Основным методом лечения распространенного рака яичников (РЯ) в настоящее время остается хирургический. Главной задачей хирурга является достижение оптимальной циторедукции. Этот постулат продиктован многочисленными исследованиями, которые показали, что размеры остаточной опухоли после первичной циторедукции являются самым важным прогностическим критерием прогноза заболевания для этой категории больных [1]. В настоящее время, согласно классификация GOG (Gynecologic Oncology Group), выделяют оптимальные и субоптимальные циторедуктивные операции. Оптимальной циторедукцией считаются операции с объемом остаточной опухоли менее 10 мм, субоптимальной – более 10 мм [2].
Стандартным объемом операции по рекомендации AОР и ESMO-ESGO 2019 г. признана экстирпация матки с придатками, удаление большого сальника и удаление всех видимых метастазов. Эти операции успешно выполняются у больных с начальной стадией РЯ, однако лечение распространенного РЯ, когда опухоль поражает другие органы малого таза и выходит за его пределы, образуя единый конгломерат, за счет которого возникают грубые топические нарушения, является трудной задачей [3]. У данной группы больных для достижения оптимальной циторедукции возникает необходимость выполнения расширенных и комбинированных хирургических вмешательств [4, 5]. Помимо основного объема операции, в последние 10 лет выполняются комбинированные операции, которые включают аппендэктомию, резекцию толстой и/или тонкой кишки, печени, тазовую и поясничную лимфаденэктомию, спленэктомию, цистэктомию и др. Также с целью увеличения операбельности и резектабельности опухоли больным проводят несколько курсов периоперационной химиотерапии.
К достоинствам проведения хирургического лечения после проведения нескольких курсов химиотерапии препаратами платины и таксанов является то, что у больной уменьшается объем опухоли и улучшается общее состояние. Уменьшение объема опухоли увеличивает шансы выполнения оптимальной и полной циторедукции даже для той группы больных, для которой первичное хирургическое вмешательство было бы эксплоративным. Улучшение общего состояния больной влечет за собой лучшую переносимость обширного и травматического вмешательства [6, 7].
Доктора, придерживающиеся позиции оперировать больных распространенным РЯ на этапе интервальной циторедукции, аргументируют свою позицию тем, что после проведения неоадъювантной химиотерапии увеличивается резектабельность опухоли и, как следствие, снижается число интраоперационных осложнений.
Однако существующие рандомизированные исследования при увеличении вдвое числа выполненных оптимальных и полных циторедукций показали низкую выживаемость данной группы больных [8].
Цель исследования – сравнительная оценка отдаленных онкологических результатов расширенных и комбинированных, а также стандартных хирургических вмешательств на этапе интервальной циторедукции.
Материалы и методы
Исследование случай–контроль (подбор исследуемой и контрольной групп в пропорции 1:4 по следующим критериям: стадия FIGO, ECOG, схема химиотерапии), основано на ретроспективном анализе проспективно собранного материала и включало 150 больных РЯ IIIС–IV стадий, которые находились на лечении в отделении комбинированных и лучевых методов лечения онкогинекологических заболеваний НМИЦ им. Н.Н. Блохина в 2010–2018 г.
В ходе исследования больные были разделены на две группы: группа А (основная), в которой больным были выполнены расширенные и комбинированные объемы хирургических вмешательств, и группа В (контрольная), в которую вошли женщины, перенесшие стандартные хирургические вмешательства. Все больные на дооперационном этапе получили 6 курсов полихимиотерапии (ПХТ) – комбинациипрепаратов платины и таксанов.
Критериями включения являлись: гистологически и цитологически подтвержденный диагноз РЯ Т3-4N0-1M0-1. Исключали из исследования женщин со статусом по шкале ECOG>2, больныхс первично-множественными опухолями, пациентов, получавших на первом этапе неоадъювантную химиотерапию.
Всем больным до проведения специального лечения выполняли: полное физикальное, бимануальное ректовагинальное исследования, электрокардиографию (ЭКГ), Эхо-КГ, спирометрию, лучевые методы исследования (рентгенологическое исследование органов грудной клетки, РКТ органов брюшной полости с внутривенным контрастированием, малого таза, органов грудной клетки, УЗИ органов брюшной полости и малого таза), эндоскопические исследования органов желудочно-кишечного тракта (эзофагогастродуоденоскопию, фиброколоноскопию); специальное лабораторное обследование: цитологическое исследование экссудатов брюшной и плевральной полостей, смывов брюшной полости и определение уровня опухолевых маркеров. Клиническая стадия заболевания расценивалась по системе FIGO 2009 г. Интраоперационные и послеоперационные осложнения стадировались в соответствии с классификацией хирургических осложнений Clavien-Dindo.
