Characteristics of the proliferative and antiproliferative activities of the cells of the endometrium in its hyperplasia concurrent with chronic endometritis
Objective. To determine the characteristics of the proliferative and antiproliferative activities of the cells of the endometrium in its hyperplasia concurrent with chronic endometritis (CE), by analyzing the expression level of Ki-67 and p16INK4a proteins in the uterine mucosa.Kazachkov E.L., Zatvornitskaya A.V., Voropaeva E.E., Kazachkova E.A., Shchegolev A.I.
Subjects and methods. Ninety patients were examined. Group 1 consisted of 29 women, in whom histological examination of endometrial biopsy specimens revealed endometrial hyperplasia (EH) without atypia concurrent with CE; Group 2 included 21 patients with EH without atypia (Comparison Group 1); Group 3 comprised 20 women with CE (Comparison Group 2); Group 4 consisted of 20 gynecologically healthy women (a control group). A comprehensive morphological and immunohistochemical study of endometrial samples was carried out according to the generally accepted criteria.
Results. Evaluation of the proliferative activity of endometrial cells in patients with EH concurrent with CE indicates that there is a high proliferative potential of the epithelial cells of endometrial glands and stroma. The higher expression of p16INK4a in Group 1 suggests that viruses may be involved in the development of the hyperplastic process. A comparison of the expression levels of Ki-67 and p16INK4a in the uterine mucosa of the women in Groups 1 and 2 showed that the expression level of p16INK4a was directly proportional to that of Ki-67.
Discussion. The endometrial cells with EH concurrent with CE are characterized by both the enhanced proliferative and antiproliferative activities. The observed coexpression of Ki-67 and p16INK4a in EH concurrent with CE may be a signal of cell cycle damage and requires a search for factors determining the simultaneous expression of these markers in this pathology. Evidence for the maximum expression levels of Ki-67 and p16INK4a in the endometrial samples of patients with a recurrent endometrial hyperplastic process can be the basis for the development of a program to assess the risk of recurrent EH.
Keywords
In recent years, there has been a significant increase in the number of patients with endometrial carcinoma [1]. Many experts believe that endometrial hyperplasia (EH) represents a precursor lesion to endometrial cancer and therefore, is associated with the risk of developing endometrial carcinoma [2]. Currently, risk factors for EH that are debated in the medical literature include unbalanced long-term stimulation with estrogens, impaired endometrial receptivity, inadequate response of endometrial cells to external factors, which is determined by the expression of proliferation markers, apoptosis, growth factors, etc. [3]. The above factors are most likely to realize in the presence of chronic endometritis (CE), which creates the prerequisites for endometrial proliferation and further malignant transformation to endometrial carcinoma [4, 5]. However, the rates of detecting bacterial pathogens of inflammation in CE have been decreasing in parallel with increasing detection rates of viral agents, including human papillomavirus (HPV), [5]. HPV is also often detected in patients with EH, [6], and every third of them has concurrent atypia and a well-differentiated endometrial adenocarcinoma [7].
Increased expression levels of a cell proliferation marker Ki-6 and a marker of antiproliferative activity cyclin-D-dependent kinase inhibitor p16INK4a in cells have been considered as a predictor of cancer [8, 9]. In normal epithelial cells, in particular, exocervical epithelial cells, p16INK4a protein is found at low expression levels, but its expression increases in patients with HPV-associated cervical diseases [10, 11]. However, studies investigating the expression of the above markers in endometrial mucosa in patients with concomitant EH and CE have been lacking and inconsistent. [12–14].
This study aimed to investigate the features of the proliferative and antiproliferative activity of endometrial cells in patients with concomitant EH and CE by analyzing the expression levels of Ki-67 and p16INK4a proteins in endometrial mucosa.
Materials and methods
This study was conducted at the Department of Obstetrics and Gynecology and the Department of Anatomic Pathology and Forensic Medicine of the South Ural State Medical University of Minzdrav of Russia, Chelyabinsk. The study comprised 90 patients, who were divided into four groups. Group 1 included 29 women with concomitant CE and EH without atypia confirmed by histological examination of endometrial biopsy specimens. Patients in group 2 (comparison group1, n = 21) had EH without atypia. Group 3 (comparison group 2) included 20 women with CE. Twenty women without gynecologic abnormalities who sought pregnancy counselling and had normal endometrial biopsies were enrolled in group 4 (control group).
