Gene transcription profiles in fibrocystic breast disease
Objective: To investigate gene transcription profiles and identify differences in gene expression between different forms of fibrocystic breast disease (FBD).Burmenskaya O.V., Kometova V.V., Smetnik A.A., Rodionov V.V., Trofimov D.Yu., Ashrafyan L.A., Sukhikh G.T.
Materials and methods: We studied 128 formalin-fixed, paraffin-embedded breast tissue samples obtained at the time of surgery with histologically confirmed non-proliferative FBD, typical ductal hyperplasia, atypical ductal hyperplasia, ductal carcinoma in situ, and normal tissue. The expression profiles of the 46 candidate genes were analyzed by real-time quantitative PCR.
Results: Depending on the histological type of the sample, we established the general patterns of mRNA expression of the genes studied in different forms of FBD, and the spectra of differentially expressed genes in proliferative FBD with atypia (18 genes) and non-proliferative forms of FBD (14 genes).
Conclusion: Proliferative FBD with atypia, which carries the highest risk of developing breast cancer, is characterized by a statistically significant increase in the mRNA expression of MKI67, CCNB1, KIF14, PTTG1, ANLN, TMEM45B, TPX2, PRLR, FGFR4, MMP11, GATA3, EXO1, TYMS, and NAT1, and a decrease in the mRNA expression of MYC, RANK, TNFA, and MMP9.
Authors' contributions: Ashrafyan L.A., Rodionov V.V., Sukhikh G.T. – conception and design of the study; Kometova V.V., Burmenskaya O.V. – data collection and analysis; Trofimov D.Yu. – organization and conduct of PCR; Burmenskaya O.V. – statistical analysis; Burmenskaya O.V., Smetnik A.A. – manuscript drafting; Rodionov V.V. – manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The work was partially supported financially within the framework of State Task "Assessment of Individual Risk of Breast Cancer in Women with Benign Breast Disease (fibrocystic breast disease)" (State Registration Number 121040600432-9).
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Burmenskaya O.V., Kometova V.V., Smetnik A.A., Rodionov V.V., Trofimov D.Yu., Ashrafyan L.A., Sukhikh G.T. Gene transcription profiles in fibrocystic breast disease.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (7): 55-65 (in Russian)
https://dx.doi.org/10.18565/aig.2023.77
Keywords
According to the WHO definition (1984), mastopathy is classified as a fibrocystic disease (FBD), which is characterized by an abnormal ratio of epithelial and connective tissue components. It encompasses a range of proliferative and regressive changes in breast tissue [1].
Statistical records of FBD are not maintained, but many authors estimate its prevalence in the female population to be approximately 50%. This makes it reasonable to consider FBD the most common breast pathology [2–4].
Although FBD is generally not considered a precancerous disease, several of its forms significantly increase the risk of developing breast cancer. In women with benign tumors and high mammographic breast density, the risk of developing breast cancer is three times higher than that in women with moderate mammographic density and without benign breast tumors [5]. The risk of developing breast cancer varies depending on the morphological changes detected during breast biopsy. Estimates suggest that the risk of breast cancer is slightly increased or equal to the population risk of non-proliferative forms of the disease. It is increased by 1.5–3.5 or 2–10 times compared to the population risk in proliferative forms of the disease without atypia and with atypia, respectively [6, 7].
Considering the importance of assessing breast cancer risk in FBD, it was deemed necessary to determine the expression profiles of genes that may be involved in neoplastic transformation. The functions of these genes and their roles in the pathogenesis of breast diseases will be discussed in more detail when describing the results obtained.
The objective of this study was to determine the transcriptional profiles of genes and to identify differential differences in gene expression depending on the form of the FBD.
Materials and methods
This study analyzed 128 formalin-fixed paraffin-embedded (FFPE) breast tissue samples obtained at the time of surgery in the Department of Breast Pathology of the V.I. Kulakov NMRC for OG&P. These included 21 samples of non-proliferative FBD, 40 samples of typical ductal hyperplasia (TDH), 20 samples of atypical ductal hyperplasia (ADH), 22 samples of ductal carcinoma in situ (DCIS), and 25 normal tissue samples (Table 1).
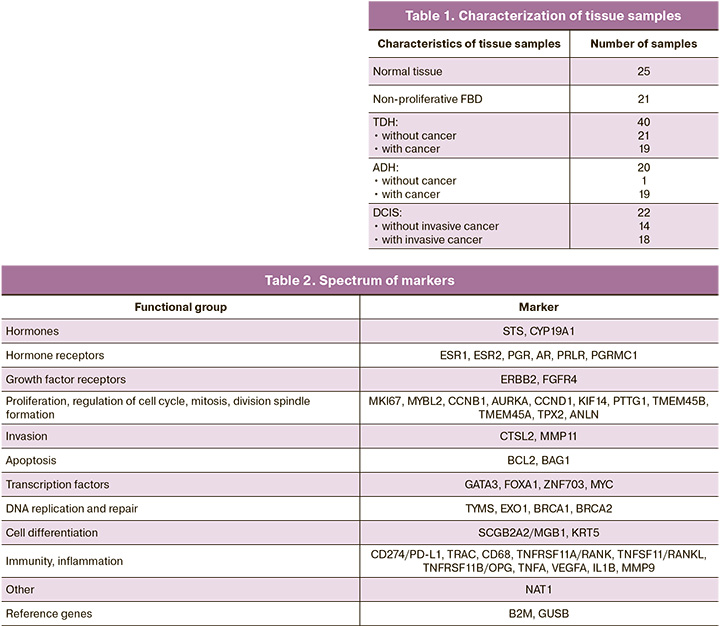
Samples of normal tissues from patients without cancer were obtained after reduction mammoplasty; no pathological changes in the breast tissue were detected according to the pathomorphological examination of the surgical material. Tissue samples with typical and atypical proliferation from patients without cancer were obtained after incisional or core biopsy in the FBD. Samples of normal tissue, TDH, ADH, and DCIS from patients with breast cancer were obtained from the surgical material at a distance from the tumor node.
