Рак молочной железы (РМЖ) занимает первое место в структуре онкологических заболеваний у женщин. Ежегодно в мире регистрируется свыше 2 млн новых случаев, одновременно смертность в 2018 г. составила 626 679 случаев [1].
РМЖ представляет гетерогенную группу заболеваний, обусловленных различными молекулярными свойствами опухолей. Основу современной классификации злокачественных опухолей молочной железы положила работа Perou C. и соавт. 2000 г., в которой при исследовании относительно небольшого числа образцов (n=65) с использованием микроматричного анализа 8102 генов были выделены четыре молекулярных (транскрипционных) подтипа РМЖ: базальноподобный, HER2-обогащенный, люминальный и вариант с экспрессией «нормального» генного профиля [2]. В результате дальнейших исследований было установлено, что профили экспрессии мРНК (транскрипционные профили) генов человека адекватно отражают биологическую гетерогенность РМЖ и могут применяться для молекулярной классификации опухолей c целью прогноза рецидива заболевания и выбора адъювантной терапии. В итоге были разработаны диагностические тест-системы, такие как Oncotype DX [3], MammaPrint [4], PAM50/Prosigna [5] и другие.
В 2011 г. подтипы РМЖ, определяемые при анализе экспрессии генов, были включены в соглашение международной группы экспертов Санкт-Галленской конференции по РМЖ (2011 St Gallen International Expert Consensus), однако в силу высокой стоимости исследования была предложена суррогатная (заменяющая истинную транскрипционную) классификация методом иммуногистохимического анализа (ИГХ) на основе определения экспрессии 4 генов: ER, PR, HER2/neu и KI67 [6].
В настоящее время методом ИГХ выделяют пять молекулярных подтипов РМЖ: люминальный А (ЛюмА), люминальный В HER2/neu – позитивный (люмВ Her2+), люминальный В HER2/neu – негативный (люмВ Her2-), HER2/neu – обогащенный (Her2+) и трижды негативный (ТН), характеристика которых хорошо представлена в работе Кулигиной Е.Ш. [7].
Несмотря на широкое использование в рутинной практике суррогатного метода ИГХ для определения подтипов РМЖ, истинный молекулярный подтип определяют транскрипционные сигнатуры генов, тем самым обеспечивая более надежный прогноз риска рецидива заболевания и выбор адъювантной терапии по сравнению с клиническими и ИГХ-прогностическими факторами [8]. Дискордантные результаты между ИГХ и молекулярно-генетической классификациями составляют от 8 до 30%, а это значит, что трети пациентов назначается некорректное лечение [9].
В связи с этим цель настоящего исследования состояла в разработке и апробации на клиническом материале отечественной транскрипционной сигнатуры генов для диагностики молекулярных подтипов РМЖ.
Материалы и методы
Для реализации поставленной цели была разработана транскрипционная панель, включающая 48 генов ESR1, PGR, AR (группа рецепторов гормонов), ERBB2, GRB7, EGFR, FGFR4 (группа рецепторов ростовых факторов), MKI67, MYBL2, CCNB1, AURKA, BIRC5, MYC, CCND1, CCNE1, CDKN2A, KIF14, PPP2R2A, PTTG1, SFRP1, TMEM45B, TMEM45A, TPX2 (группа генов, регулирующих пролиферацию, митоз, формирование веретена деления и регуляцию клеточного цикла), MMP11, CTSL2, EMSY, PAK1, ANLN (группа генов, регулирующих миграцию клеток, инвазию, организацию цитоскелета), BCL2, BAG1, PTEN (гены апоптоза), TYMS, EXO1, UBE2T, TPT1 (группа генов, регулирующих репликацию и репарацию ДНК), SCGB2A2, KRT5, MIA (маркеры дифференцировки клеток и коэкспрессированные с ними гены), GATA3, FOXA1, ZNF703, NAT1, (факторы транскрипции), CD68, TRA, CD274/PD-L1 (гены иммунной системы) и референсные гены B2M, GUSB, HPRT1. Транскрипционный профиль генов определяли методом мультиплексной полимеразной цепной реакции с обратной транскрипцией (ОТ-ПЦР) в реальном времени»с использованием оригинальных праймеров и флуоресцентно-меченных проб (Fam, Cy5), специфичных к последовательностям, определяемым РНК. Использованы олигонуклеотиды, не амплифицирующие с матриц геномной ДНК, с размером ампликонов до 100–120 пар нуклеотидов, так как в парафинизированных образцах большая часть РНК имеет длину 100–300 оснований. Использованы реактивы и детектирующие амплификаторы ДТпрайм (ООО «НПФ ДНК-Технология», Россия).
