Steroid hormone profiles in reproductive age patients with advanced endometriosis
Introduction. Investigating pathological changes in endogenous steroidogenesis associated with advanced extragenital endometriosis (AEGE) requires high-precision and sensitive methods. Aim. To investigate blood steroidome changes in AEGE. Materials and methods. Patients with AEGE and control subjects were tested for serum hormones using high-performance liquid chromatography-mass spectrometry (HPLC-MS). Results. Patients with AEGE had significant changes in the concentration of 9 steroids. Blood hormone concentrations in patients with and without AEGE were as follows: estradiol 0.18 vs. 0.12 ng/ml (p=0.05), 17-OH-progesterone 0.55 vs. 0.44 ng/ml (p=0.05), testosterone 0.81 vs. 0.43 ng/ml (p <0.01), dihydrotestosterone- 0.16 vs. 0.37 ng/ml (p<0.1), dehydroepiandrosterone 3.56 vs. 5.98 ng/ml (p<0.01), DHEA sulfate 2533.00 vs. 4425.00 ng/ml (p<0.01), androstenedione 1.35 vs. 1.31 ng/ml (p=0.29), cortisol 164.00 vs. 90.50 ng/ml (p<0.01), corticosterone 4.87 vs. 1.20 ng/ml (p<0.01), 11-deoxycorticosterone 0.87 vs. 0.37 ng/ml (p<0.01). Progesterone concentrations were below the detection limit in both groups. Four logistic regression models allowed differentiation of blood steroidome of AEGE patients from that in controls. Conclusion. Endometriotic cysts in stage III – IV AEGE have no significant effect on changes in the steroidome. HPLC-MS differences in blood steroidome allow differentiation of patients with AEGE from control subjects.Pavlovich S.V., Iurova M.V., Chagovets V.V., Frankevich V.E., Starodubtseva N.L., Chuprynin V.D., Sukhikh G.T.
Keywords
Endometriosis is a chronic systemic immune- and hormone-dependent multifactorial disease [1]. Currently, endometriosis affects about one out of every ten women of reproductive age, or 176 million people worldwide. Since the early stages of the disease may be asymptomatic, many patients (up to 7%) are not diagnosed for decades [2]. This disease is particularly important because it is associated with decreased fertility and quality of life in patients of reproductive age due to severe pelvic pain with a neuropathic component. It reduces women's workability and social activity and represents a significant burden on the health care system and economy [1, 3].
Endometriosis is the most common intraoperatively established diagnosis in patients undergoing surgery to identify the causes of chronic pelvic pain and treat infertility [4]. The disease is heterogeneous and is subdivided into several phenotypes: endometriotic ovarian cysts, superficial peritoneal, and deep infiltrative endometriosis [1, 5].
Changes in steroidogenesis are one of the critical links in the pathobiological cascade in the development of endometriosis. Assessment of endometriosis patients' hormonal profile is routinely performed for diagnostic purposes and the selection of postoperative hormone-modulating therapy. Depending on the structural features of hormones, their ability to ionize is significantly different, but due to a neutral charge, the signal intensity is usually weak. Steroids with a conjugated double bond with a carbonyl group (delta-4-steroids such as testosterone) are generally easier to ionize, making them measurable even at minimal concentrations. The opposite tendency is observed in estradiol: the content of the phenol group and extremely low concentrations of this hormone in the samples lead to real difficulties in measuring this steroid concentration [6].
Besides, steroid hormones are present in low concentrations in the systemic circulation and tissues, and therefore highly sensitive methods are required for their identification. The concentrations are measured by chromatography-mass spectrometry or by immunological methods. Immunoassays have a high sample throughput. However, this technique's specificity is limited due to the significant structural similarity of various steroids and overlap with other endogenous and exogenous components. Chromatographic methods are more specific for steroid analysis [7–11].
High-performance liquid chromatography and mass spectrometry (HPLC-MS), especially in the modification outlined in the study by M.R. Häkkinen et al., have a high sensitivity for quantitative determination of low molecular weight metabolites, including steroid hormones [7].
This study aimed to investigate serum steroid hormone changes in patients with advanced-stage III–IV extragenital endometriosis to determine the diagnostic performance of a single-step HPLC-MS and the impact of endometriotic cysts on changes in steroidome.
