The features of the course of pregnancy and its outcomes in women vaccinated against papillomavirus infection
Objective. To investigate the features of the course of pregnancy and labor and the health status of newborn babies born to HPV-positive mothers and women who had undergone vaccination against papillomavirus infection (PVI) in adolescence.Krasnopolsky V.I., Petrukhin V.A., Zarochentseva N.V., Belaya Yu.M., Bocharova I.I., Vodovatova V.A., Keshiyan L.V.
Subjects and methods. A survey was conducted in 440 women aged 18 to 36 years, who were divided into 2 groups: 1) HPV-negative patients, who undergone vaccination against PVI mainly in adolescence, as well as at reproductive age; 2) HPV-positive patients who had not been vaccinated or treated for PVI. The course of pregnancy, its complications, as well as outcomes and neonatal health status were studied in the comparison groups.
Results. Gestational and postnatal complications were found to have much less common in the group of women who had undergone quarivalent HPV vaccination than in that of HPV-positive patients who had not been vaccinated and treated for PVI during pregnancy.
Conclusion. The findings showed that HPV vaccination itself had a pronounced effect on the onset of pregnancy, its course and outcome in the mother and fetus, as well as safety. These investigations require further consideration and studies of a number of issues related to the follow-up of an infant and his/her immunity, as well as confirm the need for urgent inclusion of vaccination in Russia’s immunization schedule for teenagers.
Keywords
Human papillomavirus infection (HPV infection) is the most prevalent sexually transmitted infection. Currently, the role of human papillomavirus (HPV) in the etiology of precancerous genital lesions in women, certain types of cancer in men and women, and adverse pregnancy and perinatal outcomes has been very well established. HPV infection during pregnancy is of public health importance and clinically relevant. The overall HPV prevalence among pregnant women ranges from 30 to 65.0%, and high-risk HPV types constitute 20–30% [1]. HPV can be transmitted via the placenta and as intranatal infection (in particular, HPV types 6 and 11). The risk of infection is directly related to the infection severity (the number of viral particles) and the duration of ruptured membranes during childbirth. Caesarean delivery does not reduce the risk of HPV transmission to fetus, which suggests predominantly intrauterine infection. Throughout the pregnancy, active processes aimed at local immunosuppression occur in the fetoplacental unit. In such a situation, HPV infection is not only a high risk of developing HPV-associated diseases in the settings of physiological immunodeficiency but also the risk of mother to child transmission of HIV during childbirth [2].
Estrogens and progesterone increase the expression of HPV in the cervical epithelium leading to cell proliferation and carcinogenesis. Increased vascularization, active tissue metabolism, changes in the vaginal microbiocenosis, and compromised compensatory immune mechanisms contribute to the risk of infections. In this case, latent HPV infection may have subclinical and clinical forms. During pregnancy visible anogenital warts often recur, tend to grow, become loose, and can reach gigantic proportions. And in children, intranatal infection can lead to juvenile-onset recurrent respiratory papillomatosis.
It is possible that persistent HPV infection without clinical manifestations (two or more successive positive HPV tests for a certain period) or viral latency (eradication of HPV does not occur, but the virus cannot be detected by standard molecular diagnostic testing, and the infection may recur) [3]. In a clinically latent course, periods of viral clearance and relapse may alternate, leading to an incorrect assessment of the HPV status if the diagnosis is based on a single HPV test [4].
Although HPV infection is considered as one of the leading causes of adverse pregnancy outcomes, such as spontaneous miscarriage [5], preterm birth [6], and pregnancy complications [7], data on the mechanisms and the impact of HPV infection on pregnancy remain a subject of debate. So far, there is no consensus on the optimal management of pregnant women with HPV infection and the prevention of its possible adverse effects on pregnancy outcomes.
Several studies over the past decade have confirmed the association between HPV infection and both male and female infertility, as well as the negative effect of HPV infection on perinatal outcomes, such as missed miscarriage, spontaneous miscarriage, and preterm birth [8–10].
According to the literature, 76.8% of HPV-positive by PCR pregnant women had placental damage at the end of pregnancy, which was associated with morphological and functional signs of chronic placental insufficiency, fetal malnutrition, and neonatal complications [11, 12].