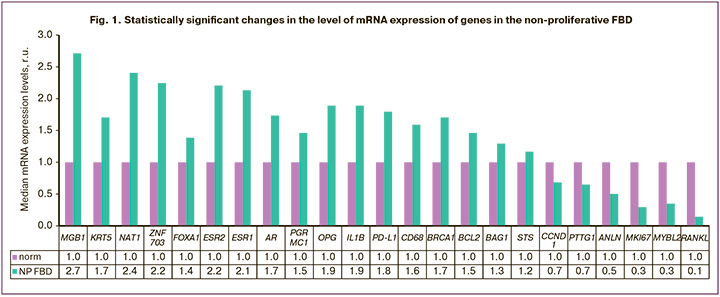
A wide range of genes, presented in Table 2, were studied using reverse transcription and polymerase chain reaction. The studies were conducted according to our previously published methodology [8].
In accordance with the traditional method for determining the level of gene expression [9], upon completion of amplification, the level of representation of transcripts was calculated by comparing indicator cycles (∆∆Ct method) with normalization relative to the normalization factor calculated from the reference genes B2M and GUSB, and relative to the median value (Me), the level of marker expression in a group of normal tissue samples as a reference level. With this normalization, the median value of the expression level in normal tissue corresponds to one, and the expression indices in the samples are calculated relative to this value.
Statistical analysis
Statistical analysis was performed using the IBM SPSS Statistics 17.0. Numerical variables were found not to be normally distributed and were reported as median (Me) and interquartile range (Q1; Q3); the groups were compared using the non-parametric Mann–Whitney U test. Differences were considered statistically significant at p<0.05.
Results and discussion
Based on the results of the mRNA analysis of the studied genes, two fundamentally different transcriptional profiles were identified compared to normal tissue: low (non-proliferative FBD) and high proliferative activity (DCIS, ADH, and TDH).
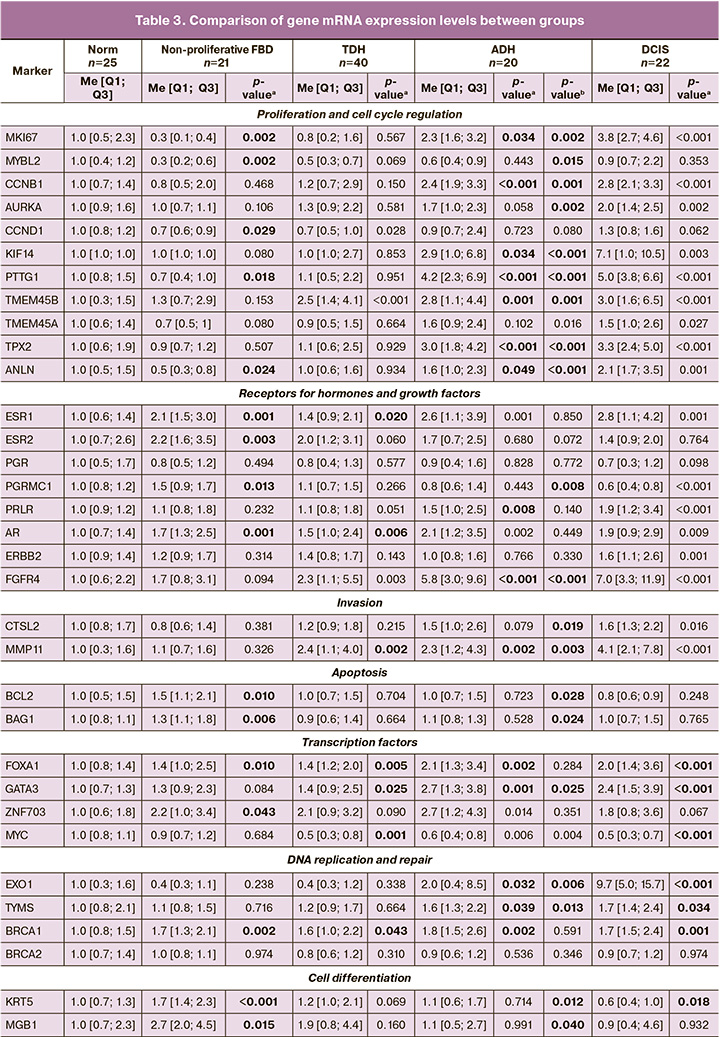
In the non-proliferative FBD, the following statistically significant changes in gene expression were revealed: increased expression of STS sulfatase mRNA, hormone receptors ESR1, ESR2, AR, progesterone-associated membrane component PGRMC1, transcription factors ZNF703 and FOXA1, apoptosis genes BCL2 and BAG1, cell differentiation markers MGB1 and KRT5, as well as the homologous recombination genes BRCA1, NAT1, PD-L1, OPG, and CD68; decreased expression of proliferation markers (MKI67, MYBL2, CCND1, PTTG1, ANLN), and RANKL (Fig. 1, Table 3). In addition, the activation of the pro-inflammatory response (increase in IL1B) was observed.