Inclusion criteria: reproductive age, normal ovulatory menstrual cycle (for groups 1, 3, and 4), and informed consent to participate in the study.
Exclusion criteria: ovulatory dysfunction (for groups 1, 3, and 4), adenomyosis, uterine fibroids requiring surgical treatment, and atypical EH.
The study design is a cross-sectional nonrando-mized retrospective study using histological, immunohistochemical, and statistical methods.
The endometrium was obtained on days18-22 of the menstrual cycle by hysteroscopically guided manual vacuum aspiration (in groups 1 and 2) or using pipelle endometrial biopsy (in groups 3 and 4).
Endometrial specimens underwent a comprehensive morphological study, and the findings were interpreted according to generally accepted criteria [15, 16]. The histological sections were prepared according to standard methods.
Histological sections were deparaffinized and stained with hematoxylin-eosin and Van Gieson’s picrofuchsin. Plasma cells were detected using murine monoclonal antibodies (MKAT) CD 138/syndecan-1 (clon B-A38, r.t.u., Cell Sigma-Aldrich - Sigma-Aldrich, Inc., USA).
The severity of CE was determined under criteria proposed by E.A. Kazachkova et al. [17] and G.Kh. Tolibova et al. [18] (to assess the level of CD 138).
The degree of CE activity was assessed by the criteria for a semi-quantitative morphological assessment of the inflammatory process in CE proposed by O.A. Alimova et al. [19]. The process was defined as inactive if neutrophilic granulocytes in the polymorphic inflammatory cell infiltrate in biopsy specimens from CE were absent or scanty.
The analysis of expression level of the Ki-67 proliferation marker was carried out using MKAT for Ki-67 (Cell Marque, clone SP6, 1: 100); the expression level of p16INK4a was determined with monoclonal anti-p16 antibody from the CINtec Histology kit (MTM Laboratories AG, Germany). Immunohistochemical markers were assessed by the presence, intensity, and distribution of brown staining of the affected cell nuclei. The study was conducted independently by two morphologists. We studied the percentage of positively stained cell nuclei per at least 1000 cells in 10 fields of view. In the absence of stain-positive cells for p16INK4a and Ki-67, expression was considered negative. Upon verification of single expression, rare single cells of glands and endometrial stroma with brown staining of nuclei were found; with a focal form of expression, a few clusters of cells with brown colored nuclei were detected; the diffuse form of expression was established upon detection of cells with brown nuclei throughout the entire thickness of the endometrium in the glands and stroma. To objectify the assessment of Ki-67 and p16INK4a expression, we used the Video Test – Morphology 5.2 imaging software for computerized morphometry (Russia).
Statistical analysis was performed using the Statistica 10 (StatSoft) statistical package. The distribution of continuous variables was tested for normality using the Kolmogorov-Smirnov test. Quantitative variables showing normal distribution were expressed as means (M) and standard deviation (SD) and presented as M (SD); otherwise the median (Me) and the quartiles Q1 and Q3 in the Me (Q1; Q3) format were reported. Qualitative variables were summarized as counts and percentages.
Kruskal–Wallis test was used for comparing numerical data between groups, followed by pairwise comparison using the Mann – Whitney U-test. Categorical variables were compared by the Chi-square test with the Yates correction. Correlation analysis was conducted by calculating Spearman’s rank correlation coefficients. The critical level of significance when testing statistical hypotheses was considered at p < 0.05.
Results and discussion
Mean age of patients in groups 1, 2, 3, and four was 39.8 (3.5), 34.8 (3.6), 34.2 (1.5), and 33.85 (0.7) years, respectively. There were no statistically significant differences in the results.
Analysis of somatic comorbidities showed that patients in group 1 were statistically significantly more likely to have cardiovascular diseases (ICD-10: I25, I11, R00, I83) (n = 11, 37.9%) than patients in group 2 (n = 4, 19.04%), 3 (n = 3, 15%), and 4 (n = 2, 10%), respectively (p = 0.035). Patients in group 2 had statistically significantly higher rate (n = 9, 42.8%) of endocrine diseases (E00-07, E65-68, E10-14) than patients in group 1 (n = 4, 13.8%), 3 (n = 2, 10%), and 4 (n = 1, 5%), respectively (p = 0,003).