Расчет отдаленных результатов лечения проводился с помощью программ статистического пакета SPSS (IBM® SPPS® Statistics v. 22). Общая выживаемость (ОВ) рассчитывалась от даты начала лечения до смерти от любой причины или до даты последнего наблюдения больного. Безрецидивная выживаемость (БРВ) рассчитывалась от даты начала лечения до верификации рецидива заболевания или до даты последнего наблюдения больного. Анализ кривых выживаемости проводили методом Каплана–Майера, сравнение кривых выживаемости проводилось методом log-rank. При использовании перечисленных методов статистики применялся 95% доверительный интервал (ДИ), достоверность различий оценивали с помощью точного критерия Фишера. Различия считали статистически достоверными при р<0,05.
Результаты
За период с 2010 по 2018 г. нами были отобраны 150 архивных историй болезней больных распространенным РЯ (IIIС–IV стадии), которые находились на лечении в отделении комбинированных и лучевых методов лечения онкогинекологических заболеваний НМИЦ онкологии им. Н.Н. Блохина. Исследуемые группы больных были однородными и сопоставимыми по возрасту, стадии заболевания, общему состоянию по шкале ECOG, по распространенности опухолевого процесса, а также по числу и схемам проведенных курсов химиотерапии (таблица).
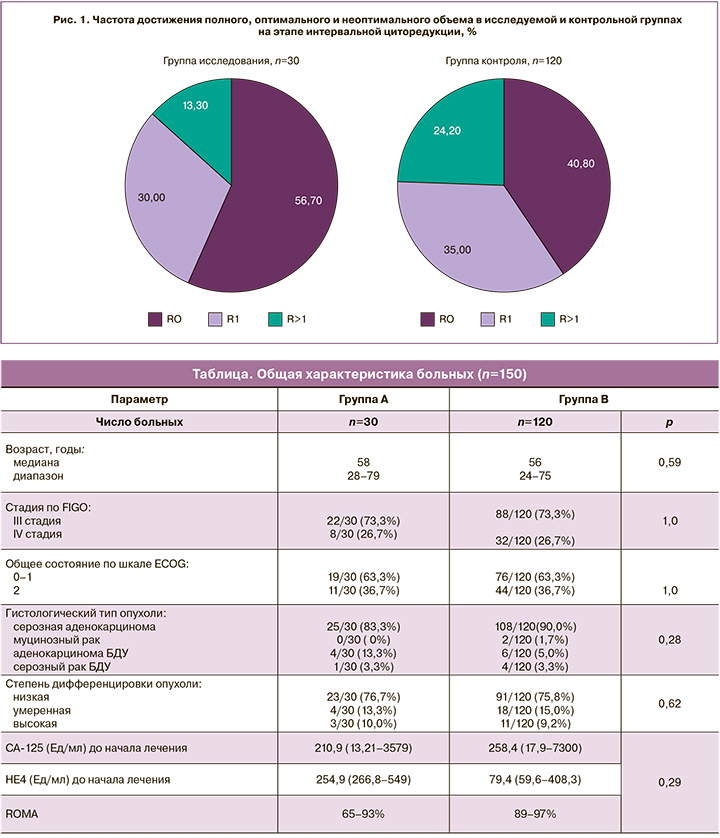
В группе исследования 17 больных из 30 (56,7%) были прооперированы в объеме полной циторедукции, 9 (30,0%) – в оптимальном объеме. На долю R>1 в исследуемой группе пришлось 4 (13,3%). В группе контроля 49 (40,8%) больных были прооперированы в объеме полной циторедукции, 42 (35,0%) – в оптимальном и 29 (24,2%) – в неоптимальном объеме. Достоверность различий составила р=0,15 (рис. 1).
Остаточная опухоль чаще всего локализовалась в верхнем этаже брюшной полости и представляла собой просовидную диссеминацию к куполам диафрагмы, капсуле печени, желудку с инфильтрацией малого сальника, в небольшом проценте была представлена тотальным канцероматозом с инфильтрацией висцеральной брюшины тонкой кишки.
