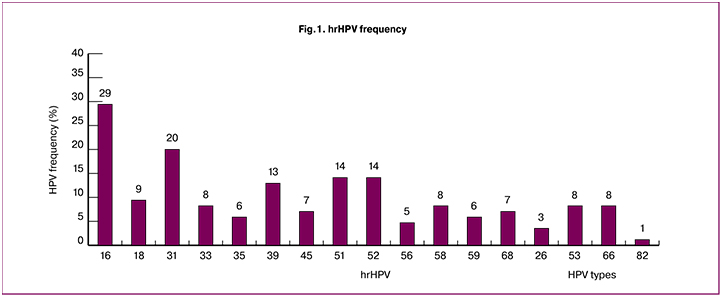
Following the results of dynamic examination, two groups of female patients were formed on the basis of the HPV development process: group Iа including 52 patients (61%) with transient papillomavirus infection and a persistent negative response, when virus elimination took place in 12 months; group Ib consisting of 12 patients (14%) with transient infection and an unstable negative response, when virus elimination occurred in 24 months after the contamination; group 2 including 21 patients (25%) with persistent hrHPV, detection of one and the same HPV type lasted for 24 months.
Full elimination of hrHPV in a 24-month period was manifested in 64 patients (75%), 52 of them showed positive results in the first 12 months, while other 12 patients had them in 24 months (Table 1).
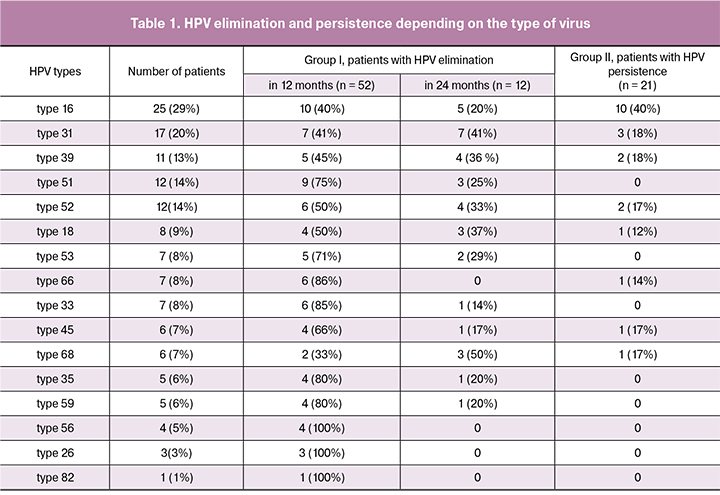
The analysis of hrHPV types elimination was carried out in 12 and 24 months. The results showed that elimination during the first 12 months occurred in 10 female patients (40%) with HPV type 16, in 9 patients (75%) with HPV type 51, in 7 patients (41%) with HPV type 31, in 5 patients (45%) with HPV type 39, in 2 patients (33%) with HPV type 68, in 6 patients (50%) with HPV type 52, in 6 patients (86%) with HPV type 66 and type 33, in 5 patients (71%) with HPV type 53, in 4 patients (66%) with HPV type 45; in 4 patients (50%) with HPV type 18.
In group II, the results showed persistence of HPV type 16 in 10 patients (40%), HPV type 31 in 3 patients (18%), HPV type 39 in 2 patients (18%), HPV type 52 in 2 patients (17%), HPV type 45 in 1 patient (17%), HPV type 68 in 1 patient (17%), HPV type 66 in 1 patient (14.3%) and HPV type 18 in 1 patient (12%). At baseline, average viral load in group Iа with persistent negative response and in group Ib with unstable negative response was 4.8 log of viral copies in the sample (with interquartile range 3.8 – 6.3). Viral load of the examined HPV types in group II was 4.5 log of viral copies in the sample (with interquartile range 3.5 – 5.7). Thus, the amount of viral load could not be considered as the persistence prediction criterion.
Cytology results in group I showed NILM (in 12 and 24 months). Group with hrHPV persistence showed that cytology results changed to more unfavorable in 3 cases: ASCUS (atypical squamous cells of undetermined significance) was revealed in 2 patients (9%) with HPV type 16 and type 31; there was LSIL in 1 patient (5%) with HPV type 16.