The formation of menstrual function in the study groups was not impaired; Mean ages at menarche were also homogeneous.
Patients in group 1 were statistically significantly more likely to report a history of pelvic inflammatory disease (n = 20, 68.97%) than women in in group 2 (n = 9, 42.86%), 3 (n = 8, 40%), and 4 (n = 0), respectively (p = 0.04). Eight (27.6%) patients in group 1had recurrent EH and earlier received gestagen therapy. In the cervical specimens of 6 (20.7%) women in group 1, HPV types 16, 31, and 58 were detected.
In group 2, statistically significantly more common was polycystic ovary syndrome (E28) (n = 8, 38.1%) compared with 3 (10.3%) in group 1, and none in groups 3 and 4 (p = 0.002). Non-inflammatory disorders of ovary (N83.0, N83.1) were more common in group 2 (n = 11, 52.4%) compared with 8 (27.6%), 6 (30%), and 2 (10%) in groups 1, 3, and 4, respectively (p = 0.03).
Histological findings of endometrial biopsies obtained in group 1 revealed the pattern of CE concomitant with EH without atypia, which included a proliferation of glands of irregular shape and size with an increase in the gland to stroma ratio, and thinning of the interacinar septa. These changes were observed against the background of a chronic inflammatory process characterized by diffuse sparse neutrophilic granulocytes, aggregates of lymphocytes, and small fields of fibroblasts in the stroma of the endometrial mucosa. When CD138 was present (2 (6.9%) cases), both scanty plasmocytes and diffusely scattered scanty plasma cells (7 (24.1%) cases), up to their focal aggregates (20 (68, 97%) cases). The above endometrial pattern indicates EH without atypia concomitant with CE of low activity and varying degrees of severity.
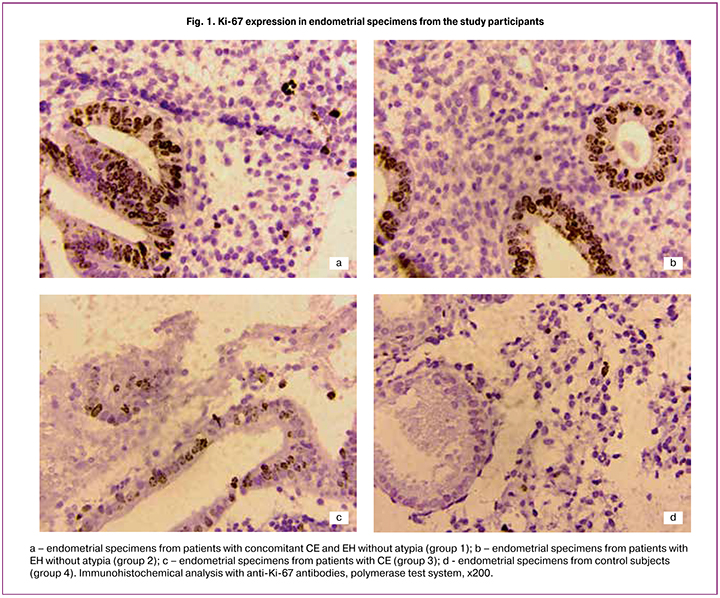
Diffuse expression of Ki-67 was found in 17 (58.6%) samples (Fig. 1), showing intense brown staining of the nuclei in both the glandular and stromal components. Focal expression of Ki-67 was found in 6 (20.7%) cases showing groups of cells with brown nuclei), and in 6 (20.7%) samples, single immunopositive cells were detected. Morphometric area of immunopositive structures in group 1 represented 29.1% (15.7%; 43.7%).
Diffuse expression of p16INK4a (Fig. 2) was detected in 23 (79.3%) samples presenting as numerous, diffusely located immunopositive cells in the stromal and glandular components. Focal expression was found in 3 (10.3%) samples showing scanty clusters of glandular and stromal cells with brown nuclei; in 3 (10.3%) samples scanty immunopositive cells were detected. Moreover, HPV 16, 31, and 58 types were detected in cervical epithelium in 6 (20.7%) group 1patients. The area of immunopositive structures was 38.1% (24.2%; 50.2%). In group 1, patients with recurrent EH had the highest expression levels of Ki-67 and p16INK4a.