Currently, HPV vaccination is the only reliable method of primary prevention of anogenital cancers and warts. Since HPV is mainly transmitted through sexual contact, the vaccines are most effective when given before the onset of sexual activity (that is, in children aged between 9 and 14 years, when most have not started sexual activity). In more than 98% of vaccinated individuals, a full course of vaccination induces the production of specific antibodies to four types of HPV (6, 11, 16, and 18). The World Health Organization (WHO) recognizes the importance of cervical cancer and other HPV-related diseases as global public health problems and recommends that HPV vaccines should be included in national immunization programs [13].
Prophylactic vaccination against high risk HPV has been recommended by the world’s leading professional associations/organizations. More than 270 million doses of HPV vaccine have been distributed since 2006 when they were licensed. More than 90 countries around the world have included the HPV vaccine in their National Immunization Programs, and a gender-neutral HPV vaccination strategy has been introduced in 20 countries [14].
In the Moscow region and 30 regions of the Russian Federation, the program “Vaccination for the prevention of HPV-associated malignancies” has been in operation since 2007. For 10 years, the Moscow region has accumulated the greatest experience in Russia in the use of the HPV vaccine. More than 22,000 teenage girls aged 12-17 and women under 45 have been vaccinated. Vaccination has resulted in a decreased incidence of anogenital warts not only in girls living in the region but also in the entire population of the Moscow region [15, 16]. The Global Advisory Committee on Vaccine Safety considers HPV vaccines to be extremely safe. No association between HPV vaccination and adverse effects concerning fertility, new-onset autoimmune disease, or death has been established [17].
Of major interest is the effect of the HPV vaccine on reproduction. In 5 clinical trials of the safety and efficacy of a quadrivalent vaccine, there were no differences between the groups in the rates of pregnancies that ended in a live birth, stillbirth, spontaneous miscarriage, or major birth defects. If the vaccine was inadvertently administered to pregnant women, tolerance was good. According to current data, more than 2000 women exposed to the HPV vaccine became pregnant without complications [18, 19].
New Zealand Study 2008–2014 showed that the HPV vaccine was associated with a significant reduction in preterm births compared with no vaccination. However, the risk of stillbirth and preeclampsia (PE) was similar in both groups [20].
Thus, the problem of HPV infection during pregnancy is relevant, and in view of the available preventive opportunities, one should not miss the chance to protect women’s reproductive health.
This study aimed to investigate the features of the pregnancy course, childbirth and health of infants born to HPV-positive mothers, and health of women who were vaccinated against HPV during adolescence.
Materials and methods
This study on the prevalence of HPV-associated diseases and gestational complications in HPV-positive and HPV-negative pregnant women after vaccination comprised 440 patients aged 18 to 36 years.
Group 1 included 320 pregnant women who were vaccinated against HPV in their teens and were HPV-negative. Group 2 included 120 HPV-positive pregnant patients who received neither vaccination nor HPV therapy. All patients underwent examination including reproductive function, pregnancy course and outcomes, and health status of their newborns at birth.
Evaluation of pregnancy course included the diagnosis of sexually transmitted infections (STIs) using PCR of exo-endocervical smears, smear microscopy, Doppler ultrasound assessment of fetal health, and immunohistochemical (IHC) examination of placental tissue. Newborns’ health at birth was evaluated using the Apgar score, signs of functional and morphological maturity, and neurosonography. All women gave birth in maternity units of the MRRIOG.
Statistical analysis was performed using the methods of descriptive and analytical statistics, including mean and relative values of numerical indicators. The probability of an error-free prediction was at least 95% (p<0.05).
Results
The mean age of patients in groups 1 and 2 was 22.5 and 26.5 years, respectively. The mean age at onset of sexual activity in groups 1 and 2 was 17 (5) and 15.5 (2) years, respectively. In group 1, 30% (n = 96) and 70% (n = 224) of the patients had their first and second or more pregnancies, respectively. In group 2, 36.7% (n = 44) and 63.3% (n = 76) of the patients had their first and second or more pregnancies, respectively (Table 1).