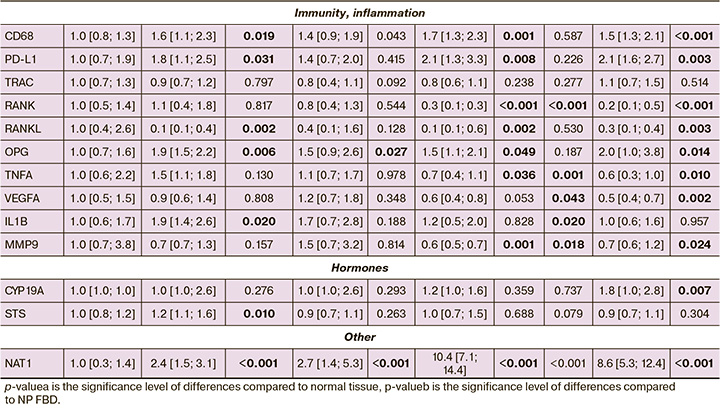
The expression of mRNA genes in the TDH and ADH groups showed trends similar to DCIS, which was more pronounced in atypia. In contrast to the non-proliferative form of the disease, changes in the transcriptional profile of genes in the ADH group were characterized by a statistically significant increase in the expression of proliferation markers (MKI67, KIF14, PTTG1, TPX2, TMEM45B, CCNB1, ANLN), DNA replication and repair (EXO1, BRCA1, TYMS), factors transcription of GATA3, FOXA1, fibroblast growth factor receptor 4 FGFR4, matrix metalloproteinase MMP11, hormone receptors (ESR1, AR, PRLR), as well as PD-L1, OPG, CD68 and NAT1; a statistically significant decrease in the expression of mRNA of the immunity and inflammation genes MMP9, TNFA, RANK, RANKL, transcription factor MYC, compared with normal tissue (Table 3, Fig. 2).
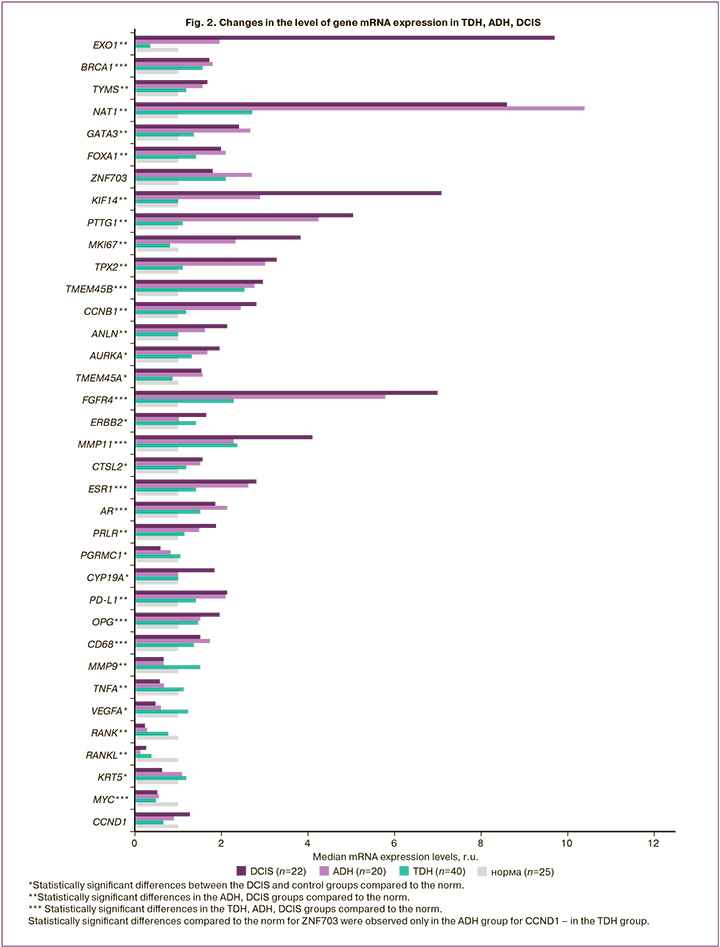
The mRNA expression of genes in the TDH group had similar trends to those in the ADH group, but statistically significant differences were obtained for a smaller number of genes. There was an increase in the mRNA expression levels of the DNA repair marker BRCA1, transcription factors GATA3, FOXA1, proliferation markers TMEM45B, CCND1, growth factor receptor FGFR4, matrix metalloproteinase MMP11, hormone receptors (ESR1, AR), as well as OPG, CD68; a statistically significant decrease in MYC mRNA expression compared to normal tissue (Table 3, Fig. 2).
High proliferative activity was accompanied by an increase in matrix metalloproteinase MMP11 by 2.4 (p=0.002), 2.3 (p=0.002), 4.1 (p<0.001) times in the TDH, ADH, and DCIS groups, respectively, as well as marker DNA replication and repair (EXO1 by 2.0 (p=0.032), 9.7 (p<0.001), and TYMS (1.6 (p=0.039), 1.7 (p=0.034) times in the ADH and DCIS groups, respectively. An increase in the expression of mRNA of the gene of homologous recombination BRCA1 was observed in all study groups:1.7 (p=0.002) times in the non-proliferative form of FBD, 1.6 (p=0.043), 1.8 (p=0.002), 1.7 (p=0.001) times in the TDH, ADH, DCIS groups, respectively.