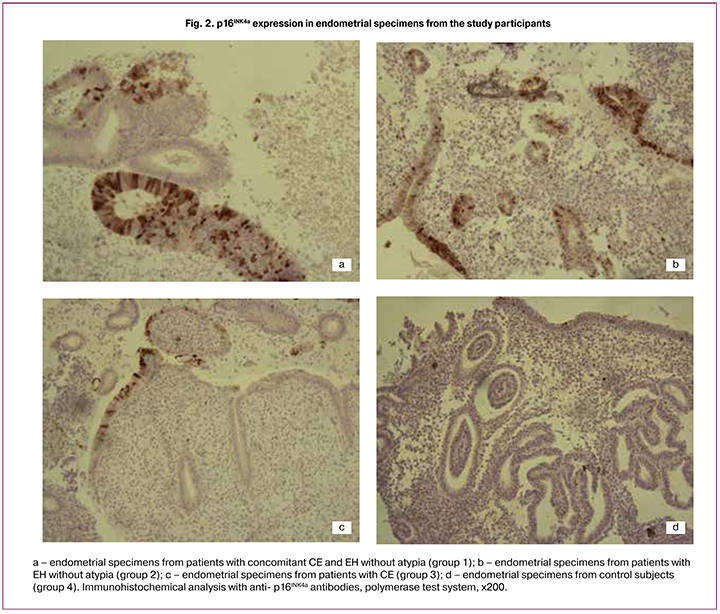
A similar pattern of EH without atypia was noted in group 2. The uterine mucosa had numerous unevenly distributed glands of various shapes and sizes, including cystically dilated glands, in separate areas with folds in the direction of the lumen of the glands. There were signs of reactive inactive inflammation presented as the absence of plasmocytes, scanty diffuse granulocytes and lymphocytes, and small clusters of fibroblasts. An immunohistochemical study of endometrial samples showed focal expression of Ki-67 in 15 (71.4%) cases; single expression was found in 6 (28.6%) samples. p16INK4a was more often detected as focal expression (13 (61.9%) cases); single expression was found in 8 (38.1%) cases. The area of immunopositive structures was 14.7% (10.2%; 26.6%) and 25.3% (16.3%; 34.1%) regarding Ki-67 and p16INK4a, respectively (Figs. 1, 2).
Samples from patient in group 3 had a pattern of low grade activity and moderate severity CE represented by scanty neutrophilic granulocytes in the thickness of the endometrium, polymorphic cell infiltration with numerous diffusely scattered lymphocytes without a tendency to focal aggregation, the presence of single plasmocytes, and fibroblast transformation of stromal cells and fibrosis seen as large fields of fibroblast cells. Analysis of proliferative activity showed a single expression of Ki-67 in 19 (95%) samples. In 1 (5%) sample, no immunopositive cells were detected. The mean area of Ki-67 immunopositive structures was 6% (1.93%; 12.93%). Evaluation of antiproliferative activity showed predominantly a single form of p16INK4a expression (19 (95%) samples), and 1 (5%) sample was negative for this marker. The mean area of p16INK4a immunopositive structures was 5.8% (1.6%; 9.8%) (Figs.1, 2).
Histologic pattern of uterine mucosa of women in control group was consistent with the middle stage of the secretion phase: Ki-67 expression was predominantly single (15 (75%) cases), and 5 (25%) samples were negative. The mean area of immunopositive structures was 1.25% (0.07%; 3.93%). The observed proliferation was probably associated with active endometrial cell division, which characterizes this phase of the menstrual cycle. Three (15%) samples had single p16INK4a expression, which is probably due to the transient nature of human papillomavirus infection. Seventeen (85%) biopsy samples of endometrial mucosa were negative; the area of p16INK4a protein immunopositive structures was 2.6% (0.7%; 4.5%) (Fig. 1, 2).
Analysis of the proliferative activity of endometrial cells in patients with concomitant EH and CE indicated a high proliferative potential of glandular epithelial cells and endometrial stroma, not only in comparison with the control group but also concerning the 1st and 2nd comparison groups suggesting actively ongoing processes of cell division. The area of Ki-67 immunopositive structures was statistically significantly larger in group 1 amounting to 29.1% (15.7%; 43.7%). In groups 2, CE group, and control group it was 14.7% (10.2%; 26.6%), 6% (1.93%; 12.93%), and 1.25% (0.07%; 3.93%), respectively (p = 0.03).