В исследование включены 168 больных РМЖ в возрасте от 29 до 82 лет, получивших лечение в отделении патологии молочной железы ФГБУ «НМИЦ АГП им. В.И. Кулакова» в период с октября 2016 по июль 2018 г.
Критерием включения был инвазивный РМЖ. К критериям исключения относилась неоадъювантная терапия. В связи с тем что в образцах должно было присутствовать не менее 80–90% опухолевых клеток, с целью контроля риска интерференции нормальной тканью взятие материала осуществлялось врачом патоморфологом.
Проведены патоморфологическое и гистологическое исследования операционного материала; для молекулярно-генетического исследования подобраны образцы парафиновых блоков опухолей и нормальной ткани без опухолевого роста. Образцы отбирали в течение 30 минут после удаления опухоли и фиксировали в течение 48 ч в нейтральном забуференном формалине, после фиксации образцы помещали в автоматический микроволновой гистопроцессор LOGOS (Milestone, Италия) для ускоренной проводки тканей. Полученные срезы толщиной 4–5 мкн окрашивали гематоксилин-эозином. Микроскопические препараты исследовали методом обзорной микроскопии, которую осуществляли на световом микроскопе Olympus BX46 (Olympus Corp., Япония). ИГХ-диагностику проводили на иммуногистостейнере BenchMark ULTRA (Ventana, Roche) на фиксированных формалином парафиновых срезах толщиной 4 мкн с использованием антител к рецепторам эстрогенов (ER, клон SP1, VENTANA), прогестерона (PgR, клон 1E2, VENTANA), к Ki-67 (клон MIB–1, VENTANA), к онкопротеину гена эпидермального фактора роста 2 типа человека (c-erbB2).
Для проведения молекулярно-генетического исследования парафиновые срезы толщиной 4-5 мкн в количестве 3 штук помещали в сухие пластиковые пробирки объемом 1,5 мл. Перед выделением тотальной РНК проводили предварительную изоляцию парафина и обработку образцов протеиназой К, далее использованы наборы реагентов для выделения РНК «Проба НК-плюс», предусматривающие спиртовое осаждение нуклеиновых кислот.
Полученные препараты РНК сразу использовали для постановки реакции обратной транскрипции со смесью специфичных для каждого гена олигонуклеотидов. Реакцию проводили при температуре 40 ºС в течение 30 минут, с последующей инактивацией обратной транскриптазы при 95 °С в течение 5 минут. Для увеличения объемов образцов после обратной транскрипции кДНК разводили в 10 раз в ТЕ-буфере.
Амплификацию осуществляли в режиме реального времени с в объеме 12 мкл по следующей программе: 1 цикл – 80 °С 30 с, 94 °С 5 минут; 5 циклов – 94 °С 30 с, 64 °С 15 с; 45 циклов – 94 °С 10 с, 64 °С 20 с; 10 °С – хранение 94 °С. «Горячий старт» обеспечивался использованием Taq-полимеразы, активность которой блокировалась антителами и восстанавливалась при прогреве 94 °С. Измерение уровня флуоресценции проводили на каждом цикле при температуре 64 °С по каналам Fam и Cy5. Реакцию ставили в двух повторах для каждой точки.
По завершении амплификации уровень представленности транскриптов рассчитывали методом сравнения индикаторных циклов (метод ∆Ct) с нормировкой относительно референсных генов B2M, GUSB, HPRT1. Данные показатели были использованы для построения модели классификации опухолей молочной железы.
В качестве меры центральной тенденции в группах исследования рассчитывали медиану (Me), учитывали также интерквартильный размах (25%–75%). Данные по группам исследования представлены в виде Me (25%–75%). При этом значение медианы в нормальной ткани принималось равным единице, относительно этого значения рассчитывали показатели экспрессии для различных типов опухолей (метод ∆∆Ct). Для оценки значимости межгрупповых различий применяли U-критерий Манна–Уитни.
Корреляционные связи устанавливали с использованием метода ранговой корреляции по Спирмену. Силу предполагаемой взаимосвязи между величинами определяли по значению коэффициента корреляции и уровню достоверности корреляции (р<0,05). При оценке силы связи коэффициентов корреляции использовали шкалу Чеддока и учитывали слабые (от 0,3 до 0,5), средние (от 0,5 до 0,7), высокие (от 0,7 до 0,9) и очень высокие (более 0,9) положительные корреляционные связи. При отрицательной корреляции значения силы связи между переменными соответственно меняли на противоположные.