Materials and methods
Study design
This case-control study was conducted at the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia from March 2018 to April 2020 and included 65 reproductive-age women. The patients were divided into three groups. Group 1 included 16 patients aged 23–37 years with deep infiltrative stage III-IV endometriosis according to the revised AFS classification of endometriosis [6] and endometriotic ovarian cyst (mean cyst diameter 4.4±1.1 cm). Group 2 included 23 patients aged 27–39 years with deep infiltrative endometriosis. The patients underwent laparoscopic surgery. The diagnosis of AEGE was confirmed surgically and verified by histological examination of surgical specimens.
The third group (control group) consisted of 26 healthy female volunteers aged 23–34 years with successfully realized reproductive function. Based on clinical and laboratory (anamnesis, examination, clinical and biochemical testing) and instrumental (expert level ultrasound examination) examination, they were found to have no ovarian masses. They also had no complaints; blood concentrations of steroid hormones, investigated by immunological diagnostic methods, were within the reference range. HPLC-MS hormone levels in this group were within the reference range recommended for laboratory equipment.
Serum profiles of steroid hormones in all patients were determined during the proliferative phase of the menstrual cycle. At the time of blood sampling, patients received no hormonal therapy for three months or more. Comorbidities, including uterine fibroids, hyperproliferative endometrial conditions, were criteria for exclusion [1].
All patients signed informed consent for providing blood samples. The Research Ethics Committee of the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia approved this study.
Reagents
Calibration lines were constructed using a Jasem Steroid Hormones standards set. In 200 μl of the standard, 20 μl of the Jasem internal standard (a set of deuterium-labeled steroids) was added. Proteins were precipitated using 450 μl of acetonitrile, the supernatant solution was decanted, and steroids were extracted with 1 ml of methyl tertiary butyl ether. An aliquot of 800 μL was taken, dried in a nitrogen flow, and the sample was reconstructed with 100 μL methanol/water (v/v=1/1). Serum samples were prepared similarly. For one analysis, 20 μl of the sample was used.
Mass spectrometric analysis conditions
HPLC-MS was used for single-step quantitative analysis of serum samples of patients with AEGE and the control group. The analysis included estrogens (estradiol, E2), androgens (testosterone, T; dihydrotestosterone, androstenedione, A4; dehydroepiandrosterone, DHEA; dehydroepiandrosterone sulfate, DHEAS), progestogens (17 progesterone-4, P4 F; corticosterone, B), and mineralocorticoids (11-deoxycorticosterone, S; aldosterone, A). The studies were carried out on a QTRAP 5500 mass spectrometer, ABSciex (ABSciex, Canada), with an Agilent 1260 chromatographic system (Agilen, USA). Ionization was achieved using electrospray in positive mode (ESI+), voltage 5500 V; nitrogen was used as spraying and drying gases, temperature 350°C, scanning type – monitoring of multiple reactions. Hormone separation was conducted on a Jasem Steroids reverse phase column (Turkey). The mobile phase consisted of deionized water (A) and acetonitrile (B) (99.9%), containing 0.01% formic acid. Elution was carried out at a rate of 400 μl/min, 0.5 minutes – 15% B. Then the content of the organic phase was linearly raised to 60% within 13.5 minutes, the column was washed for 1 minute with 85% B at a flow rate of 800 μl/min, and the analytical column was equilibrated for 4 minutes with 15% B.
Statistical analysis
Statistical analysis was performed using scripts written in the R language [R CoreTeam (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/] at RStudio [RStudio Team (2016). RStudio: Integrated Development for R. RStudio, Inc., Boston, MA URL http://www.rstudio. com/]]. The distribution of continuous variables was tested for normality using the Shapiro–Wilk test. The statistical significance of between-group differences for continuous variables was tested with Mann–Whitney test. The critical level of significance when testing statistical hypotheses was considered at p<0.05. Logistic regression models were developed to classify patients based on the studied parameters. All possible combinations of steroid hormones were considered as dependent variables in the models. The patient's group membership was used as an independent variable. Of all the models developed, the four with the largest area under the ROC curve (AUC) were selected. For each model, Wald's test, 95% confidence interval (CI), odds ratio (OR), and confidence interval were calculated. The developed models' quality was determined by constructing the ROC curve, defining the area under the ROC curve, and calculating the sensitivity and specificity.
Results
Serum steroid profiles (12 hormones) of patients with AEGE (groups 1 and 2) and control subjects were determined by HPLC-MS.