 The increased expression of p16INK4a in the group 1 patients suggests the possible role of viruses in EH development. In group 1, the mean area of p16INK4a immunopositive structures was statistically significantly larger and reached 38.1% (24.2%; 50.2%); in groups 2, 3, and control group it was 25.3% (16, 3%; 34.1%), 5.8% (1.6%; 9.8%), an 2.6% (0.7%; 4.5% ), respectively (p = 0.002).
The increased expression of p16INK4a in the group 1 patients suggests the possible role of viruses in EH development. In group 1, the mean area of p16INK4a immunopositive structures was statistically significantly larger and reached 38.1% (24.2%; 50.2%); in groups 2, 3, and control group it was 25.3% (16, 3%; 34.1%), 5.8% (1.6%; 9.8%), an 2.6% (0.7%; 4.5% ), respectively (p = 0.002).
There was a strong direct correlation (Spearman’s correlation coefficient 0.869, p = 0.0001) between the expression levels of p16INK4a and Ki-67 in endometrial mucosa of women in groups 1 and 2 (Fig. 3). The expression levels of these markers in groups 3 and 4 were weak, and statistical comparisons were not possible.
Conclusion
In concomitant endometrial hyperplasia and chronic endometritis, endometrial cells are characterized by both increased proliferative and antiproliferative activity. The observed co-expression of Ki-67 and p16INK4a in EH concomitant with CE can be a sign of cell cycle damage and requires searching for factors determining the simultaneous expression of these markers. The finding of highest expression levels of Ki-67 and p16INK4a in endometrial samples from patients with recurrent EH may serve as a rationale for developing an EH recurrence risk assessment program.
References
- Каприн А.Д., Старинский В.В., Петрова Г.В., ред. Злокачественные новообразования в России в 2017 году (заболеваемость и смертность). М.: МНИОИ им. П.А. Герцена 2018. 250 c. [Kaprin A.D., Starinsky V.V., Petrova G.V. Malignant neoplasms in Russia in 2017 (morbidity and mortality). M.: Moscow them. P.A. Herzen 2018; 250. (In Russ.)]
- Kurman R.J., Carcanglu M.L., Herrington C.S., Young R.H. WHO Classification of tumors of female reproductive organs. 4th ed. Lyon: IARC Press, 2014.
- Леваков С.А., Шешукова Н.А., Кедрова А.Г., Федотова А.С., Обухова Е.А. Молекулярно-биологические профили гиперплазии эндометрия и эндометриальной интраэпителиальной неоплазии. Опухоли женской репродуктивной системы. 2018; 2: 76-81. [Levakov S.A., Sheshukova N.A., Kedrova A.G., Fedotova A.S., Obukhova E.A. Molecular biological profiles of endometrial hyperplasia and endometrial intraepithelial neoplasia. Tumors of the female reproductive system. 2018; No. 2: 76-81. (In Russ.)]
- Ткаченко Л.В., Свиридова Н.И. Двухэтапный метод лечения хронического эндометрита у женщин с гиперпластическими процессами эндометрия в перименопаузе. Гинекология. 2016; 1: 40-4. [Tkachenko L.V., Sviridovа N.I. A two-step method for the treatment of chronic endometritis in women with endometrial hyperplastic processes in perimenopause. Gynecology 2016; 1: 40-4. (In Russ.)]
- Радзинский В.Е., Ордиянц И.М., Добрецова Т.А. Эндометрий в огне. Острое и хроническое воспаление эндометрия: от новых взглядов к новым стратегиям. Status Praesens. Гинекология, акушерство, бесплодный брак. 2016; 2: 126-32. [Radzinsky V.E., Ordiyants I.M., Dobretsova T.A. Endometrium on fire. Acute and chronic inflammation of the endometrium: from new perspectives to new strategies. Status Praesens. Gynecology, obstetrics, barren marriage. 2016; 2: 126-132. (In Russ.)]
- Громова А.М., Афанасьева Е.Е., Громова А.Л., Мартыненко В.Б., Нестеренко Л.А. Роль инфекций, передающихся половым путем, в развитии гиперплазии эндометрия. Мир медицины и биологии. 2014; 3: 29-32. [Gromova A.M., Afanasyeva E.E., Gromova A.L., Martynenko V.B., Nesterenko L.A. The role of sexually transmitted infections in the development of endometrial hyperplasia. The world of medicine and biology. 2014; 3: 29-32. (In Russ.)]