Разработку алгоритмов молекулярно-генетического подтипа опухоли осуществляли с использованием дискриминантного анализа с помощью программ статистической обработки результатов Statistica 10 и SPSS 17.
Результаты и обсуждение
Обследованы 168 больных РМЖ в возрасте от 29 до 82 лет. При определении подтипа РМЖ методом ИГХ в 72 (43%) случаях был установлен люминальный А подтип, в 52 (31%) – люминальный В Her2-негативный, в 9 (5%) случаях – люминальный В Her2-позитивный, в 11 (7%) случаях – Her2-обогащенный, в 24 (14%) – ТН подтип РМЖ.
Возрастное распределение и морфологическая характеристика опухолей (TNM классификация) в зависимости от молекулярного подтипа представлены в табл. 1.
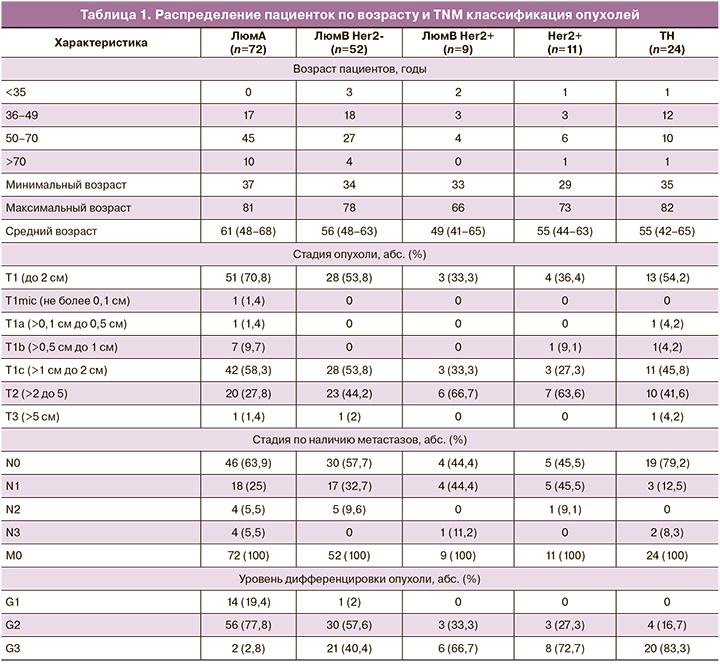
Размеры опухоли менее 2 см были зарегистрированы в 70,8% случаев при люминальном А подтипе, в 54% случаев – при люминальном В Her2-негативном и ТН подтипах, в 33,3 и 36,4% случаях в Her2-позитивных люминальных В и Her2-обогащенных опухолях соответственно. Размеры опухоли более 5 см отмечены в единичных случаях. Во всех случаях заболевания отсутствовали отдаленные метастазы. Метастазы в региональные лимфатические узлы не были диагностированы более чем в половине случаев в Her2-негативных опухолях (57,7–79,2%), включая ТН опухоли, и менее чем в половине случаев (44–45%) в Her2-позитивных опухолях.
Люминальный А подтип включал в основном образцы с низкой и умеренной степенью злокачественности (Grade 1, 2), люминальный В HER-2-негативный подтип – образцы с умеренной и высокой степенью злокачественности (Grade 2, 3). Большая часть образцов других подтипов были высокой степени злокачественности (Grade 3).
Уровни представленности транскриптов были использованы для построения модели классификации опухолей молочной железы, позволяющей выделить 6 молекулярно-генетических подтипов: люминальный А, люминальный В ERBB2-негативный, люминальный В ERBB2-позитивный, ERBB2-обогащенный, ТН подтипы РМЖ и соответствующий норме.
Для разработки алгоритмов молекулярно-генетического подтипа опухоли использовали дискриминантный анализ, позволивший для каждого образца вычислить значение пяти классифицирующих функций, определяющих транскрипционный профиль данного образца. Значения классифицирующих функций вычисляли по формуле 1:
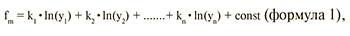
где f – значение классифицирующей функции для исследуемого образца;
y1 ... yn – определенные уровни экспрессии генов, n – порядковый номер гена, от 1... до 45; k1 ... kn – коэффициенты, значения которых подобраны статистически, исходя из экспериментальных данных; const – константы, значения которых подобраны статистически, исходя из экспериментальных данных; m – порядковый номер классифицирующей функции, от 1 до 5.