DNA replication and repair factors ensure accurate DNA synthesis at checkpoints in the cell cycle by correcting errors in nucleotide sequences. Normally, they are "guardians of the genome" and prevent genomic instability. With high proliferative activity, the control of the accuracy of DNA replication is enhanced. At the same time, BRCA1 is a polyfunctional protein involved in the regulation of transcription, chromatin remodeling and ubiquitination, and controls the cell cycle. The increase in BRCA1 mRNA in non-proliferative FBD may be due to its involvement in processes other than homologous recombination. In this regard, of note is the study by Chiang H.C. et al., in which the mechanisms of BRCA1 influence on the expression of ESR1 and transcription of genes responsible for the differentiation of the luminal epithelium are considered and proved in detail [10], suggests that this function is supported by BRCA1 in non-proliferative forms of the disease.
Due to the increase in the ratio of the epithelial and connective tissue components in the non-proliferative FBD, we observed an increase in the expression levels of both cytokeratin KRT5 by 1.7 times (<0.001) and mammoglobin MGB1 by 2.7 times (p=0.015) as markers of differentiation of the luminal epithelium. In contrast, in in situ carcinoma, KRT5 decreases by 1.6 times (p=0.018) because of the loss of epithelial differentiation.
Considering the hormone-dependency of FBD, we studied the expression levels of hormones (STS – sulfatase, СYP19A1 – aromatase) and hormone receptors (ESR1 – α-estrogen, ESR2 – β-estrogen, PGR – progesterone, AR – androgen, PRLR – prolactin, and the progesterone-associated membrane component PGRMC1).
There was a statistically significant 1.2-fold increase in STS in the non-proliferative FBD group (p=0.010) and CYP19A1 in the DCIS group by 1.8 times (p=0.007). The sulfatase gene STS encodes a hydrolysis protein for 3-beta-hydroxysteroid sulfates, which are metabolic precursors of estrogens, androgens, and cholesterol. The aromatase gene CYP19A1 encodes cytochrome P450 protein, which catalyzes the last step of estrogen biosynthesis.
Estrogens play a key role in the pathogenesis of breast diseases and breast cancer by mediating their action through their receptors, transcription factors, and other factors that control the activity of genes, including those involved in cell growth or differentiation. In the non-proliferative FBD group, there was a statistically significant increase in the expression of both α and β estrogen receptors by 2.1 times (p=0.001) and 2.2 times (p=0.003), respectively. In the TDH, ADH, and DCIS groups, only the α-estrogen receptor expression was 1.4 (p=0.020), 2.6 (p=0.001) and 2.8 (p=0.001), respectively. Such changes during proliferative processes (TDH, ADH, and DCIS groups) lead to an increase in the ESR1/ESR2 ratio, which can be considered a risk factor for neoplastic transformation. Rodionova M.V. et al. in their review noted that the effect of estrogens depends on the ratio of different types of receptors in the cell; at the same time, β-estrogen receptors can suppress the function of α receptors [11].
As has been shown in human breast cancer cells co-expressing both types of estrogen receptors, a proliferative response to estrogens is observed only at a low level of β-estrogen receptor expression [12].
Previously, we noted strong and moderate positive correlations between ESR1 expression and the transcription factors FOXA1, GATA3, and ZNF703 in breast cancer [8]. These transcription factors are involved in the cellular response to estradiol stimulation and in the proliferation, growth, and differentiation of breast cells. FOXA1, GATA-binding protein 3 (GATA3), and ESR1 are believed to be responsible for maintaining the luminal tumor phenotype, and model experiments have demonstrated a synergistic effect of ESR1 and FOXA1 on target genes [13].
A statistically significant increase in the level of FOXA1 expression compared to normal tissue was observed in all groups: 1.4 times (p=0.010) in the non-proliferative FBD, 1.4 times (p=0.005), 2.1 (p=0.032), and 2 (p<0.001) times for TDH, ADH and DCIS, respectively; the non-proliferative form and ADH DCIS were characterized by an increase in the expression level of GATA3 mRNA by 1.4 (p=0.025); and 2.7 (p=0.001) times, respectively; and the non-proliferative form and ADH by an increase in ZNF703 by 2.2 (p=0.043) and 2.7 (p=0.014) times, respectively. ZNF703 is a well-known marker of the luminal B subtype of breast cancer, and its overexpression in tumors is associated with gene amplification [14].
However, our understanding of this marker is changing. Immunohistochemistry showed that 34.2% of triple-negative tumors expressed this marker, and knockdown of this gene led to cell cycle arrest in the G1 phase [15].
This study is the first to identify ZNF703 as a potentially important FBD marker.
In addition to ESR1, proliferation can also be activated by other hormonal receptors. In all groups, we observed a statistically significant increase in the expression of androgen receptor AR mRNA by 1.7 (p=0.001), 1.5 (p=0.006), 2.1 (p=0.002), 1.9 (p=0.009) times, respectively, for the non-proliferative FBD, TDH, ADH, and DCIS groups. Increased prolactin receptor mRNA expression by 1.5 (p=0.008) and 1.9 (p<0.001) times was noted only in the ADH and DCIS groups, respectively). It is believed that prolactin (PRL) promotes both differentiation of the mammary glands and oncogenesis [16].