At the baseline examination, 80 (66.7%) patients in group 2 and no patients group 1 were found to have anogenital warts. In 38 patients with anogenital warts in group 2, genital warts were removed by radiofrequency ablation during gestation; one patient had giant condylomas of Buschke-Löwenstein, which required additional hospitalization and two-stage surgical intervention with a positive outcome and the possibility of full-term vaginal delivery.
The results of laboratory testing showed statistically significant between-group differences in detection rates of STIs and disorders of vaginal biocenosis (Table 2).
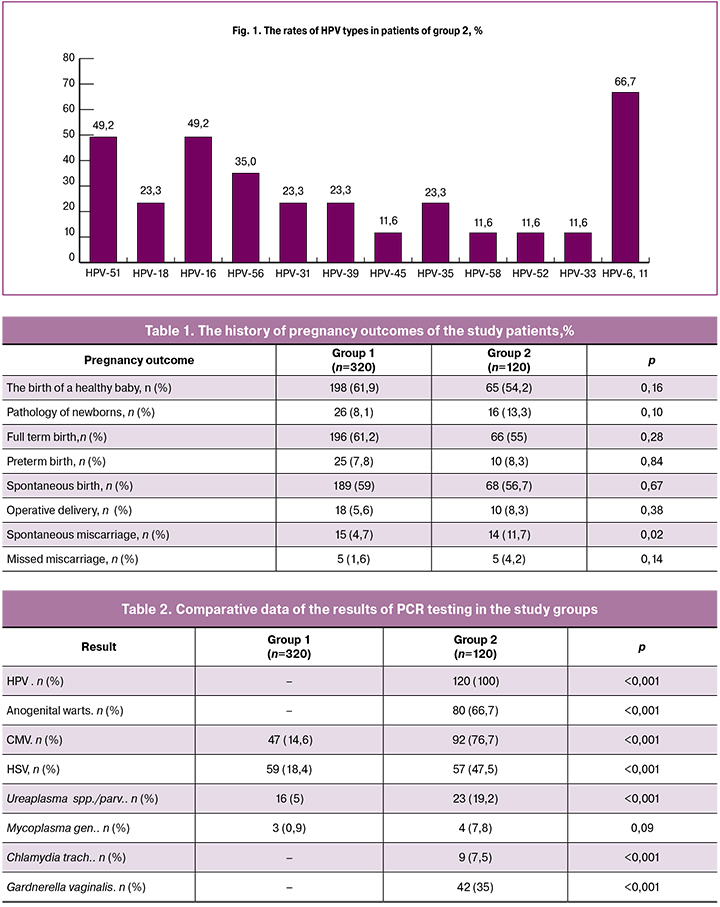
PCR analysis of HPV revealed that patients in group 2 had one or more types of HPV (Fig. 1).
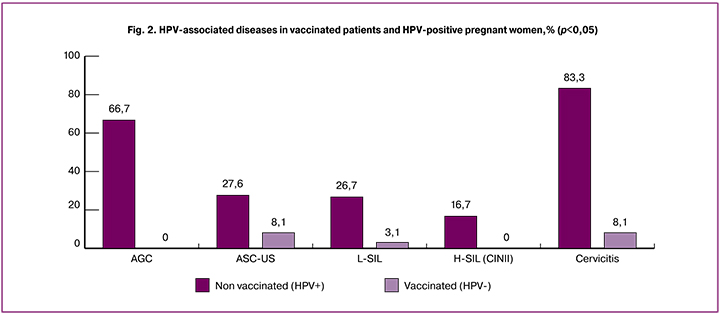
Cytology of exo-endocervical samples detected ASC-US in 27.5% (n = 33) and 8.1% (n = 26) of patients in groups 2 and 1, respectively; L-SIL in 26.7% (n = 32) and 3.1% (n = 10) of patients in groups 2 and 1, respectively; H-SIL in 16.7% (n = 20) of patients group 2 and were not found in group 1; signs of cervicitis were found in 83.3% (n = 100) and 8.1% (n = 26) of patients in groups 2 and 1, respectively (p <0.001 for all comparisons) (Fig. 2).
The groups did not differ significantly in parameters of pregnancy course.