Comparison of serum steroid profiles of patients in the study and control groups
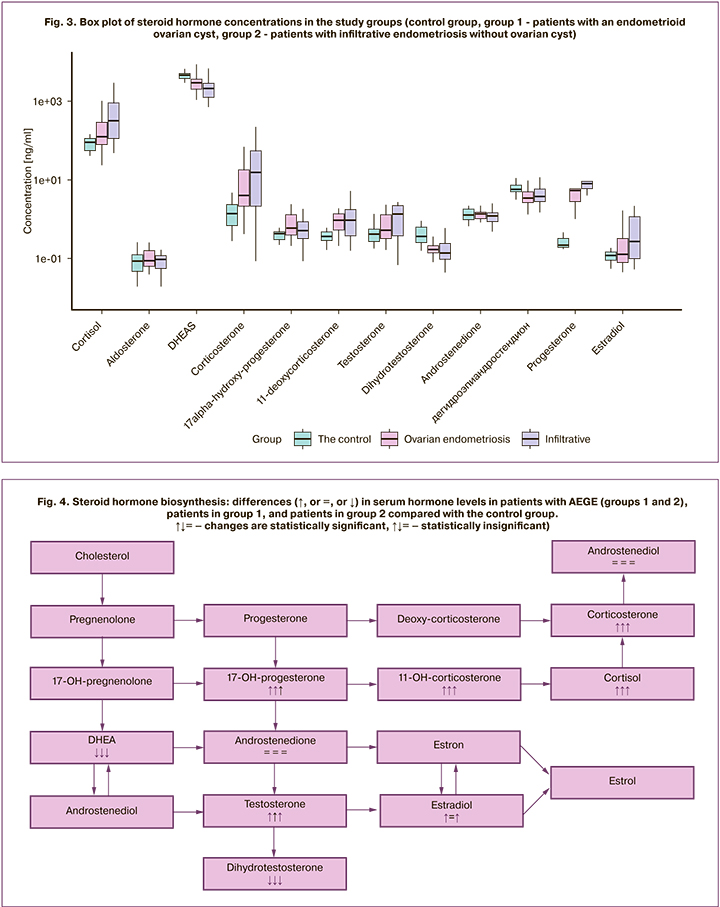
Concentrations of E2, P4, 17-OHP4, T, dihydrotestosterone, DHEA, DHEAS, S, B, and F were statistically significantly different between patients with AEGE and the control group (Table 1).
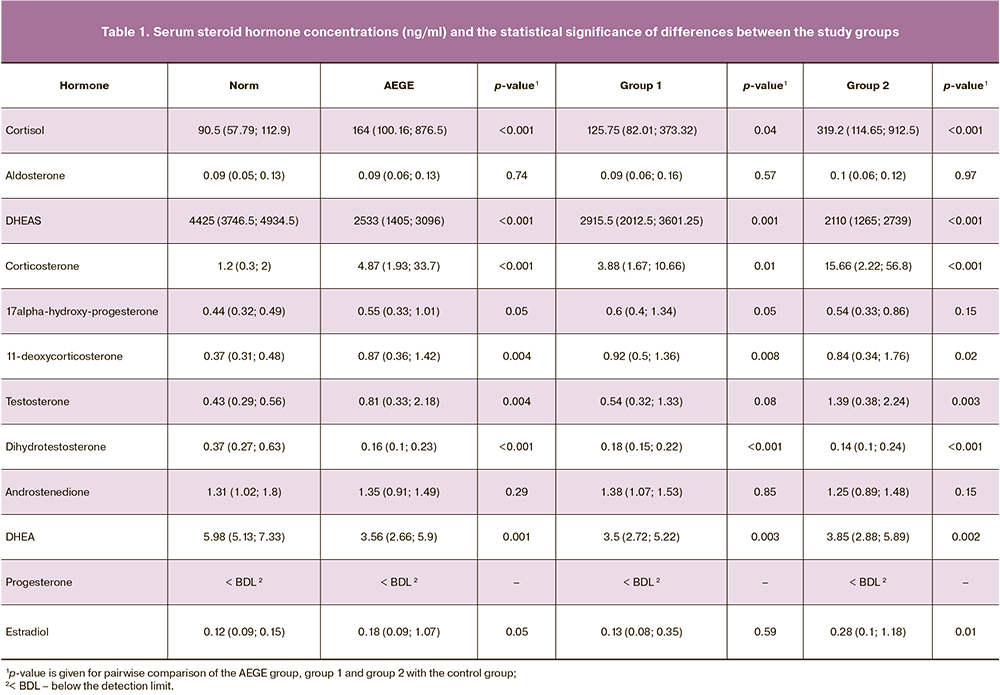
Estrogens
In patients with AEGE, E2 concentrations [0.18 (0.09–1.07)] were significantly higher than in the control group [0.12 (0.09–0.15)] ng/ml (p=0.04).