In order to assess cervical epithelium state, all the women underwent extended colposcopy twice with an interval of 12 months. After the first visit there were satisfactory colposcopy results (transformation zone, type 1) in 40 patients (77%) from group Iа with a persistent negative response, in 5 patients (42%) from the group Ib with unstable negative response, in 12 patients (57%) from group II with persistent hrHPV. Unsatisfactory colposcopy results were detected in 19 women (30%) from group I and in 9 women (43%) from group II. Colposcopy changes predominated in group II with persistent hrHPV. There were ill-defined colposcopy changes at baseline in 8 patients (38%) from group II, well-defined changes were not observed; in 24 months there were ill-defined changes in 11 patients (52%) and well-defined in 1 patient (5%) (Table 2). In 12 months 3 patients (25%) of group Ib with colposcopy changes underwent targeted cervical biopsy. All cases demonstrated histologically verified benign transformations: chronic cervicitis in 2 patients, cervical leukoplakia in a 1 patient.
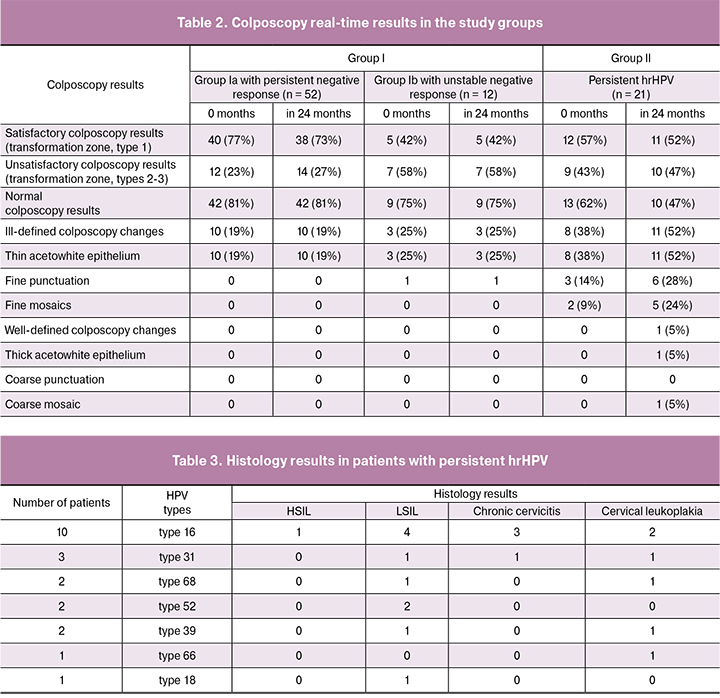
Patients of group II with persistent hrHPV underwent targeted cervical biopsy: there were ill-defined colposcopy changes (thin acetowhite epithelium with fine mosaic in 5 patients (24%), fine punctuation in 6 patients (28%)) and well-defined changes (thick acetowhite epithelium and coarse mosaic in 1 patient (5%)). Histology results detected LSIL in 10 patients (47%), HSIL in 1 patient (5%), cervical leukoplakia in 6 patients (28%), chronic cervicitis in 4 patients (19%). Considering the results of the study, extended colposcopy in patients with persistent hrHPV (with normal cytology) is of high importance and helps reveal the group of women requiring targeted cervical biopsy for SIL verification.
Study results demonstrated that hrHPV persistence causes the risk of SIL development in 52% of women. Patients with histologically verified LSIL and HSIL showed the domination of HPV type 16, in 4 patients (19%) and in 1 patient (5%), respectively; LSIL was also connected to HPV type 31 in 3 patients (14%), to HPV type 52 in 2 patents (9%), to HPV type 68 in 1 patient (5%), to HPV type 39 in 1 patient (5%) (Table 3). These HPV types, according to the IARC classification, are believed to be major ones in the development of severe precancerous lesions and cervical cancer.
The same data were obtained during Dillner meta-analysis in 2015, which demonstrated that HPV types 16, 31, 39, 45 caused cervical cancer among HPV patients more frequently [10]. It should be noted that some researchers consider HPV types 51, 56, 59 and 66 as those which have minimal morbidity rate in cervical cancer. Our research has shown that elimination of HPV types 56 and 59 happened in 100% of cases during a 24-month period. However, HPV type 66 demonstrated persistence with ill-defined colposcopy changes, which resulted in targeted cervical biopsy. Histological diagnosis confirmed benign cervical epithelium changes.