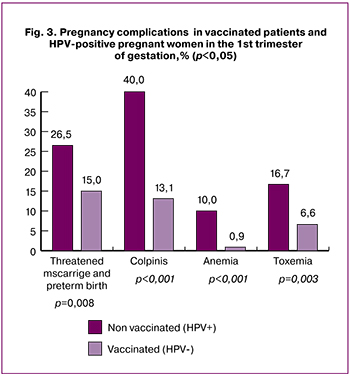 During the first trimester, patients in group 2 were 11.5% more likely to have threatened miscarriage than patients in group 1 [(26.5% (n = 32) and 15% (n = 48), respectively], (p = 0.008). Colpitis was diagnosed in 40% (n = 48) and 13.1% (n = 42) of patients in groups 2 and 1, respectively, p <0.001). Patients in group 2 were 9.1 % more likely to have anemia than patients in group 1 [(10% (n = 12) and 0.9% (n = 3), respectively], (p = 0.001). Patients in group 2 were 10.1% more likely to have toxemia of pregnancy than patients in group 1 [(10.1% (16.7% (n = 20) and 6.6% (n = 21), respectively], (p = 0.003) (Fig. 3).
During the first trimester, patients in group 2 were 11.5% more likely to have threatened miscarriage than patients in group 1 [(26.5% (n = 32) and 15% (n = 48), respectively], (p = 0.008). Colpitis was diagnosed in 40% (n = 48) and 13.1% (n = 42) of patients in groups 2 and 1, respectively, p <0.001). Patients in group 2 were 9.1 % more likely to have anemia than patients in group 1 [(10% (n = 12) and 0.9% (n = 3), respectively], (p = 0.001). Patients in group 2 were 10.1% more likely to have toxemia of pregnancy than patients in group 1 [(10.1% (16.7% (n = 20) and 6.6% (n = 21), respectively], (p = 0.003) (Fig. 3).
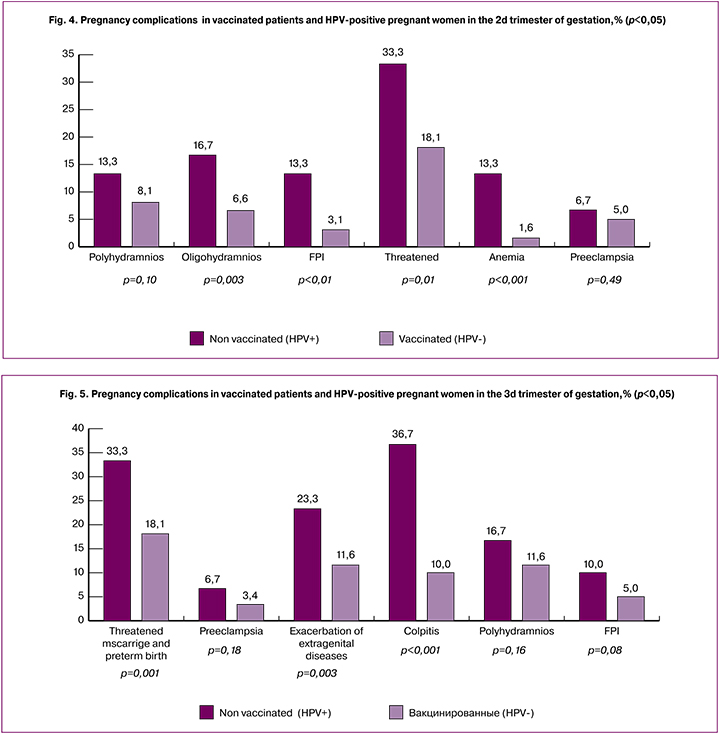
During the second trimester, patients in group 2 also had more pregnancy complications than in group 1. Polyhydramnios was found in 13.3% (n = 16) of patients in group 2, which is 5.2% more than in group 1 (8.1% (n = 26)) (p = 0.10). Oligohydramnios was found in 16.7% (n = 20) and 6.6% (n = 21) of patients in groups 2 and 1, respectively (p = 0.003). In group 2, 10.2% more patients were diagnosed with FPI compared with group 1 [(13.3% (n = 16) and 3.1% (n = 10), respectively], p <0.001. In group 2, 15.2% more patients had threatened miscarriage (33.3%; n = 40) compared with group 1, (18.1%; n = 58), (p = 0.001). Anemia was diagnosed in 1.6% (n = 5) and 13.3% (n = 16) of patients groups 1 and 2, respectively (p <0.001). No statistically significant between-group differences were observed in the rates of PE, which were 5% (n = 16) and 6.7% (n = 8) in patients in groups 1 and 2, respectively (p = 0.49) (Fig. 4).