Progestogens
In patients with AEGE, the P4 concentrations in both groups were less than the lowest measurement limit (0.00–6.09 ng/ml).
Concentrations of 17-OHP4 in patients with AEGE (0.55 [0.33–1.01] ng/ml) were statistically significantly different than in the control group (0.44 [0.32–0.49] ng/ ml) (p=0.05).
Androgens
In patients with AEGE, T concentrations [0.81 [0.33– 2.18] were higher than in the control group [0.43 (0.29– 0.56)] ng/ml, (p<0.01).
In patients with AEGE, concentrations of dihydrotestosterone were significantly reduced 0.16 [0.10–0.23] ng/ml compared to the control group [0.37 (0.27–0.63)] ng/ml (p<0.01).
In patients with AEGE, concentrations of DHEA [(3.56 (2.66–5.90) ng/ml] and DHEAS [2533.00 (1405.00–3096.00) ng/ml] were significantly lower (p<0.01 and p<0.01) than in the control group [5.98 (5.13–7.33) ng/ml and 4425.00 (3746.50–4934.50)] ng/ml, respectively.
There were no statistically significant between-group differences regarding A4: 1.35 (0.91–1.49) ng/ml in patients with AEGE and 1.31 (1.02–1.80) ng/ml in control subjects (p=0.29).
Glucocorticosteroids
In patients with AEGE, concentrations of F were significantly higher than in the control group [164.00 (100.16–876.502)] and [90.50 (57.79–112.90)] ng/ml, respectively; p<0.01). Similar data were obtained when comparing B levels [4.87 (1.93–33.70)] and [1.20 (0.30–2.00)] ng/ml, respectively; p<0.01).
Mineralocorticoids
In patients with AEGE, S concentrations were significantly higher than in the control group: 0.87 [0.36–1.42] and 0.37 [0.31–0.48] ng/ml, p<0.01.
There were no statistically significant differences in concentrations of A in patients with AEGE [0.09 (0.06– 0.13)] ng/ml and in control subjects [0.09 90.05–0.13)] ng/ml, (p=0.74).
Therefore, statistically significant differences in serum hormone concentrations in patients with AEGE and the control group were obtained for E2, F, DHEA, and DHEAS, B, 17-OHP4, S, T, and dihydrotestosterone (Fig. 1).
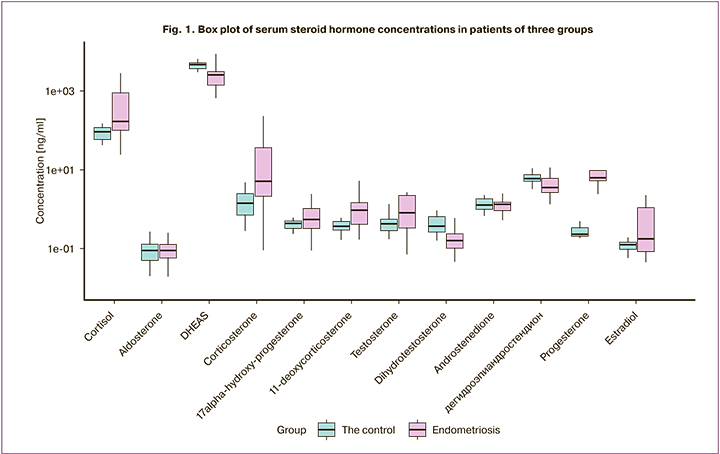
Based on serum steroid hormone concentrations of control and AEGE patients, logistic regression models were constructed (Table 2).