The mRNA expression of the progesterone-associated membrane component, PGRMP1, was significantly higher in the non-proliferative form by 1.5 times (p=0.013) and decreased by 1.7 times (p<0.001) in the DCIS group. PGRMC1 mediates the effects of steroid hormones, including estrogen and progesterone, and plays an important role in the ovaries and uterus in maintaining female fertility and pregnancy. However, its function in mammary glands has not been fully elucidated. Experimental data obtained from mice indicated that PGRMC1 is required for mammary gland development during puberty and pregnancy, regardless of progesterone receptor expression [17].
It is known that PGRMC1 significantly increases estrogen-dependent breast cell proliferation, and in patients with breast cancer, its high expression is associated with a worse prognosis [18].
There are no data in the literature on PGRMC1 expression in FBD. Our data showing increased expression in non-proliferative FBD and decreased expression in DCIS are in contrast to the known function of PGRMC1 in estrogen-dependent proliferation. We do not fully appreciate the role and involvement of PGRMC1 in various biological processes. It is known that PGRMC1 is involved in the modulation of fatty acid synthesis, mitochondrial function, regulation of glycolysis/gluconeogenesis, regulation of spindle-mediated meiotic/mitotic chromosome division, induction of cell and tissue maturation, epigenetic regulation of CpG methylation, and genome packaging [19]. In non-proliferative FBD, the increased expression of PGRMC1 may be associated with its participation in the maturation of the luminal epithelium.
They enhance proliferative activity and interactions between growth factors and their receptors. In proliferative forms of FBD, an increase in the expression of mRNA of the fibroblast growth factor receptor FGFR4 was revealed: 2.3 times (p=0.003) with TDH, 5.8 times (p<0.001) with ADH, 7 times (p<0.001) with DCIS. FGFR4 is a tyrosine kinase. It is believed that its aberrant activation due to genetic changes is involved in the development and progression of several types of cancers, including breast cancer [20].
We also considered RANKL/RANK/OPG signaling as a potential system for controlling epithelial proliferation. These compounds play a key role in the regulation of osteoclast differentiation and calcium metabolism. The RANKL/RANK/OPG system consists of three main signaling molecules: the RANK activator that binds to RANKL (nuclear factor (NF) receptor activator – kB ligand) and osteoprotegerin (OPG). RANK activates osteoclasts by interacting with RANKL. OPG is a soluble receptor that acts as a negative regulator of this system, competing with RANK for binding to its ligand (RANKL). Osteoprotegerin inhibits osteoclast activation and stimulates osteoclast apoptosis in vitro. The RANK cascade is an important regulator of the interaction between dendritic and T cells during the T cell immune response. This system plays a special role in the physiology of the mammary glands, particularly in the hormone-dependent growth of the epithelium during pregnancy. Evidence suggests that progesterone induces RANKL expression in the breast]. RANKL controls the proliferation of mammary epithelial cells under physiological conditions associated with higher serum progesterone levels, such as the luteal phase of the menstrual cycle and pregnancy [21].
The RANKL/RANK system is considered to be a possible downstream mediator of the progesterone effect aimed at the proliferation of mammary gland epithelial cells, potentially contributing to the initiation and progression of pathological processes in the mammary gland.
This pathway of epithelial proliferation activation was probably not involved. Contrary to expectations, a statistically significant decrease in RANK mRNA by 3.6 (p<0.001), 3.3 (p<0.001) times in the ADH and DCIS groups, respectively) and RANKL mRNA by 2.6 (p=0.002), in 7.8 (p=0.002), 3.8 (p=0.003) times in the non-proliferative FBD, ADH, DCIS groups, respectively. Statistically significant increases in the levels of OPG osteoprotegerin mRNA expression by 1.9 (p=0.006), 1.5 (p=0.027), 1.5 (p=0.049), and 2 (p=0.014) times in the non-proliferative form groups, TDH, ADH, and DCIS, respectively, indicated the involvement of this system in apoptosis. In the non-proliferative FBD group, the levels of other markers of apoptosis, BCL2 and BAG1, were also increased by 1.5 (p=0.010) and 1.3 (p=0.010) times, respectively.
One of the most striking changes in expression levels was observed for the arylamine N-acetyltransferase NAT1 gene in all study groups compared to that in normal tissue. NAT mRNA expression was 2.4 (p<0.001), 2.7 (p<0.001), 10.4 (p<0.001), 8.6 (p<0.001) times higher in the groups of the non-proliferative FBD, TDH, ADH, DCIS, respectively. NAT1 is a drug-metabolizing enzyme. It would be possible to assume the influence of the drugs used during the operation; however, all tissues were exposed to this effect, including the control, and the changes were not identical. It is believed that NAT1 affects the proliferation and survival of tumor cells and promotes invasion both in vitro and in vivo; however, the exact mechanisms underlying this effect have not been established [22].
Interestingly, in a study of 1904 breast tumor samples, Li P. et al. found a negative correlation between NAT1 and MMP9 expression [23].
In our study, ADH and DCIS were associated with a 1.5-fold decrease in MMP9 mRNA against the background of a significant increase in NAT1 mRNA expression, which is consistent with the data of Li P. et al. [23].
When planning the study, we predicted an increase in MMP9. Similar to other matrix metalloproteinases, MMP9 may play a significant role in tumor invasion, as it is responsible for the degradation of the extracellular matrix in the vicinity of proliferating tumor cells. MMP9 is found in over 97% of invasive ductal carcinomas, and its expression increases with increasing histological grade of malignancy [24].