During the third trimester, patients in group 2 also had more pregnancy complications than in group 1. Threatened miscarriage was observed 15.2% more often in group 2compared with group 1 [33.3% (n = 40) vs.18.1% (n = 58)], (p = 0.001), PE occurred 2 times more often [6.7% (n = 8) and 3.4% (n = 11)], p = 0.18), as well as exacerbation of extragenital comorbidities (chronic pyelonephritis, bronchial asthma, chronic gastroduodenitis) [(11.6% (n = 37) vs. 23.3% (n = 28) of patients, p = 0.003), colpitis was diagnosed in 36.7% (n = 44) and 10% (n = 32) of patients in groups 2 and 1 (p < 0.001), polyhydramnios was detected in 16.7% (n = 20) and (11.6% ( n = 37) of patients in groups 2 and 1 (p = 0.16); FPI was also twice more common in patients of group 2 (5%; n = 16) and (10%; n = 12), p = 0.08) (Fig. 5).
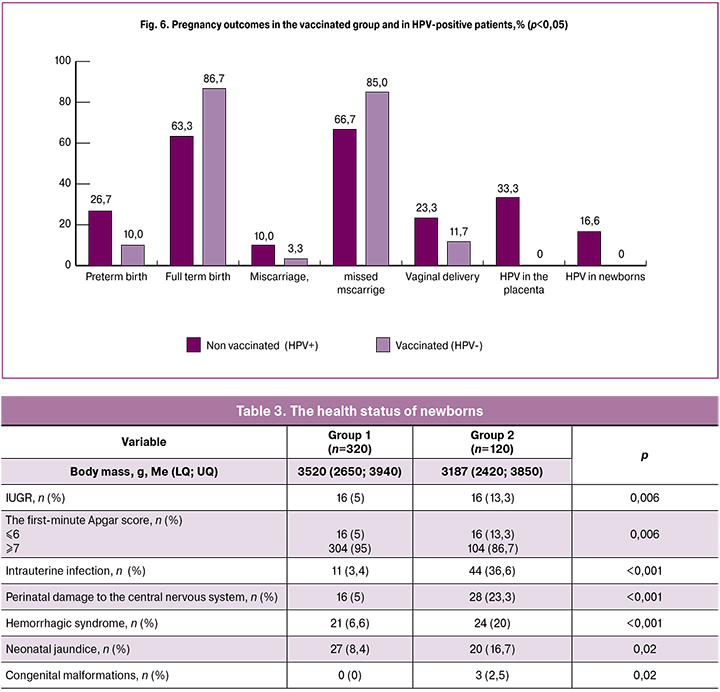
Pregnancy outcomes also differed between the two groups. Patients in group 2 were 16.7% more likely to have preterm birth than patients in group 1 [26.7% (n = 32) and 10% (n = 32), respectively], p <0.001); more women in group 1 had full-term birth [(86.7 (n = 277) and 63.3% (n = 76), respectively], p <0.001). Forty (33.3%) patients in group 2 and none of the HPV-negative women were found to have HPV in the placentas. The presence of HPV in the placenta was associated with focal lymphocytic leukocyte infiltration while maintaining a sufficient level of compensatory reactions (angiomatosis, an increase in syncytiocapillary membranes in terminal villi). The more frequent identification of the dissociated development of cotyledons, the thinning of the villi syncytiotrophoblast, and the decrease in blood flow through the vessels of the supporting villi revealed an initial degree of chronic placental insufficiency or, in other terms, compensated chronic renal failure. This general morphological assessment was also confirmed by IHC analysis of HPV, which characterized the position of placental tissue as part of inflammatory infiltrates, intermediate and invasive cytotrophoblast, and also in maternal macrophages in the intervillous space of the placenta, which indicated that some women in group 2 were undoubtedly infected with this virus.