Далее полученный транскрипционный профиль образца, представляющий систему пяти координат, сравнивали со «средним транскрипционным профилем» каждого подтипа РМЖ: люминальный А, люминальный В ERBB2-негативный, люминальный В ERBB2-обогащенный, ERBB-обогащенный не люминальный, ТН и подобный норме (центроиды подтипов).
«Средний транскрипционный профиль» каждого подтипа определяли аналогично указанному выше алгоритму для образцов тканей молочной железы как среднее значение для каждой из пяти классифицирующих функций для каждого подтипа (центроид).
При сравнении транскрипционного профиля образца со «средними транскрипционными профилями» каждого подтипа РМЖ вычисляли расстояние от образца к каждому из центроидов подтипов по формуле 2:
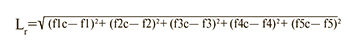
где f1c….f5c – значения функций центроида молекулярного подтипа РМЖ; f1….f5 – значения классифицирующих функций для исследуемого образца; r – подтип РМЖ.
Минимальное расстояние L к центроиду группы определяло принадлежность образца к определенному молекулярному подтипу опухоли.
При сравнении результатов, полученных методом ОТ-ПЦР, совпадения с ИГХ получены в ≈80% случаев при люминальных Her2-негативных подтипах опухолей, в 100% случаев при Her2-обогащенных подтипах опухолей, в 96% – при ТН подтипе опухоли и в 98% в нормальной ткани (табл. 2).
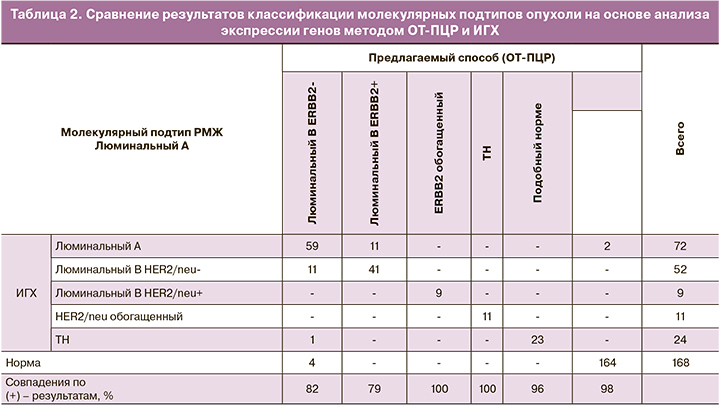
Уровень экспрессии мРНК четырех «ключевых» ИГХ-маркеров полностью соотносился с традиционными принципами классификации опухолей молочной железы на основе ИГХ (табл. 3).
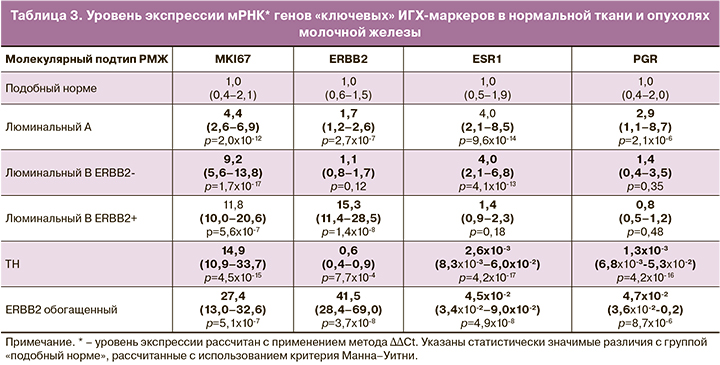
При определении корреляционных связей учитывались главным образом средние (от 0,5 до 0,7), высокие (от 0,7 до 0,9) и очень высокие (более 0,9) положительные связи. Отрицательные корреляционные связи были представлены в основном слабыми (от -0,3 до -0,5), реже – средними (от -0,5 до -0,7).
Исследование корреляционных связей позволило выявить коэкспрессированные с ключевыми ИГХ-маркерами гены («гены-дублеры» или подтверждения результата) и определить группы маркеров, экспрессирующихся сходным образом при различных подтипах опухолей:
- пролиферативная активность (MKI67), инвазия, репликация и репарация ДНК;
- дифференцировка клеток;
- гормональная рецепция (ESR1, PGR), апоптоз, факторы транскрипции;
- ERBB2 и коэкспрессированные гены.