In addition, MMP9 can recruit inflammatory cells that enhance tumor progression [25] and play a role in tumor angiogenesis [26].
Based on our results, MMP9 is probably not involved in pathological processes in FBD or in the early stages of carcinogenesis. The decrease in MMP9 in the ADH and DCIS groups, according to our data, was accompanied by a decrease in the expression levels of mRNA of the tumor necrosis factor TNFA by 1.5 (p=0.036), 1.7 (p=0.010) times, respectively, as well as the vascular endothelial growth factor VEGFA 2.1 times (p=0.002) with DCIS. Our findings are consistent with those of Vandooren J. [25] and Vu T.H. [26].
A pro-inflammatory background was observed only in the non-proliferative FBD group, which was associated with a 1.9-fold increase in IL1B mRNA expression (p=0.020). In all groups macrophage recruitment occurred, which was reflected in 1,6-fold increase in the level of CD68 mRNA expression (p=0.019), 1.4 (p=0.043), and 1.7 (p=0.001), and 1.5 (p<0.001) times increase in the non-proliferative form of the disease, TDH, ADH and DCIS groups, respectively. At the same time, there was no pronounced recruitment of T cells, and no statistically significant changes in the expression level of the α-chain of the T-cell receptor (TRAC) were detected. Moreover, in the non-proliferative FBD, ADH, and DCIS groups, there was a statistically significant increase in PD-L1 expression level of 1.8 (p=0.031), 2.1 (p=0.008), and 2.1 (p=0.003) times, respectively). PD-L1 is expressed mainly on antigen-presenting cells of the immune system and participates in the mechanisms of self-tolerance formation. Its expression has also been observed in tumor cells. PD-L1 blocks the excessive activation of effector T lymphocytes by interacting with the PD-1 receptor, a protein involved in programmed cell death. An increase in the expression of PD-L1 protein allows cells to avoid the body's immune response. In oncology, this contributes to the high aggressiveness of tumors and increases the risk of death. The interaction of PD-L1 with PD-1 leads to a decrease in the proliferation of PD-1-bearing cells, a decrease in the production of cytokines and cytolytic activity, and induces apoptosis of T-lymphocytes, which leads to functional inactivation or depletion of T cells, and consequently to suppression of the antitumor immune response [27, 28].
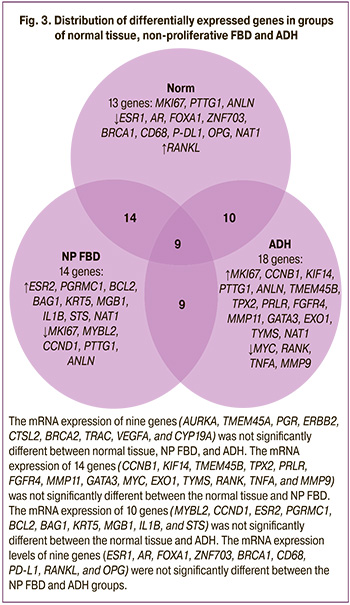
When comparing the ADH group and non-proliferative FBD, as forms with the maximum and minimum risk of developing breast cancer, a statistically significant increase in mRNA expression in ADH was noted for 17 genes and a decrease for 10 genes, compared with the non-proliferative FBD (Table 3).
Summing up, 18 genes were selected as markers that distinguish the proliferative form of the disease with atypia from non-proliferative forms of FBD and the norm, marking a high-risk group for neoplastic transformation: MKI67, CCNB1, KIF14, PTTG1, ANLN, TMEM45B, TPX2, PRLR, FGFR4, MMP11, GATA3, EXO1, TYMS, NAT1, MYC, RANK, TNFA, MMP9 (Fig. 3).
Conclusion
When studying the transcriptional profiles of genes in breast tissue samples with various forms of FBD, the most pronounced changes in gene expression were noted in atypical ductal hyperplasia (ADH). In proliferative FBD with atypia, which has the highest risk of developing breast cancer, 18 differentially expressed genes have been identified. The mRNA transcription profile in the TDH group showed trends similar to those in the ADH group, but less pronounced, with a change in the level of expression of a significantly smaller number of genes. The expression profile in tissue samples with the non-proliferative FBD was the opposite and was characterized by a high level of transcriptional activity, cell differentiation, hormonal reception, apoptosis, and a pro-inflammatory immune response against the background of a decrease in proliferative activity.
References
- Каприн А.Д., Рожкова Н.И., ред. Маммология. Национальное руководство. 2-е изд. М.: ГЭОТАР-Медиа; 2016. 496с. [Kaprin A.D., Rozhkova N.I., eds. Mammology. National guide. 2nd ed. Moscow: GEOTAR-Media; 2016. 496p. (in Russian)].
- Dyrstad S., Yan Y., Fowler A., Colditz G.A. Breast cancer risk associated with benign breast disease: systematic review and meta-analysis. Breast Cancer Res. Treat. 2015; 149(3): 569-75. https://dx.doi.org/10.1007/s10549-014-3254-6.
- Onstad M., Stuckey A. Benign breast disorders. Obstet. Gynecol. Clin. North Am. 2013; 40(3): 459-73. https://dx.doi.org/10.1016/j.ogc.2013.05.004.