Miscarriage was observed 6.7% more often in patients of group 2 [(10 (n = 12) and 3.3% (n = 11), respectively], p = 0.0) (Fig. 6).
The analysis of newborn health (Table 3) showed that infants born to HPV-positive mothers had higher all-cause morbidity in the early neonatal period than those born to vaccinated women (Table 3). Among newborns of mothers in group 2, twice as many infants were born with birth asphyxia and with manifestations of IUGR compared with newborns in group1 [(13.3% (n = 16) and 5% (n = 16), respectively], p = 0.006 ). Symptoms of damage to the central nervous system were diagnosed statistically significantly more often in newborns of HPV-positive mothers than in infants of vaccinated mothers [(23.3% (n = 28) and 5% (n = 16), respectively], p <0.001). In 50% of newborns in group 2, even clinically healthy ones, neurosonography revealed pathological changes in the form of small cysts in the lateral ventricle plexus of the brain and increased resistance of the cerebral vessels. Cutaneous hemorrhage, as manifestations of a local hemorrhagic syndrome, occurred in 6.6% (n = 21) and 20% (n = 24) of newborns in groups 1 and 2, respectively, (p < 0.001). Neonatal jaundice requiring phototherapy was twice as common among newborns of HPV-positive mothers compared with infants of vaccinated women [(16.7 (n = 20) and 8.4% (n = 27), respectively], p = 0, 02). Intrauterine infection defined by the sum of clinical and laboratory symptoms such as leukocytosis and/or leukopenia, an increase in the neutrophil index, anemia, thrombocytopenia, and an increased C-reactive protein level was diagnosed in 44 (36.6%) newborns of HPV-positive mothers and only in 11 (3.4%) newborns whose mothers were vaccinated against HPV (p<0.001). Mild congenital malformations (hypospadias, polydactylism, and hydrocele) were detected in 3 (2.5%) newborns in group 2 and were not observed in group 1 (p = 0.02).
Conclusion
Analysis of pregnancies and their outcomes in untreated HPV-positive mothers and that of HPV-negative vaccinated women showed statistically significant differences in favor of vaccinated women, thus demonstrating the benefit of the integrated approach to antenatal care of adolescents and young women.
Prophylactic HPV vaccination of adolescents before sexual debut provides protection against HPV infection since the early age of sexual activity without compromising the reproductive potential of women and reduces the risk of developing HPV-associated diseases and pregnancy complications, improves perinatal outcomes, and prevents HPV infection in neonates who were born to HPV-positive mothers.
Newborns who were born to HPV-positive mothers should be examined to detect HPV and ensure proper follow-up and prevention of future infectious complications.
WHO recognizes the importance of cervical cancer and other HPV-related diseases as global public health problems and reiterates the recommendation that HPV vaccines should be included in national immunization programs. Therefore, prophylactic HPV vaccination as a part of the adolescent vaccination calendar should be a national healthcare priority to help improve the reproductive health of the population both in the Moscow region and in Russia as a whole.
References
- Юнусова Е.И., Данилова О.В., Юсупова Л.А., Мавлютова Г.И., Гараева З.Ш. Папилломавирусная инфекция и беременность. Особенности диагностики и тактики ведения. Лечащий врач. 2018; 3: 56–9. [Yunusovа E.I., Danilovа O.V., Yusupova L.A., Mavlyutova G.I., Garaeva Z.Sh. HPV infection and pregnancy. Features of diagnostics and management tactics. Lechahij vrach. 2018; 3: 56–9.(in Russian)]
- Woodhall U.K., Jeet M., Soldan K., et al. Effect of genital warts: the loss of guality of life and cost of treatment in eight sex clinics in the UK. Sex and the transmissions. 2011; 87: 458–63. doi: 10.1136/sextrans-2011-050073
- Rositch A.F., Koshiol J., Hudgens M.G., et al. Patterns of persistent genital human papillomavirus infection among women worldwide: a literature review and meta-analysis. Int. J. Cancer. 2013; 133(6): 1271–85. doi: 10.1002/ijc.27828.
