Metabolomic profile of follicular fluid and embryo culture media in patients with extragenital endometriosis
Objective: To identify specific markers in follicular fluid (FF) and embryo culture media to assess embryo quality and implantation potential in patients with extragenital endometriosis (EGE).Ibragimova L.K., Smol'nikova V.Yu., El'darov Ch.M., Bobrov M.Yu., Agadzhanyan D.S., Romanov E.A., Kalinina E.A.
Materials and methods: We performed comparative genomic hybridization to analyze FF and embryo culture media samples from patients with EGE and control subjects undergoing the IVF (ICSI) with preimplantation genetic testing. Spent culture medium metabolites from individually cultured embryos were detected using the HPLC-MS in the positive ion detection mode. After identifying chromatographic peaks and aligning chromatograms, partial least squares discriminant analysis was performed to better visualize the metabolite profiles of the compared samples.
Results: Metabolite profiling revealed differences in FF and culture medium compo-sitions of patients with EGE and control subjects. Comparative analysis of metabolites showed changes in lipid metabolism and decreased antioxidant levels in patients with EGE. These findings suggest an essential role of oxidative stress and lipid metabolism regulation, especially fatty acids performing structural, nutritional, and signaling func-tions in EGE pathogenesis.
Conclusion: Further analysis of the study findings and the search for markers at-tributable to embryonic development features, characteristic of EGE, remain relevant.
Keywords
Recent estimates suggest that the prevalence of infertility among couples of reproductive age ranges from 10 to15% [1]. Multiple unsuccessful attempts to conceive are associated with subsequent psychological distress, which has significant adverse impacts on the mental and personal characteristics of this patient category [2]. Due to the high prevalence of infertility, the World Health Organization has recognized it as a global health and social issue affecting families and communities in a wide variety of ways [3]. Assisted reproductive technologies (ART) are often the only way to have one's healthy child. Although ARTs have advanced dramatically, the effectiveness of in vitro fertilization (IVF) on average does not exceed 40%, and the live births rate is 33.3% per one selective embryo transfer [4].
Infertility is a multifactorial pathology, which requires a personalized approach to the treatment of each married couple [5]. One of the most interesting and still not fully understood factors contributing to infertility is extragenital endometriosis (EGE). The concern over this problem is explained by the high incidence of the disease, the co-occurrence of multiple reproductive disorders, and late and often ineffective use of ART [6, 7].
EGE is a genetically determined pathological process characterized by the heterotopic presence of endometrium, including endometrial epithelium and endometrial stroma outside the uterus [8, 9]. It is important to note that not all women with endometriosis are infertile. However, the prevalence of EGE is much higher among women unable to conceive (~ 30–50%) than among the fertile population (10–15%) [10]. The adverse effect on fertility is associated with a local inflammatory reaction with subsequent damage to lipids, proteins, and nucleic acids [11]. This toxic environment affects both the quality of gametes and the fertilization process, reducing the likelihood of natural conception and harming the quality of embryos and their capacity to implant and develop [12].
It is becoming evident that new noninvasive alternatives to embryo investigation are needed to increase implantation and pregnancy rates in cycles of selective embryo transfer in ART programs.
The human embryo culture medium at various stages of embryo development and follicular fluid (FF) obtained during transvaginal follicular puncture are unique objects for noninvasive investigation. Metabolic profiling of FF and culture media can provide information on energy metabolism, metabolic activity, and the state of the embryo's signaling systems. Analysis of culture media molecular composition can offer promising markers of successful implantation and facilitate the selection of the best embryo with the subsequent development of clinical pregnancy and the birth of a healthy child.
In modern metabolomics, liquid chromatography coupled with mass spectrometry (HPLC-MS) has emerged as a powerful analytical technique.
This study analyzed metabolite profiles in FF and embryo culture media of PGT-A confirmed euploid embryos of different morphological groups obtained from patients with EGE and control subjects. The study aimed to identify specific markers in follicular fluid and embryo culture media to assess embryo quality and implantation potential in patients with extragenital endometriosis.
Materials and methods
This cross-sectional study with parallel groups analyzed samples of FF and spent culture media used to culture human embryos of different morphological groups obtained from patients with and without EGE (control group). The study included 31 patients aged 23 to 37 undergoing the IVF (ICSI) using gonadotropins and PGT by comparative genomic hybridization.
Were selected FF (n=72) and embryo culture medium (n=29) samples of excellent and good categories, according to the Gardner embryo grading system [13, 14]. After obtaining PGT data for a comparative analysis of metabolic profile results, samples from euploid embryos of the EGE and the control groups were selected, including 27 FF samples (21 – EGE, 6 – control) and 19 culture media samples (13 – EGE, 6 – control). All patients signed informed consent for the use of biomaterial.