According to some researches, viral persistence depends on the type of virus. Soto-De Leon and co-authors analyzed elimination and persistence of the most frequent hrHPV types using Kaplan-Meier estimator. The results showed that persistence was detected in patients with HPV types 31 and 18, elimination was revealed in patients with HPV type 33 [5, 11]. The results of our study demonstrated that elimination of HPV type 33 occurred in 85.3% of cases for the first 12 months, and in 100% of cases during a 24-month period of examination, which correlates to the meta-analysis results.
The analysis of the study results showed that LSIL developed in 10 out of 21 women with hrHPV persistence and HSIL developed in 1 patient. According to integral assessment criterion of mRNA expression of the MKI67, CDKN2A (р16), PGR and BCL2 genes, risk of CIN development was determined as high in 1 patient during the first visit, later it developed into HSIL; and in 2 patients with the following LSIL. High risk of CIN development during the following visits in 12 and 24 months was revealed in 3 patients with low-grade dysplasia, which can be described as the risk group for CIN progression. Due to the high risk of CIN development and progression, the level of mRNA expression of MKI67, CDKN2A genes increases while the level of mRNA expression of PGR and BCL2 genes falls, which is taken into account using the sum assessment criterion (mentioned above in Materials and Methods Section). Risk index was not determined as high in the group with persistent or unstable viral elimination. Colposcopy results together with integral assessment criterion of four markers of mRNA expression can be used to detect risk groups for CIN development and progression in women with persistent hrHPV.
Thus, not only the existence, but also the persistence of one and the same hrHPV type for a 2-year period or more (with normal cytology results) should become the sign of CIN development for the clinician. HPV persistence of types 16, 31, 39, 18, 68 and 52 caused neoplastic cervical processes in the examined patients. Viral load was the same and it could not be the major cause of SIL development. However, the change in mRNA expression of the MKI67, CDKN2A, PGR, and BCL2 genes in patients with persistent hrHPV allowed us to detect timely the group of patients with low-grade and high-grade dysplasia; this step is of great importance for prevention of cervical cancer.
Conclusion
Persistent hrHPV and changes in the mRNA expression of the MKI67, CDKN2A, PGR, and BCL2 genes and in the colposcopic pattern are considered as markers for SIL progression. Viral load amount is not considered to be a criterion for persistent HPV and CIN development prediction.
References
1. Saslow D., Solomon D., Lawson H.W., Killackey M., Kulasingam S.L., Cain J. et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am. J. Clin. Pathol. 2012; 137(4): 516-42.
2. Soto-De León S.C., Del Río-Ospina L., Camargo M., Sánchez R., Moreno-Pérez D.A., Pérez-Prados A. et al. Persistence, clearance and reinfection regarding six high risk human papillomavirus types in Colombian women: a follow-up study. BMC Infect. Dis. 2014; 14: 395.
3. Xiao S.S., Fan J.L., He S.L., Li Y.R., Wang L.Y., Yu K.N. et al. Analysis of human papillomavirus infection in 16,320 patients from a gynecology clinic in Central South China. J. Low. Genit. Tract Dis. 2016; 20(4): 327-31.
4. Kang W.D., Ju U.C., Kim S.M. Is human papillomavirus genotype important in predicting disease progression in women with biopsy-proven negative or CIN1 of atypical squamous cell of undetermined significance (ASC-US) cytology? Gynecol. Oncol. 2018; 148(2): 305-10. https://doi.org/10.1016/j.ygyno.2017.11.025
5. Quaas J., Reich O., Frey Tirri B., Küppers V. Explanation and use of the colposcopy terminology of the IFCPC (International Federation for Cervical Pathology and Colposcopy) Rio 2011. Geburtshilfe Frauenheilkd. 2013; 73(9): 904-7.