- Gravitt P.E. The known unknowns of HPV natural history. J. Clin. Invest. 2011; 121(12): 4593–99. doi: 10.1172/JCI57149.
- Giakoumelou S., Wheelhouse N., Cuschieri K. et al. The role of infection in miscarriage.Hum Reprod Update. 2016; 22(1): 116–33. doi: 10.1093/humupd/dmv041.
- Manuck T.A., Esplin M.S., Biggio J., et al. The phenotype of spontaneous preterm birth: application of a clinical phenotyping tool. Am. J. Obstet. Gynecol. 2015; 212(4): 487.e1–487.e11. doi: 10.1016/j.ajog.2015.02.010.
- Rustveld L.O., Kelsey S.F., Sharma R. Association between maternal infections and preeclampsia: a systematic review of epidemiologic studies. Matern Child Health J. 2008; 12(2): 223–42. http://dx.doi.org/10.1007/s10995-007-0224-1
- Tiatou Souho Human, Mohamed Benlemlih, Bahia Bennani. Papillomavirus Infection and Fertility Alteration: A Systematic Review. PLoS One doi:10.1371/journal.pone.0126936 May 18, 2015 Fez,Morocco.
- Cho G., Min K.J., Hong H.R., Kim S., Hong J.H., Lee J.K. High-risk human papillomavirus infection is associated with premature rupture of membranes. BMC Pregnancy Childbirth. 2013; 13:173. doi: 10. 1186/1471-2393-13-173
- Conde-Ferráez L., Chan M.A.A., Carrillo-Martínez J.R., Ayora-Talavera G., González-Losa M.R. Human papillomavirus infection and spontaneous abortion: a case-control study performed in Mexico. Eur J Obstet Gynecol Reprod Biol. 2013; 170: 468–73. doi: 10.1016/j.ejogrb.2013.07.002
- Воробцова И.Н., Тапильская Н.И., Гайдуков С.Н. Результаты обследования новорожденных, рожденных от матерей с различными формами папилломавирусной инфекции. Педиатр. 2011; 2(4): 72–75. [Vorobtsova I.N., Tapilskaya N.I., Gaidukov S.N. Result Of inVestigation newborn from born mothers with human papilloma Virus. Pediatr. 2011; 2(4): 72-75. (In Russian)]
- Чистяков М.А. Патоморфология папилломавирусной инфекции в системе «мать-плацента-плод». Автореф. дис. … к.м.н. М., 2008. 25 с. [Chistyakov M.A. Patomorfologiya papillomavirusnoy infektsii v sisteme mat’-platsenta-plod: Avtoref. dis. ... kand. med. nauk. M., 2008. 24 s. (in Russian)].
- WHO. Human papillomavirus (HPV) and cervical cancer. 24.01.2019 https://www.who.int/ru/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer.
- Вакцины против вируса папилломы человека: документ по позиции ВОЗ. 2017; 92: 241–68. [WHO. Vaccine in National Immunization Programm. Update February 2019. Available at: https://www.who.int/immunization/monitoring_surveillance/VaccineIntroStatus.pptx Accessedon 22.03.2019.
- Зароченцева Н.В., Белая Ю.М. Опыт реализации программ первичной профилактики заболеваний, вызываемых вирусом папилломы человека, в Московской области. Эпидемиология и вакцинопрофилактика. 2017; 6(97): 59–65. [Zarochentseva N.V., Belaiya J.M. Experience in the Implementation of Programs for Primary Prevention of Human Papillomavirus-associated Diseases in the Moscow region. Epidemiology and Vaccinal Prevention. 2017; 16(6): 59–65. (In Russian)]. https://doi.org/10.31631/2073-3046-2017-16-6-59-65.