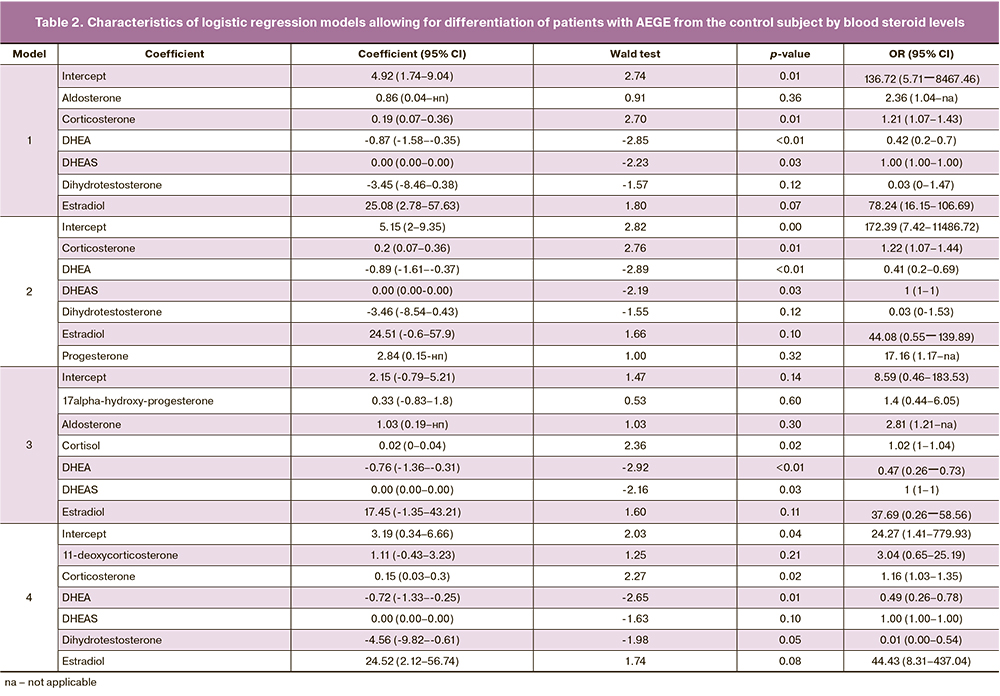
Logistic regression model:
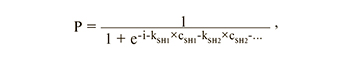
where i is a constant; kSH1, kSH2,… – coefficients for hormone concentrations; cSH1, cSH2, ... – hormone concentrations.
Based on these models, ROC curves were constructed (Fig. 2).
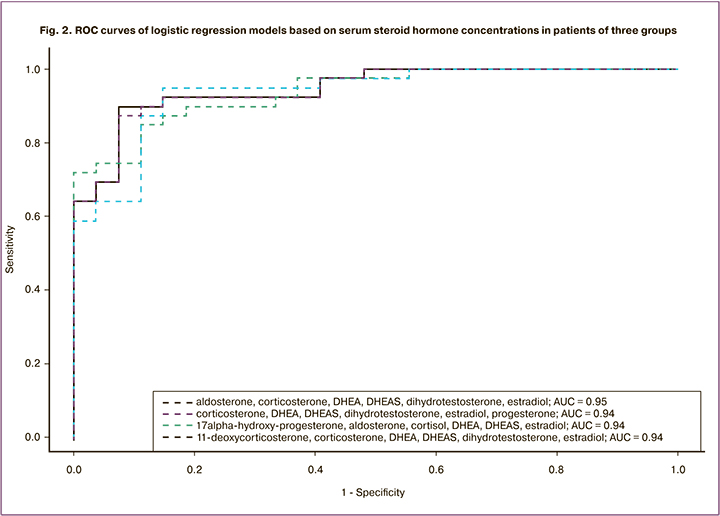
E2, dihydrotestosterone, DHEA, DHEAS, B, A: sensitivity = 0.90, specificity = 0.93, AUC=0.95.
E2, P4, dihydrotestosterone, DHEA, DHEAS, B: sensitivity = 0.87, specificity = 0.93, AUC=0.94.
E2, 17-OHP4, F, DHEA, DHEAS, A: sensitivity = 0.85, specificity = 0.89, AUC=0.94.
E2, dihydrotestosterone, DHEA, DHEAS, B, S: sensitivity = 0.95, specificity = 0.85, AUC=0.94.
Pairwise comparison of serum steroid profiles of patients in the study and control groups
Comparison of hormone concentrations in each study group and control group (Mann-Whitney test without multiple comparisons correction) showed the following results (Table 1).
Comparison of the serum steroid profile of patients with ovarian and infiltrative AEGE
There were no statistically significant differences in steroid hormone levels in groups 1 and 2 (Table 1).
Concentrations of the analyzed steroid hormones in the study groups are shown in box plots (Fig. 3).
Discussion
Several studies have formed a stable model of the etiology and pathogenesis of endometriosis. It hypothesizes that a change in hormone biosynthesis (hyperestrogenism, resistance to progesterone and overexpression of aromatase, cytochrome CYP19 family) is not only a consequence but also the cause of the disease progression since it correlates with the activity of proinflammatory cascades, neoangiogenesis, and neurogenesis [12, 13].