Investigation of follicular fluid composition
Before HPLC-MS, the metabolites were extracted by adding 19 volumes of extraction mixture of methanol, chloroform, and methyl tertiary butyl ether (in a ratio of 4:3:3 by volume) to 100 μl of FF. After that, they were stirred for 20 minutes and centrifuged at 13,000 g for 15 min. The supernatant was transferred into clean vials and dried in a nitrogen flow, after which the dry residue was dissolved in 45 μL of a mixture of acetonitrile and isopropanol (1:1).
For HPLC-MS analysis, 5 μL of an internal standard with a final concentration of 5 μM was added to 45 μL of the extract of each sample; the separation of samples was carried out on an ACQUITY UPLC BEH HILIC Column, 1.7 µm, 1.0 mm x 150 mm (Waters, USA) using the chromatographic UltiMate 3000 Nano LC system (Thermo Scientific, USA).
Elution of the sample components was performed by chromatography of hydrophobic interactions in an isocratic solution of 5% mobile phase "B" (5 μM of ammonium acetate water solution) and 95% phase A (100% acetonitrile) for 15 minutes, then in a gradient of 5–30 % mobile phase B for 10 minutes at a flow rate of 50 μl/min. Then it was washed for 5 minutes (95% of phase B), after which within 1 minute, the initial concentration of phase B was returned to 5%, and the column was equilibrated for 3 minutes. The total time of chromatographic analysis of one sample was 34 minutes. Metabolites were detected in two measurements per sample on a Bruker MaXis Impact hybrid quadrupole-time-of-flight mass spectrometer (Bruker Daltoniks, Germany). Mass spectra were collected at a resolution of 50,000 in the range of 100–3000 m/z, in the mode of positively charged ions.
Peaks were detected, grouped, and retention time corrected using the XCMS software package [15]. Peak detection was performed using the Centwave algorithm [16] with the following parameters: m/z spread 15ppm; the minimum and maximum peak widths 10 and 50 seconds, respectively. Peak grouping for all samples was performed using the Peak Density method [17] with default parameters. The HMDB database (www.hmdb.ca) was used to identify potential metabolites with the corresponding molecular weights.
Investigation of embryo culture media
The embryos were cultured in separated 30 μl drops of Irvine CSC culture medium. On day 5 of culture, a morphological assessment of the obtained embryos was conducted, followed by sampling in equal volumes of spent culture media, marked and frozen (-80°C).
Before HPLC-MS, the metabolites were extracted by adding three volumes of methanol to one volume of the incubation medium. After stirring, the precipitate underwent centrifugation at 14,000 g, and the supernatant was used for analysis.
For HPLC-MS analysis, 18 μL of the extract of each sample was taken, 2 μL of an internal standard with a final concentration of 5 μM was added, samples were separated on an Atlantis T3 column (150 mm length×1.0 mm inner diameter×3 μm particle size) (Waters, USA) using Ultimate 3000 Nano LC System (Thermo Scientific, USA).
Elution of the sample components was conducted by reversed-phase chromatography in isocratic mobile phase B solution of 5% (0.1% solution of formic acid in acetonitrile) and 95% phase A (0.1% solution of formic acid in water) for 15 minutes, then in a gradient of 5–95% mobile phase B for 10 minutes at 40 μl/min flow rate. Then it was washed for 5 minutes (95% of phase B), after which within 1 minute, the initial concentration of phase B was returned to 5%, and the column was equilibrated for 3 minutes. The total chromatography time for one sample was 34 minutes. Metabolites were detected on a Bruker MaXis Impact hybrid quadrupole-time-of-flight mass spectrometer (Bruker Daltoniks, Germany) in two measurements per sample. Mass spectra were collected at a resolution of 50,000 in the range of 50–1,500 m/z, in the mode of positively charged ions. Peak detection, grouping, and retention time correction were performed similarly to the FF analysis. The HMDB database was also used for the primary identification of metabolites.
Statistical analysis
Data of FF and embryo culture media mass spectrometry analysis was performed by the orthogonal partial least squares discriminant analysis (OPLS-DA) [18]. Specific metabolites' relative concentrations (mean integrated peak areas) were compared with Student’s t-test. Differences between the groups were considered statistically significant at p<0.05with correction for multiple comparisons. An additional selection criterion for potential biomarkers was the fold change in concentration with at least two-fold change between groups. For functional analysis of mass spectra, the mummichog algorithm was used [19]. Categorical variables of metabolic pathways were compared using Fisher’s exact test. Statistical analysis was performed on the Metaboanalyst v5.0 platform [20].