С уровнем экспрессии MKI67 высоко коррелировали маркеры регуляции клеточного цикла (MYBL2 (rS=0,86; р=1,0×10-82), AURKA (rS=0,80; р=1,8×10-61), CCNB1 (rS=0,85, р=2,4×10-80), PTX2 (rS=0,88; р=2,3×10-91), BIRC5 (rS=0,83, р=1,1×10-69), CCNE1 (rS=0,75; р=2,5×10-49), формирования веретена деления, цитокинеза клеток (KIF14 (rS=0,89; р=8,3×10-83), PTTG1 (rS=0,86; р=4,2×10-84), ANLN (rS=0,90; р=3,7×10-103), репликации, репарации ДНК (EXO1 (rS=0,88; р=7,2×10-88), TYMS (rS=0,69; р=2,5×10-40), убиквитинирования белков UBE2T (rS=0,82; р=3,4×10-68). Установлены средние и низкие положительные связи MKI67 с маркерами инвазии MMP11 (rS=0,51; р=2,5×10-19), CTSL2 (rS=0,40; р=3,8×10-12) и отрицательные корреляционные связи с маркерами дифференцировки клеток (EGFR (r=-0,45; р=3,7×10-15), SFRP1(r=-0,42; р=2,5×10-13), KRT5 (r=-0,38; р=6,9×10-11), SCGB2A2 (r=-0,30; р=3,6×10-7) (табл. 4).
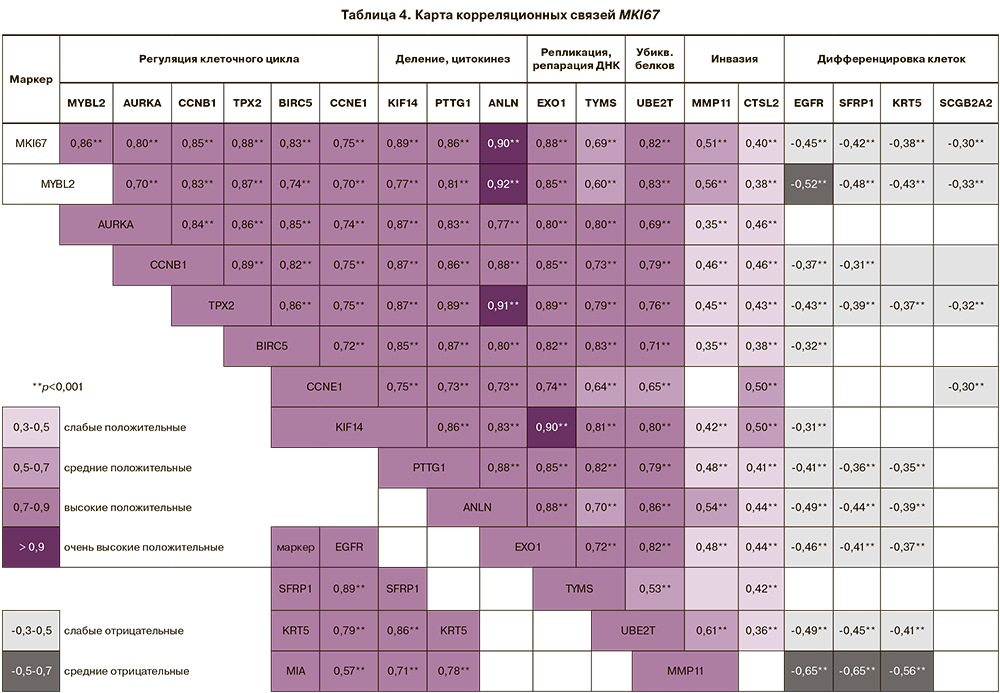
Высокие положительные корреляционные связи установлены между четырьмя маркерами дифференцировки клеток EGFR, SFRP1, KRT5 и MIA (р<0,001), экспрессия которых понижена в опухолях в десятки раз (см. табл. 4). Необходимо отметить, что данная группа маркеров не входит в ИГХ-исследование.