Recently, some genetic aspects of the pathogenesis of endometriosis have been clarified. Found genes have been linked to controlling the activity of specific intracellular specific enzymes responsible for forming a particular steroid. The epigenetic mechanism of DNA methylation affects estrogen receptors and the HOXA10 and NR5A1 genes, the latter of which is responsible for the aromatase overexpression and the synthesis of a peptide that regulates steroidogenesis (steroidogenesis regulatory peptide, CRP), which is characteristic of endometriosis [14]. The methylated NR5A1 gene expresses specific transcription factor 1 (STF-1), the interaction of which with promoters activates enhancers and stimulates the pathological synthesis of aromatase and CRP. In normal endometrial tissue, there are no such signaling pathways mediated by cyclic adenosine monophosphate or stimulated by prostaglandin E2, and multimeric complexes do not contain SFT-1 [15]. These processes are manifested by difficultly identifiable local hyperestrogenism [16].
In 1991 F. Labrie introduced the concept of intracrinology, which focused on pathways and mechanisms of androgens and estrogens, the formation from precursors, and the biological response of the cells to the hormones secreted by them (peptides, proteins, steroids) [17]. A significant contribution to this type of homeostasis belongs to intracrine regulation. The synthesis of active steroids occurs in peripheral target tissues where steroid action is exerted in the same cells, where synthesis occurs without releasing the active hormones in the extracellular space and the general circulation. The change in concentration does not correlate with their actual activity. Besides, several biological fluids are characterized by a concentration that is insufficient for identification by standard methods [18]. Several studies have shown that the analysis of the reproductive organs' hormonal activity with intracrine regulation can be more informative using the HPLC-MS [8, 15].
In the study, patients with AEGE were found to have hyperandrogenemia in addition to hyperestrogenism. In women, the intracrine pathway of formation and exposure to T and 5α-dihydrotestosterone from DHEA dominates, one of the essential precursors of E2 and bioactive androgens, into which it is converted in peripheral tissues [6, 18, 19]. Revealing the nature of enzymatic disturbances in systemic and local steroidogenesis is essential for diagnosing the source of hypo- and hyperandrogenemia.
Determination of serum T is the most informative for assessing the androgen-synthesizing function of the ovary in endometriosis. According to K. Huhtinen et al., T concentrations established using liquid mass spectrometry of serum and tissue samples were higher in the group of patients with endometriosis, mainly when heterotopies were localized in ovarian tissue, which may be associated with an increase in HSD3B2 (isoenzyme 3β-hydroxysteroid dehydrogenase), CYP11A1 and CYP17A1 (Cholesterol side chain-cleavage enzyme), as well as with the increased expression of androgen-activating genes PRUNE2, HGD, PDGFRL [15]. In our study, an increase in T was noted, on the contrary, in patients without endometriotic cysts, but it did not reach a statistically significant level. Thus, it can be assumed that an increase in the concentration of androgens is associated with an increase in the function of the adrenal glands against the background of severe pain syndrome, chronic stress (as evidenced by the established increase in the concentration of F), caused by the disease.
The obtained models suggest the high diagnostic performances of steroid hormones in the studied panel (Fig. 2, 4). A significant change in 9 steroid hormones was observed in AEGE.
There were no statistically significant differences between groups 1 (AEGE III–IV stages, ovarian cyst) and 2 (AEGE III–IV stages) in concentrations of estrogens, progestogens, androgens, glucose- and mineralocorticoids. This observation suggests that the involvement of ovarian tissue in the process is not a factor in the intensity of steroidogenesis. This may indicate the unity of endometriosis as a pathological process, the substrate of which is the activity, rather than localization of heterotopic tissue (Fig. 4). These results may indicate the absence of an additional effect of endometrioid cysts on steroidogenesis in AEGE. The study findings lend support for further studies with a larger sample of patients.
Limitations
It should be borne in mind that a change in serum steroid hormone concentrations can be associated not only with a change in their synthesis rate (steroidogenesis) or secretion but also with a change in the content of the corresponding blood transport proteins, including globulin binding sex hormones, transcortin, etc. Their liver formation and blood concentration largely depend on steroid hormones' total content and their metabolically active free forms.
When analyzing the hormonal activity of the reproductive organs with an intracrine function, the HPLC-MS of the cell compartment steroidome can be more informative than the analysis of changes in the peripheral blood. A detailed study of these aspects and a multivariate analysis to minimize the influence of confounders constitute real prospects for the continuation of this study on a larger sample of patients.
Conclusion
The study presents the feasibility of single-step metabolomic analysis by an expanded panel measuring serum levels of 12 steroid hormones using liquid chromatography coupled to mass spectrometry.