- Чайка В.К., Холодняк Т.Н. Новый подход к диагностике и прогнозированию гиперпластических процессов эндометрия. Медико-социальные проблемы семьи. 2003; 8(1): 94-100. [Chaika V.K., Kholodnyak T.N. A new approach to the diagnosis and prediction of endometrial hyperplastic processes. Medical and social problems of the family. 2003; 8 (1): 94-100. (In Russ.)]
- Nakanishi A., Kitagishi Y., Ogura Y., Matsuda S. The tumor suppressor PTEN interacts with p53 in hereditary cancer (Review). Int. J. Oncol. 2014; 44(6): 1813-29. https://doi.org/10.3892/ijo.2014.2377.
- Song M.S., Salmena L., Pandolfi P.P. The functions and regulation of the PTEN tumour suppressor. Nat. Rev. Mol. Cell. Biol. 2012; 13(5): 283-96. https://doi.org/10.1038/nrm3330.
- Коган Е.А., Ли Д.Д., Файзулина Н.М., Козаченко А.В. Экспрессия oct-4, p53, p16 и ki67 при ВПЧ-ассоциированном предраке и раке шейки матки. Клиническая практика. 2016; 1: 34-8. [Kogan E.A., Lee DD, Fayzulina N.M., Kozachenko A.V. Expression of oct-4, p53, p16 and ki67 in HPV-associated precancer and cervical cancer. Clinical practice. 2016; 1: 34-8. (In Russ.)]
- Евстигнеева Н.П., Кузнецова Ю.Н., Бальберт Н.А., Потапова А.Л., Медведева Ю.А. Экспрессия вирусных онкогенов p16, ki67 и вирусная нагрузка при различных вариантах течения урогенитальной папилломавирусной инфекции. Акушерство и гинекология. 2013; 9: 41-5. [Evstigneeva NP, Kuznetsova Yu.N., Balbert N.A., Potapova A.L., Medvedeva Yu.A. Expression of viral oncogenes p16, ki67 and viral load in different variants of the course of urogenital papillomavirus infection. Obstetrics and gynecology. 2013; 9: 41-5. (In Russ.)]
- Аникина Т.А., Сысоева В.Ю., Рубина К.А., Радзинский В.Е. Экспрессия маркеров клеточного цикла мезенхимными клетками нормального эндометрия и эндометрия при пролиферативных заболеваниях матки. Акушерство и гинекология. 2016; 9: 79-86. [Anikina T.A., Sysoeva V.Yu., Rubina K.A., Radzinsky V.E. Expression of cell cycle markers by normal endometrial and endometrial mesenchymal cells in uterine proliferative diseases. Obstetrics and gynecology. 2016; 9: 79-86. (In Russ.)]
- Казачкова Э.А., Гошгарлы А.В., Воропаева Е.Е., Казачков Е.Л., Рогозина А.А. Экспрессия белка p16INK4a при гиперплазии эндометрия, ассоциированной с хроническим эндометритом. Уральский медицинский журнал. 2018; 2: 97-100. [Kazachkova E.A., Goshgarly A.V., Voropaeva E.E., Kazachkov E.L., Rogozina A.A. Expression of p16INK4a protein in endometrial hyperplasia associated with chronic endometritis. Ural Medical Journal. 2018; 2: 97-100. (In Russ.)]
- Думановская М.Р., Чернуха Г.Е., Бурменская О.В., Донников А.Е., Трофимов Д.Ю. Вероятность неопластической трансформации при различных типах гиперплазии эндометрия. Акушерство и гинекология. 2013; 8: 56-62. [Dumanovskaya M.R., Chernukha G.E., Burmenskaya O.V., Donnikov A.E., Trofimov D.Yu. The likelihood of neoplastic transformation in various types of endometrial hyperplasia. Obstetrics and gynecology. 2013; 8: 56-62. (In Russ.)]
- Кондриков Н.И., Баринова И.В. Патология матки. Руководство для врачей. 2-е изд. М.: Практическая медицина; 2019. 352 с. [Kondrikov N.I., Barinova I.V. Pathology of the uterus: a guide for doctors. 2nd ed. M.: Practical medicine, 2019. 352 р. (In Russ.)]