Высокие, средние и слабые положительные корреляционные связи установлены в экспрессии рецепторов гормонов: ESR1 c PGR (rS=0,63; р=2,8×10-34), AR (rS=0,45; р=1,7×10-17), с транскрипционными факторами (FOXA (rS=0,71; р=2,5×10-47), NAT1 (rS=0,73; р=2,5×10-51), GATA3 (rS=0,65; р=3,7×10-37), ZNF703 (rS=0,55; р=1,6×10-25), с маркерами апоптоза (BCL2 (rS=0,61; р=2,2×10-32) и BAG1 (rS=0,53; р=6,2×10-23), с циклином D ССND1 (rS=0,54; р=1,6×10-24), являющимся маркером пролиферации, а также с ERBB2 (rS=0,41; р=8,0×10-14) (табл. 5). Положительные корреляционные связи гормональных рецепторов, вероятно, обосновывают функциональное повышение экспрессии ERBB2 при люминальном А подтипе опухоли, не связанное с амплификацией гена.
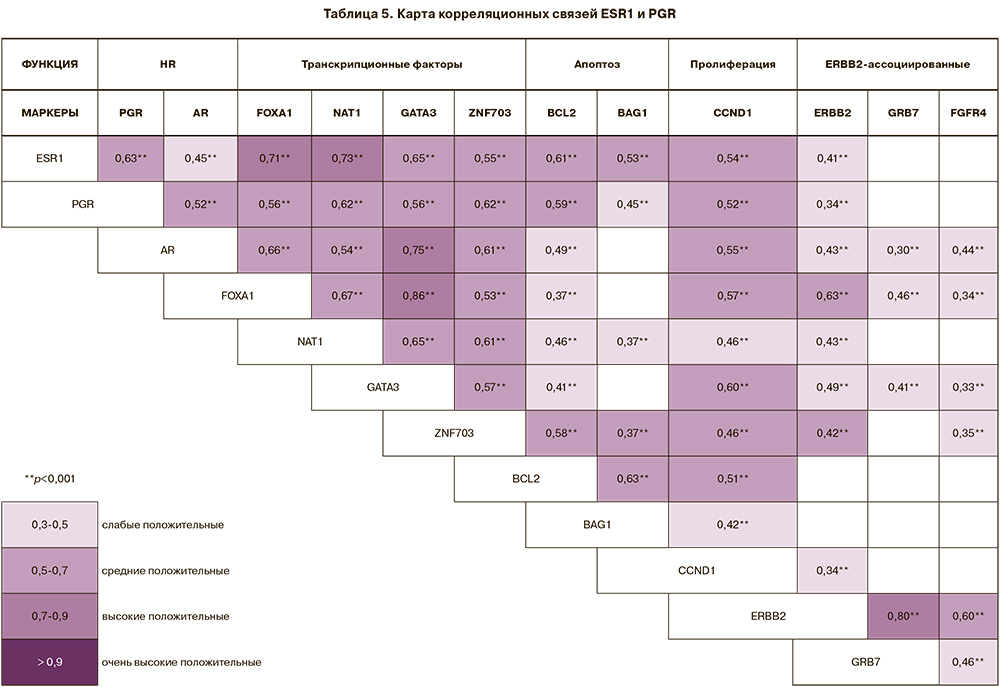
Гиперэкспрессия мРНК гена ERBB2 является результатом амплификации локуса 12 длинного плеча 17 хромосомы (17q12) и сопровождается амплификацией близко расположенного гена GRB7, использование которого также предусмотрено в предполагаемом алгоритме. С уровнем экспрессии мРНК гена ERBB2, помимо GRB7 (rS=0,80; р=7,4×10-69), коррелирует экспрессия гена FGFR4 (rS=0,60; р=3,6×10-28). FGFR4 локализован на 5 хромосоме (5q35.2), представляет тирозинкиназу и рецептор клеточной поверхности для факторов роста фибробластов.
Таким образом, использование «дублеров» может повышать точность интегральной оценки молекулярного подтипа опухоли.
Наиболее сложным в плане ИГХ-диагностики является разделение люминальных подтипов опухоли. При этом Ki-67, определяемый методом ИГХ, является ключевым и единственным маркером дифференциальной диагностики люминальных А и В молекулярных подтипов РМЖ. На 15 международной Санкт-Галленовской конференции группа экспертов вновь подняла вопрос об осторожности использования Ki-67, определяемого ИГХ-методом для принятия решения о назначении адъювантной химиотерапии из-за низкой межлабораторной воспроизводимости результатов данного исследования и необходимости стандартизации метода [10–12].
Заключение
Полученные результаты свидетельствуют о хороших перспективах внедрения способа определения молекулярно-генетических подтипов РМЖ на основе метода ОТ-ПЦР в клиническую практику, а использование в предлагаемом методе целого комплекса установленных нами коэкспрессированных или коррелированных маркеров повышает надежность диагностики молекулярных подтипов РМЖ.