- Рожкова Н.И., Меских Е.В., Бурдина Л.М., Сметник В.П., Бурдина И.И. Лекарственная патогенетическая коррекция доброкачественных заболеваний молочной железы. Опухоли женской репродуктивной системы. 2008; 2: 48-54. [Rozhkova N.I., Meskikh Y.V., Burdina L.M., Smetnik V.P., Burdina I.I. Medical pathogenetic correction of benign breast disorders. Tumors of Female Reproductive System. 2008; (2): 48-54. (in Russian)].https://dx.doi.org/10.17650/1994-4098-2008-0-2-48-54.
- Román M., Louro J., Posso M., Alcántara R., Peñalva L., Sala M. et al. Breast density, benign breast disease, and risk of breast cancer over time. Eur. Radiol. 2021; 31(7): 4839-47. https://dx.doi.org/10.1007/s00330-020-07490-5.
- Керчелаева С.Б., Сметник А.А., Беспалов В.Г. Мастопатия и профилактика рака молочной железы как междисциплинарная проблема. РМЖ. Мать и дитя. 2016; 24(15): 1018-25. [Kerchelaeva S.B., Smetnik A.A., Bespalov V.G. Mastopathy and breast cancer prevention as interdisciplinary problem. RMJ. 2016; 24(15): 1018-25. (in Russian)].
- Hartmann L.C., Sellers T.A., Frost M.H., Lingle W.L., Degnim A.C., Ghosh K. et al. Benign breast disease and the risk of breast cancer. N. Engl. J. Med. 2005; 353(3): 229-37. https://dx.doi.org/10.1056/NEJMoa044383.
- Бурменская О.В., Трофимов Д.Ю., Кометова В.В., Сергеев И.В., Маерле А.В., Родионов В.В., Сухих Г.Т. Разработка и опыт использования транскрипционной сигнатуры генов в диагностике молекулярных подтипов рака молочной железы. Акушерство и гинекология. 2020; 2: 132-40. [Burmenskaya O.V., Trofimov D.Yu., Kometova V.V., Sergeev I.V., Maerle A.V., Rodionov V.V., Sukhikh G.T. Development and experience of using the transcriptional gene signature in the diagnosis of molecular breast cancer subtypes. Obstetrics and Gynecology. 2020; (2): 132-40. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.2.132-140.
- Schmittgen T.D., Zakrajsek B.A., Mills A.G., Gorn V., Singer M.J., Reed M.W. Quantitative reverse transcription–polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal. Biochem. 2000; 285(2): 194-204. https://dx.doi.org/10.1006/abio.2000.4753.
- Chiang H.C., Zhang X., Li J., Zhao X., Chen J., Wang H.T. et al. BRCA1-associated R-loop affects transcription and differentiation in breast luminal epithelial cells. Nucleic Acids Res. 2019; 47(10): 5086-99.https://dx.doi.org/10.1093/nar/gkz262.
- Родионова M.В., Воротников И.К., Родионов В.В., Чхиквадзе Н.В., Дудко Е.А., Рябчиков Д.А., Ошкина Е.В., Богуш Т.А. Эстрогеновые рецепторы бета как маркеры эффективности гормональной терапии рака молочной железы. Российский биотерапевтический журнал. 2015; 14(2): 39-40. [Rodionova M.V., Vorotnikov I.K., Rodionov V.V., Chkhivladze N.V., Dudko E.A., Ryabchikov D.A., Oshkina E.V., Bogush T.A. Role of estrogen beta in the development and treatment of breast cancer. Russian Biotherapeutic Journal. 2015; 14(2): 39-40. (in Russian)].
- Sotoca A.M., van den Berg H., Vervoort J., van der Saag P., Ström A., Gustafsson J.-A. et al. Influence of cellular ERalpha/ERbeta ratio on the ERalpha-agonist induced proliferation of human T47D breast cancer cells. Toxicol Sci. 2008; 105(2): 303-11. https://dx.doi.org/10.1093/toxsci/kfn141.
- Chaudhary S., Krishna B.M., Mishra S.K. A novel FOXA1/ ESR1 interacting pathway: A study of ncomine breast cancer omicroarrays. Oncol. Lett. 2017; 14(2): 1247-64. https://dx.doi.org/10.3892/ol.2017.6329.
- Sircoulomb F., Nicolas N., Ferrari A., Finetti P., Bekhouche I., Rousselet E.et al. ZNF703 gene amplification at 8p12 specifies luminal B breast cancer. EMBO Mol. Med. 2011; 3(3): 153-66. https://dx.doi.org/10.1002/emmm.201100121.
- Zhang X., Mu X., Huang O., Wang Z., Chen J., Chen D., Wang G. ZNF703 promotes triple-negative breast cancer cells through cell-cycle signaling and associated with poor prognosis. BMC Cancer. 2022; 22(1): 226.https://dx.doi.org/10.1186/s12885-022-09286-w.
- Clevenger C.V., Rui H. Breast cancer and prolactin - new mechanisms and models. Endocrinology. 2022; 163(10): bqac122. https://dx.doi.org/10.1210/endocr/bqac122.
- Kim G., Lee J.G., Cheong S.A., Yon J.M., Lee M.S., Hong E.J., Baek I.J. Progesterone receptor membrane component 1 is required for mammary gland development. Biol. Reprod. 2020; 103(6): 1249-59. https://dx.doi.org/10.1093/biolre/ioaa164.
- Li X., Ruan X., Gu M., Mueck A.O. PGRMC1 can trigger estrogen-dependent proliferation of breast cancer cells: estradiol vs. equilin vs. ethinylestradiol. Climacteric. 2019; 22(5): 483-8. https://dx.doi.org/10.1080/13697137.2019.1582624.