Results
Follicular fluid examination
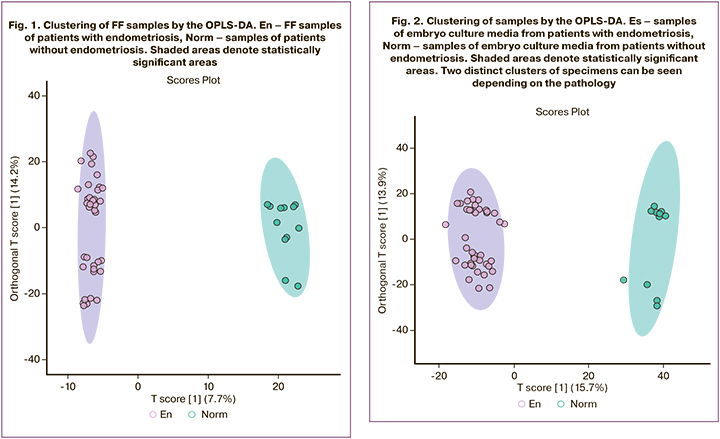
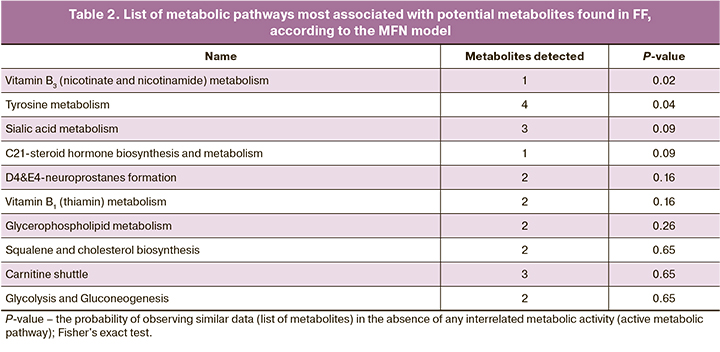
After the initial processing of the mass spectra, 3103 molecular ions were detected. OPLS-DA showed clustering of samples into distinct areas corresponding to the study groups. At the same time, the differences were smaller than in culture medium analysis (7.7% of changes were due to the first main component, versus 15.7% in culture media (Fig. 1). This result seems logical since FF is generally more homeostatic and has a limited physiological range of possible composition fluctuations. The search for FF components statistically significantly different in the comparison groups with the fold change ≥2 revealed 285 molecular ions. To identify these molecular ions, we searched the human metabolite chemical structure database (HMDB), which resulted in a list of potential biomarkers, in particular, many lipids of various classes (Table 1.)
To identify metabolic pathways associated with differences between groups, molecular ions contributing to group differences were analyzed using OPLS-DA. The identified metabolic pathways are shown in Table 2. The p-value corresponds to the probability of randomly obtaining such an intersection of a set of experimentally observed metabolites with metabolites of a particular metabolic pathway, according to Fisher's exact test. Among the identified pathways, the predominant pathways are those associated with the metabolism of vitamins, lipids (glycerophospholipids and docosahexaenoic acid derivatives – neuroprostanes), and glycans glycolipids (power acids). Of the amino acids, only the tyrosine metabolic pathway is represented.
Analysis of human embryo culture medium
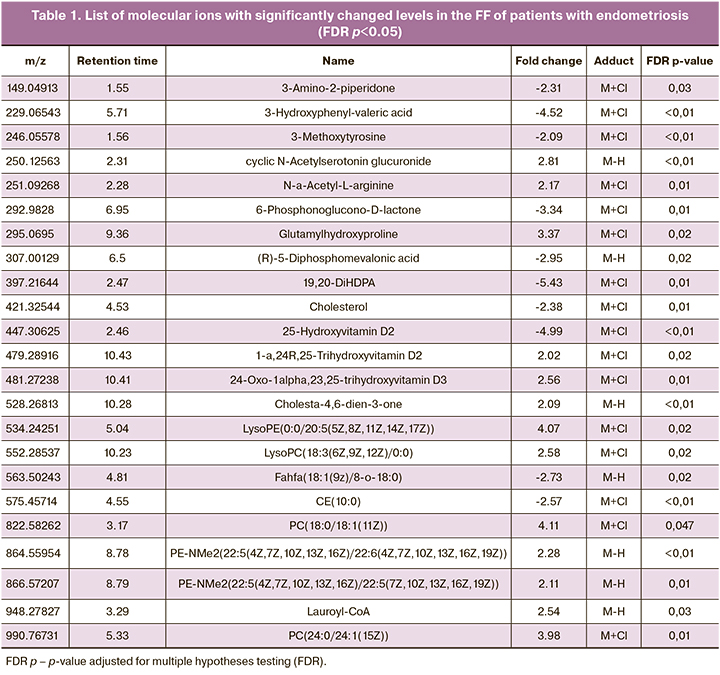
Initial processing of the mass spectra resulted in the detection of 6923 molecular ions. OPLS-DA findings showed good clustering of samples from patients with and without endometriosis (Fig. 2). This observation indicates marked differences between the study groups regarding culture medium metabolic profiles. To identify potential biomarkers responsible for the observed differences, we selected molecular ions with significant (p<0.05) differences in concentrations (integrated areas of the corresponding chromatographic peak) between the groups by an average of 2 or more times. A total of 1213 such molecular ions were selected. The Human Metabolite Database (HMDB) was searched for identification.