The study identified pathological changes in steroidogenesis in reproductive age women with AEGE, including a significant increase in the concentrations of E2, T, 17-α-OHP4, S, B, and F, a decrease in DHEA, DHEAS, and dihydrotestosterone.
Logistic regression models allowed differentiation of the blood steroidome of patients with AEGE from the control group. The four combinations of steroid hormones have the largest area under the ROC-curve (AUC), sensitivity, and specificity. However, the model based on a combination of E2, dihydrotestosterone, DHEA, DHEAS, B, and A should be noted. The calculated operating characteristics indicate the feasibility of using this combination of hormones to diagnose stages I-IV AEGE, which requires further validation.
The study findings showed no differences between groups 1 and 2; that is, the blood steroidome does not reflect the involvement of ovarian tissue in endometriosis. Therefore we can assume the unity of endometriosis as a pathological process, the substrate of which is the activity, and not the localization of heterotopic tissue. This study confirms the feasibility of using the HPLC-MS for the quantitative determination of steroid hormone precursors and metabolites. This has led us to indirect conclusions about the causes for the disruption of steroidogenesis enzyme activity.
In general, mass spectrometry, which has a high specificity and sensitivity, is a promising analytical technique for measuring steroids both in basic research and clinical settings when classical tests show borderline results.
References
- Chapron C., Marcellin L., Borghese B., Santulli P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat. Rev. Endocrinol. 2019; 15(11): 666-82. https://dx.doi.org/10.1038/s41574-019-0245-z.
- Schenken R.S., Barbieri R.L., Eckler K. Endometriosis: pathogenesis, clinical features, and diagnosis. UpToDate. 2019. Available at: https://www.uptodate.com/contents/endometriosis-pathogenesis-clinical-features-and-diagnosis
- Zondervan K.T., Becker C.M., Missmer S.A. Endometriosis. N. Engl. J. Med. 2020; 382(13): 1244-56. https://dx.doi.org/10.1056/NEJMra1810764.
- Kalogera E., Pistos C., Provatopoulou X., Christophi C.A., Zografos G.C., Stefanidou M. et al. Bioanalytical LC-MS method for the quantification of plasma androgens and androgen glucuronides in breast cancer. J. Chromatogr. Sci. 2016; 54(4): 583-92. https://dx.doi.org/10.1093/chromsci/bmv190.
- Vercellini P., Somigliana E., Vigano P., Abbiati A., Barbara G., Fedele L. et al. “Blood on the tracks” from corpora lutea to endometriomas. BJOG. 2009; 116(3): 366-71. https://dx.doi.org/ 10.1111/j.1471-0528.2008.02055.x.
- Appendix: the revised AFS classification of endometriosis. In: Timmerman D., Deprest J., Bourne T. Ultrasound and endoscopic surgery in obstetrics and gynecology. A combined approach to diagnosis and treatment. London: Springer-Verlag; 2003: 216-7.
- Häkkinen M.R., Heinosalo T., Saarinen N., Linnanen T., Voutilainen R., Lakka T. et al. Analysis by LC-MS/MS of endogenous steroids from human serum, plasma, endometrium and endometriotic tissue. J. Pharm. Biomed. Anal. 2018; 152: 165-72. https://dx.doi.org/10.1016/j.jpba.2018.01.034.
- Palermo A., Botrè F., de la Torre X., Zamboni N. Non-targeted LC-MS based metabolomics analysis of the urinary steroidal profile. Anal. Chim. Acta. 2017; 964: 112-22. https://dx.doi.org/ 10.1016/j.aca.2017.01.055.
- Higashi T., Ogawa S. Chemical derivatization for enhancing sensitivity during LC/ESI-MS/MS quantification of steroids in biological samples: a review. J. Steroid Biochem. Mol. Biol. 2016; 162: 57-69. https://dx.doi.org/10.1016/j.jsbmb.2015.10.003.
- Fülöp I., Vari C.E., Miklos A., Imre S. LC-MS/MS ESI methods for the determination of oestrogens and androgens in biological matrix – a minireview. Farmacia. 2017; 65(4): 485-93.
- Jeanneret F., Tonoli D., Rossier M.F., Saugy M., Boccard J., Rudaz S. Evaluation of steroidomics by liquid chromatography hyphenated to mass spectrometry as a powerful analytical strategy for measuring human steroid perturbations. J. Chromatogr. A. 2016; 1430: 97-112. https://dx.doi.org/ 10.1016/j.chroma.2015.07.008.
- Yilmaz B.D., Bulun S.E. Endometriosis and nuclear receptors. Hum. Reprod. Update. 2019. 25(4): 473-85.https://dx.doi.org/10.1093/humupd/dmz005.