- Cahill М.А., Neubauer H. PGRMC proteins are coming of age: a special issue on the role of PGRMC1 and PGRMC2 in metabolism and cancer biology. Cancers (Basel). 2021; 13(3): 512. https://dx.doi.org/10.3390/cancers13030512.
- Helsten T., Elkin S., Arthur E., Tomson B.N., Carter J., Kurzrock R. The FGFR landscape in cancer: analysis of 4,853 tumors by next-generation sequencing. Clin. Cancer Res. 2016; 22(1): 259-67. https://dx.doi.org/10.1158/1078-0432.CCR-14-3212.
- Infante M., Fabi A., Cognetti F., Gorini S., Caprio M., Fabbri A. RANKL/RANK/OPG system beyond bone remodeling: involvement in breast cancer and clinical perspectives. J. Exp. Clin. Cancer Res. 2019; 38(1): 12.https://dx.doi.org/10.1186/s13046-018-1001-2.
- Tiang J.M., Butcher N.J., Minchin R.F. Effects of human arylamine N-acetyltransferase I knockdown in triple-negative breast cancer cell lines. Cancer Med. 2015; 4(4): 565-74. https://dx.doi.org/10.1002/cam4.415.
- Li P., Butcher N.J., Minchin R.F. Arylamine N-acetyltransferase i regulates expression of matrix metalloproteinase 9 in breast cancer cells: role of hypoxia-inducible factor 1-α. Mol. Pharmacol. 2019; 96(5): 573-9.https://dx.doi.org/10.1124/mol.119.117432.
- Merdad A., Karim S., Schulten H.J., Dallol A., Buhmeida A., Al-Thubaity F. et al. Expression of matrix metalloproteinases (MMPs) in primary human breast cancer: MMP-9 as a potential biomarker for cancer invasion and metastasis. Anticancer Res. 2014; 34(3): 1355-66.
- Vandooren J., Van den Steen P.E., Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): the next decade. Crit. Rev. Biochem. Mol. Biol. 2013; 48(3): 222-72.https://dx.doi.org/10.3109/10409238.2013.770819.
- Vu T.H., Shipley J.M., Bergers G., Berger J.E., Helms J.A., Hanahan D. et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998; 93(3): 411-22.https://dx.doi.org/10.1016/s0092-8674(00)81169-1.
- Dong H., Strome S.E., Salomao D.R., Tamura H., Hirano F., Flies D.B. et al. Tumorassociated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 2002; 8(8): 793-800. https://dx.doi.org/10.1038/nm730.
- Боголюбова А.В., Ефимов Г.А., Друцкая М.С., Недоспасов С.А. Иммунотерапия опухолей, основанная на блокировке иммунологических контрольных «точек» («чекпойнтов»). Медицинская иммунология. 2015;17(5): 395-406. [Bogolyubova A.V., Efimov G.A., Drutskaya M.S., Nedospasov S.A. Cancer immunotherapy based on the blockade of immune checkpoints. Medical Immunology (Russia). 2015; 17(5): 395-406.(in Russian)].
Received 27.03.2023
Accepted 16.06.2023
About the Authors
Olga V. Burmenskaya, Dr. Bio. Sci., Head of the Laboratory of Oncological Genetics, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecologyand Perinatology, Ministry of Health of Russia, +7(495)438-22-92, o_bourmenskaya@oparina4.ru, https://orcid.org/0000-0003-2842-3980,
117997, Russia, Moscow, Ac. Oparina str., 4.
Dmitry Yu. Trofimov, Corresponding Member of the RAS, Professor, Dr. Med. Sci., Director of the Institute of Reproductive Genetics, Academician V.I. Kulakov National
Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-49-51, d_trofimov@oparina4.ru,
https://orcid.org/0000-0002-1569-8486, 117997, Russia, Moscow, Ac. Oparina str., 4.
Vlada V. Kometova, PhD, Head of Oncopathology Department, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology,
Ministry of Health of Russia, +7(905)183-77-83, vladakometova@gmail.com, https://orcid.org/0000-0001-9666-6875, 117997, Russia, Moscow, Ac. Oparina str., 4.
Valery V. Rodionov, Professor, Dr. Med. Sci., Head of Breast Cancer Department, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and
Perinatology, Ministry of Health of Russia, +7(926)629-34-00, dr.valery.rodionov@gmail.com, https://orcid.org/0000-0003-0096-7126,
117997, Russia, Moscow, Ac. Oparina str., 4.
Antonina A. Smetnik, PhD, Head of the Department of Gynecological Endocrinology, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology
and Perinatology, Ministry of Health of Russia, +7(905)540-09-11, a_smetnik@oparina4.ru, https://orcid.org/0000-0002-0627-3902,
117997, Russia, Moscow, Ac. Oparina str., 4.
Lev A. Ashrafyan, Dr. Med. Sci., Professor, Academician of RAS, Head of the Institute of Oncogynecology and Mammology, Deputy Director, Academician V.I. Kulakov
National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(926)740-27-45, L_Ashrafyan@Oparina4.ru,
https://orcid.org/0000-0001-6396-4948, 117997, Russia, Moscow, Ac. Oparina str., 4.
Gennady T. Sukhikh, Academician of RAS, Professor, Dr. Med. Sci., Director of the Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-18-00, g_sukhikh@oparina4.ru, https://orcid.org/0000-0002-7712-1260,
117997, Russia, Moscow, Ac. Oparina str., 4.