- Зароченцева Н.В., Белая Ю.М. Современный взгляд на остроконечные кондиломы. Возможности лечения и профилактики. Российский вестник акушера-гинеколога. 2017; 1: 109–12. [Zarochentseva N.V., Belaya Yu.M. Modern view on genital warts. Opportunities for treatment and prevention. Rossijsky vestnik akushera-ginekologa/The Russian bulletin of the obstetrician-gynecologist. 2017; 1: 109–112. (in Russian)]. doi:10.17116/rosakush2017171109-112
- Crowe E., Pandeya N., Brotherton J.M. Effectiveness of quadrivalent human papillomavirus vaccine for the prevention of cervical abnormalities: case–control study nested within a population based screening programme in Australia. BMJ. 2014; 348: 1458. doi: 10.1136/bmj.g1458.
- Koskimaa H.M., Waterboer T., Pawlita M., Grénman S. Human papillomavirus genotypes present in the oral mucosa of newborns and their concordance with maternal cervical human papillomavirus genotypes. J Pediatr. 2012; 160: 5: 837–43. doi: 10.1016/j.jpeds.2011.10.027.
- Principles and considerations for adding a vaccine to a national immunization programme: from decision to implementation and monitoring. World Health Organization, 2014. http://apps.who.int/iris/bitstream/10665/111548/5/9789244506899_rus.pdf
- Lawton B., Howe A.S., Turner N., Filoche S., Slatter T., Devenish C., Hung N.A. Association of prior HPV vaccination with reduced preterm birth: A population based study. Vaccine. 2018; 36: 134–40. doi: 10.1016/j.vaccine.2017.11.020
Received 23.08.2019
Accepted 04.10.2019
About the Authors
Vladislav I. Krasnopolsky, MD, Acad. RAS, Professor, President, GBUZ MO MONIIAG, tel .: +7 (495) 624-8808. ORSID ID: orsid.org/0000-0002-6857-9130Address: 101000 Russia, Moscow, ul. Pokrovka, 22A.
Petrukhin Vasily A., MD, professor, director of GBUZ MO MONIIAG, tel .: +7 (495) 624-88-08. ORSID ID: orsid.org/0000-0002-9920-2643
Address: 101000 Russia, Moscow, ul. Pokrovka, 22A.
Zarochentseva Nina V., MD, Professor of the Russian Academy of Sciences, Deputy Director for Research, GBUZ MO MONIIAG,
tel.: +7 (495)624-88-08, e-mail: ninazar11@mail.ru; ORSID ID: orsid.org/0000-0001-6155-788X
Address: 101000 Russia, Moscow, ul. Pokrovka, 22A.
Yulia M. Belaya, PhD, researcher at the outpatient department of GBUZ MO MONIIAG, tel. + 7 (495)624-88-08,
e-mail: belajay@yandex.ru ORSID ID: orsid.org/0000-0001-9864-2914
Address: 101000 Russia, Moscow, ul. Pokrovka, 22A.
Irina I. Bocharova, MD, Leading Researcher, MONIIAG, Doctor of the highest qualification category in the specialty “Neonatology”,
tel.: +7 (495)624-88-08, e-mail: 567891@mail.ru ORSID ID: orcid. org/0000-0002-5486-9794
Address: 101000 Russia, Moscow, ul. Pokrovka, 22A.
Valeriya A. Vodovatova, Junior Researcher, MONIIAG, neonatologist, resuscitation anesthetist,
tel.: +7 (495)624-88-08; e-mail: leleronik@gmail.com ORSI ID: orcid.org/0000-0001-9255-3785
Address: 101000 Russia, Moscow, ul. Pokrovka, 22A.
Lyudmila V. Keshiyan, PhD, head physician, GBOZ MO “Naro-Fominsky Perinatal Center”,
tel.: +7 (499)405-00-03, e-mail: lkeschyan@mail.ru ORSID ID: orcid.org/0000 -0002-8799-4540
Address: Russia, Moscow region, Naro-Fominsk. st. Kalinina, d. 30,
For citation: Krasnopolsky V.I., Petrukhin V.A., Zarochentseva N.V., Belaya Yu.M., Bocharova I.I., Vodovatova V.A., Keshiyan L.V. The features of the course of pregnancy and its outcomes in women vaccinated against papillomavirus infection.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 1: 146-54. (In Russian).
https://dx.doi.org/10.18565/aig.2020.1.146-154