- Reis F.M., Petraglia F., Taylor R.N. Endometriosis: hormone regulation and clinical consequences of chemotaxis and apoptosis. Hum. Reprod. Update. 2013; 19(4): 406-18. https://dx.doi.org/10.1093/humupd/dmt010.
- Qi Q.M., Guo S.W., Liu X.S. Estrogen biosynthesis and its regulation in endometriosis. Reprod. Dev. Med. 2017; 1(1): 55-61. https://dx.doi.org/10.4103/2096-2924.210698.
- Huhtinen K., Saloniemi-Heinonen T., Keski-Rahkonen P., Desai R., Laajala D., Ståhle M. et al. Intra-tissue steroid profiling indicates differential progesterone and testosterone metabolism in the endometrium and endometriosis lesions. J. Clin. Endocrinol. Metab. 2014; 99(11): E2188-97. https://dx.doi.org/10.1210/jc.2014-1913.
- Attar E., Bulun S.E. Endometriosis. In: Fazlebas A., ed. Endometrium. 2nd ed. Taylor and Francis Book Ltd.; 2008: 691-710.
- Labrie F. Intracrinology. Mol. Cell. Endocrinol. 1991; 78(3): C113-8. https://dx.doi.org/10.1016/0303-7207(91)90116-a.
- Gibson D.A., Simitsidellis I., Collins F., Saunders P.T.K. Endometrial intracrinology: oestrogens, androgens and endometrial disorders. Int. J. Mol. Sci. 2018; 19(10): 3276. https://dx.doi.org/ 10.3390/ijms19103276.
- Kalogera E., Pistos C., Provatopoulou X., Athanaselis S., Spiliopoulou C., Gounaris A. Androgen glucuronides analysis by liquid chromatography tandem-mass spectrometry: could it raise new perspectives in the diagnostic field of hormone-dependent malignancies? J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013; 940: 24-34. https://dx.doi.org/10.1016/j.jchromb.2013.09.022.
Received 13.07.2020
Accepted 12.02.2021
About the Authors
Stanislav V. Pavlovich, Ph.D., Academic Secretary, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia; Professor at the Department of Obstetrics, Gynecology, Perinatology and Reproductology, the Faculty of Postgraduate Professional Training of Physicians, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University). Tel.: +7(495)438-20-88. E-mail: s_pavlovich@oparina4.ru.117997, Russia, Moscow, Ac. Oparina str., 4.
Mariia V. Iurova, Specialist, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia; Ph.D. Student, the Chair of Obstetrics, Gynecology, Perinatology and Reproductology, the Faculty of Postgraduate Professional Training of Physicians, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University). Tel.: +7(495)438-20-88.
E-mail: m_yurova@oparina4.ru. ORCID: 0000-0002-0179-7635. 117997, Russia, Moscow, Ac. Oparina str., 4; 119991, Russia, Moscow, Trubetskaya str., 8-2.
Vladimir E. Frankevich, Ph.D., Head of Department of System Biology in Reproduction, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(495)438-07-88 ex. 2198. E-mail: v_frankevich@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Nataliya L. Starodubtseva, Ph.D., Head of Laboratory of Proteomics of Human Reproduction, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(916)463-98-67. E-mail: n_starodubtseva@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Vitaliy V. Chagovets, Ph.D., Senior Researcher at the Laboratory of Proteomics and Metabolomics in Human Reproduction, Department of System Biology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(926)562-65-90. E-mail: vvchagovets@gmail.com. 117997, Russia, Moscow, Ac. Oparina str., 4.
Vladimir D. Chuprynin, Ph.D., Head of the Department of Surgery, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(495)438-35-75.
E-mail: v_chuprynin@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Gennady T. Sukhikh, Dr. Med. Sci., Professor, Academician of the RAS, Director of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia; Head of the Department of Obstetrics, Gynecology, Perinatology and Reproductology, Faculty of Postgraduate Professional Training of Physicians, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University). E-mail: g_sukhikh@oparina4.ru. 17997, Russia, Moscow, Ac. Oparina str., 4.
For citation: Pavlovich S.V., Iurova M.V., Chagovets V.V., Frankevich V.E., Starodubtseva N.L., Chuprynin V.D., Sukhikh G.T. Steroid hormone profiles in reproductive age patients with advanced endometriosis.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 3: 90-100 (in Russian)
https://dx.doi.org/10.18565/aig.2021.3.90-100