- Murdock T.A., Veras E.F.T., Kurman R.J., Mazur M.T. Diagnosis of endometrial biopsies and curretings. 3rd ed. Springer; 2019.
- Казачкова Э.А., Казачков Е.Л., Хелашвили И.Г., Воропаева Е.Е. Хронический эндометрит и рецептивность эндометрия (монография). Челябинск: ГБОУ ВПО ЮУГМУ Минздрава России; 2015. 148 с. [Kazachkova E.A., Kazachkov E.L., Khelashvili I.G., Voropaeva E.E. Chronic endometritis and endometrial receptivity (monograph). Chelyabinsk: State Budgetary Educational Institution of Higher Professional Education SUSMU of the Ministry of Health of Russia, 2015. 148 р. (In Russ.)]
- Толибова Г.Х., Траль Т.Г., Клещёв М.А., Кветной И.М., Айламазян Э.К. Эндометриальная дисфункция: алгоритм гистологического и иммуногистохимического исследования. Журнал акушерства и женских болезней. 2015; 64(4): 69-77. [Tolibova G.Kh., Tral T.G., Kleschev M.A., Kvetnoy I.M., Aylamazyan E.K. Endometrial dysfunction: an algorithm for histological and immunohistochemical studies. Journal of Obstetrics and Women’s Diseases. 2015; 4: 69-77. (In Russ.)]
- Алимова О.А., Воропаева Е.Е., Казачкова Э.А. Полуколичественная морфологическая оценка активности воспалительного процесса при хроническом эндометрите. В кн.: Материалы Всероссийской научно-практической патологоанатомической конференции «Актуальные проблемы патологоанатомической службы муниципальных учреждений здравоохранения». Челябинск; 2008. С. 198-201. [Alimova O.A., Voropayeva E.E., Kazachkova E.A. Semi-quantitative morphological assessment of the activity of the inflammatory process in chronic endometritis: Materials All-Russian. scientific-practical pathoanatomical conf. “Actual problems of the pathoanatomical service of municipal health care institutions.” Chelyabinsk, 2008. Р. 198-201. (In Russ.)]
Received 04.03.2019
Accepted 19.04.2019
About the Authors
Kazachkov Evgeny L., MD, professor, head of the Department Pathological anatomy and forensic medicine, SUSMU Ministry of Health of Russia.454092, Russia, Chelyabinsk, Vorovskiy St, 64; Phone: +7 (351) 232-01-45. E-mail: doctorkel@yandex.ru
Zatvornitskaya Alexandra V., postgraduate student of the Department of Obstetrics and Gynecology, SUSMU Ministry of Health of Russia.
454092, Russia, Chelyabinsk, Vorovskiy St, 64; Phone: +7 (919) 400-75-35. E-mail: monostyle@list.ru
Voropaeva Ekaterina E., MD, Associate Professor, Professor of the Department of Pathological anatomy and forensic medicine, SUSMU Ministry of Health of Russia.
454092, Russia, Chelyabinsk, Vorovskiy St, 64; Phone: +7 (351) 232-01-45. E-mail: katya_voropaeva@mail.ru
Kazachkova Ella A., MD, professor, professor of the Department of Obstetrics and Gynecology, SUSMU Ministry of Health of Russia.
454092, Russia, Chelyabinsk, Vorovskiy St, 64; Phone: +7 (351) 772-84-01. E-mail: doctorkel@yandex.ru
Schegolev Alexander I., MD, professor, prof. Department of Pathological Anatomy and Clinical Pathological Anatomy RNRMU Ministry of Health of Russia.
Phone: +7 (495) 536-91-54; E-mail: ashegolev@oparina4.ru 117997 Russia, Moscow, Ostrovityanova str., 1.
For citation: Kazachkov E.L., Zatvornitskaya A.V., Voropaeva E.E., Kazachkova E.A., Shchegolev A.I. Characteristics of the proliferative and antiproliferative activities of the cells of the endometrium in its hyperplasia concurrent with chronic endometritis.
Akusherstvo i ginekologiya/Obstetrics and Gynecology. 2019;(8): 100-106(in Russian).
https://dx.doi.org/10.18565/aig.2019.8.100-106