6. Burd E.M. Human papillomavirus laboratory testing: the changing paradigm. Clin. Microbiol. Rev. 2016; 29(2): 291-319.
7. Стародубцева Н.Л., Назарова Н.М., Зардиашвили М.Д., Бурменская О.В., Бугрова А.Е., Чаговец В.В., Кононихин А.С., Трофимов Д.Ю., Франкевич В.Е., Сухих Г.Т. Комбинация протеомного и транскриптомного подходов для определения риска малигнизации неоплазий шейки матки при папилломавирусной инфекции. Акушерство и гинекология. 2017; 5: 64-71. [Starodubtseva N.L., Nazarova N.M., Zardiashvili M.D., Bourmenskaya O.V., Bugrova A.E., Chagovets V.V., Kononikhin A.S., Trofimov D.Y., Frankevich V.E., Sukhikh G.T. Predicting the risk of cervical intraepithelial neoplasia associated with HPV infection malignisation using the combination of proteomics and transcriptomics. Obstetrics and Gynecology. 2017; (5): 64-71. (in Russian)]
8. Бурменская О.В., Назарова Н.М., Прилепская В.Н., Мзарелуа Г.М., Бестаева Н.В., Трофимов Д.Ю., Сухих Г.Т. Прогнозирование риска развития и прогрессирования цервикальных интраэпителиальных неоплазий, ассоциированных с папилломавирусной инфекцией. Акушерство и гинекология. 2016; 2: 92-8. [Burmenskaya O.V., Nazarova N.M., Prilepskaya V.N., Mzarelua G.M., Bestaeva N.V., Trofimov D.Yu.,Sukhikh G.T. Prediction of the risk and progression of cervical intraepithelial neoplasias associated with papillomavirus infection. Obstetrics and Gynecology. 2016; (2): 92-98. (in Russian)]
9. Arbyn M., Tommasino M., Depuydt C., Dillner J. Are 20 human papillomavirus types causing cervical cancer? J. Pathol. 2014; 234(4): 431-5.
10. Halec G., Alemany L., Lloveras B., Schmitt M., Alejo M., Bosch F.X. et al. Pathogenic role of the eight probably/possibly carcinogenic HPV types 26, 53, 66, 67, 68, 70, 73 and 82 in cervical cancer. J. Pathol. 2014; 234(4): 441-51.
11. Dillner J. Prevention of human papillomavirus-associated cancers. Semin. Oncol. 2015; 42(2): 272-83. http://dx.doi.org/10.1053/j.seminoncol.2014.12.028
Received 23.05.2018
Accepted 22.06.2018
About the Authors
Sycheva, Elena G., doctor, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of Ministryof Healthcare of Russian Federation.
117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +79104925455. E-mail: el.bona@mail.ru
Nazarova, Niso M., MD, PhD, Senior Researcher, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I.Kulakov
of Ministry of Healthcare of Russian Federation.
117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954381403. E-mail: E-mail: grab2@yandex.ru
Burmenskaya, Olga V., Doctor of Biological Sciences, Senior Researcher of Laboratory molecular genetic Methods, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I.Kulakov of Ministry of Healthcare of Russian Federation.
117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +79167531709. E-mail: bourmenska@mail.ru
Prilepskaya, Vera N., MD, PhD, Professor, Deputy Director for Science, National Medical Research Center for Obstetrics, Gynecology and Perinatology named
after Academician V.I.Kulakov of Ministry of Healthcare of Russian Federation. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954386934. E-mail: VPrilepskaya@mail.ru
Trofimov, Dmitry Yu., Professor Doctor of Biological Sciences, Head of the Department of Clinical and Molecular Genetics, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I.Kulakov of Ministry of Healthcare of Russian Federation.
117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954382292. E-mail: d.trofimov@dna-tech.ru
Sykhikh, Gennady T., MD, Professor, Academical of the Russian Academy of Sciences, Director of the National Medical Research Center for Obstetrics,
Gynecology and Perinatology named after Academician V.I.Kulakov of Ministry of Healthcare of Russian Federation.
117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954381866. E-mail: g_sukhikh@oparina4.ru
For citations: Sycheva E.G., Nazarova N.M., Burmenskaya O.V., Prilepskaya V.N., Trofimov D.Yu., Sukhikh G.T. High-risk human papillomavirus persistence and other molecular genetic predictors for cervical intraepithelial neoplasias. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; (12): 104-10. (in Russian)
http://dx.doi.org/10.18565/aig.2018.12.104-110