Медиана длительности операции в исследуемой группе составила 220 минут (120–480 минут), в группе контроля – 150 минут (60–420 минут), р=0,01. Медиана кровопотери составляла 1900 мл в группе исследования и 900 мл в группе контроля, р=0,05. В группе исследования в 39,6% случаев, в группе контроля – в 14,1% случаев потребовалась заместительная терапия компонентами крови.
На долю осложнений в группе исследования приходилось 10 (33,3%), из них интраоперационные осложнения были отмечены у 2 (6,7%) больных, остальные 8 (26,7%) приходились на послеоперационные осложнения. Послеоперационные осложнения оценивали по классификации Clavien-Dindo, из них I–II степени тяжести – 6 (20,0%) случаев, в 1 случае – серома передней брюшной стенки и у 5 (16,7%) больных тромбоз вен нижних конечностей. Осложнения III–IV степеней тяжести отмечены у 2 (6,7%) больных – пельвиоперитонит вследствие несостоятельности колоректального анастомоза.
В группе контроля осложнения были отмечены у 45 (37,5%) больных, их них 16 (13,3%) приходилось на интраоперационные осложнения, включающие ранение селезенки, вскрытие просвета кишки, вскрытие просвета мочевого пузыря, ранение мочеточников. Послеоперационные осложнения оценивали по классификации Clavien-Dindo, из них I–II степени тяжести – 22 (18,3%) случая, 7 (5,8%) случаев осложнения III–IV степеней тяжести, включающие острую перфоративную язву двенадцатиперстной кишки – у 2 (1,7%) больных, кровотечение из ложа удаленной опухоли – у 1 (0,8%) больной и пельвиоперитонит вследствие несостоятельности колоректального анастомоза – у 4 (3,3%) больных.
В большинстве случаев послеоперационные осложнения в обеих группах развивались на 6-е сутки, в группе исследования 2 (6,7%) больным, а в группе контроля 7 (5,8%) больным потребовалось экстренное хирургическое вмешательство, 3 больным в группе исследования и 14 больным в группе контроля потребовалось нахождение в раннем послеоперационном периоде в ОРИТ. Медиана койко-дня в ОРИТ составила 3 дня в обеих группах. Страховочный дренаж в среднем был удален на 7-е сутки в группе исследования и на 9-е – в группе контроля. Медиана продолжительности приема наркотических анальгетиков в послеоперационном периоде у больных обеих групп составила 3 дня. Длительность послеоперационного периода в группе исследования составила 12 суток, в группе контроля – 14 суток.
Оценка эффективности лечения произведена у 150 больных, получивших хирургическое лечение после неоадъювантной химиотерапии, 30 были выполнены расширенные и комбинированные хирургические вмешательства, 120 – стандартные хирургические вмешательства. Медиана наблюдения в группе исследования – 39 месяцев, в группе контроля – 29 месяцев, медиана БРВ в исследуемой группе не достигнута, в контрольной группе – 10,48 месяца (ДИ 95% 8,233–12,66). В группе исследования годичная БРВ – 77%, 3-летняя БРВ – 59%, 5-летняя БРВ – 59%. В контрольной группе годичная БРВ – 28%, 3-летняя БРВ – 21%, 5-летняя БРВ – 21% (рис. 2).

Рецидив заболевания чаще всего локализовался по брюшине латеральных каналов в виде отдельных или сливных диссеминатов в группе контроля у 43 больных, в группе исследования – у 2.
Медиана ОВ в исследуемой группе не достигнута, в контрольной группе 37,3 месяцев (ДИ 95% 30,51–44,07). В исследуемой группе годичная ОВ – 89%, 3-летняя ОВ – 79 месяцев, 5-летняя ОВ – 56%. В контрольной группе годичная ОВ – 70%, 3-летняя ОВ – 43%, 5-летняя ОВ – 21% (рис. 3).
Заключение
Следует отметить, что преимуществами данного исследования является тщательно отобранный клинический материал и высокая прослеженность пациентов. На репрезентативной исследуемой группе нам удалось продемонстрировать важность выполнения полной циторедукции и экстраполировать данные мировой литературы на российскую популяцию пациентов. К недостаткам можно отнести ретроспективный характер исследования. Результаты проведенного ретроспективного исследования свидетельствуют о том, что если состояние больной позволяет выполнить ей операцию, а хирургическая бригада владеет необходимыми навыками, то выполнение максимальной циторедукции является обязательным условием хирургического вмешательства. Выполнение расширенных и комбинированных хирургических вмешательств при распространенном РЯ на этапе интервальной циторедукции является обоснованным.