As a result, potential biomarkers of most significant interest were selected (Table 3). Noteworthy is the absence of different masses of amino acids in the lists, which indicates a similar level of their uptake from the environment by embryos of both groups. At the same time, the analysis identified many lipids and their derivatives, including fatty acids, phosphatidylethanolamines, phosphatidylserine, mono- and diglycerides, and many other biologically active molecules.
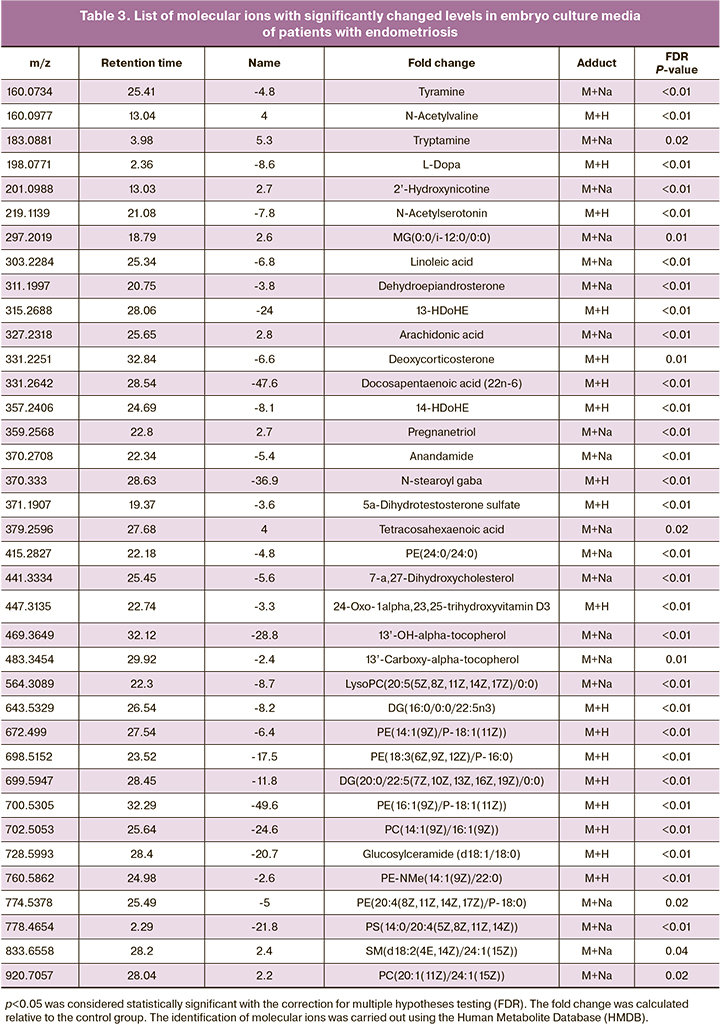
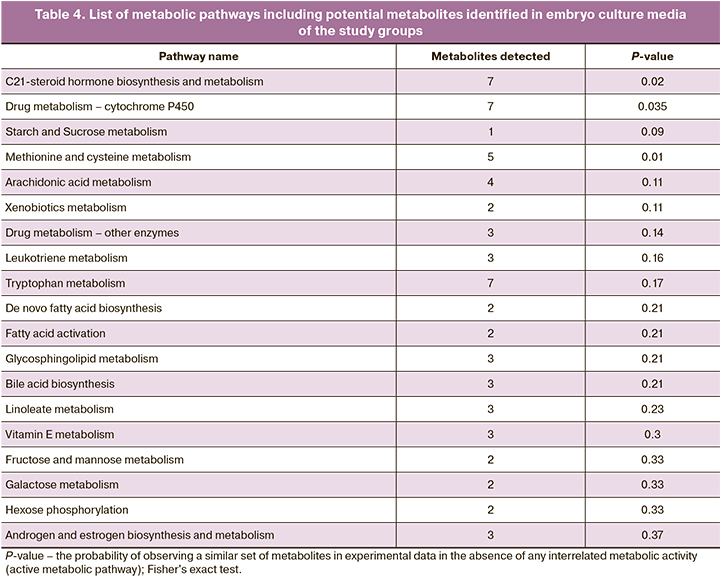
A functional analysis was performed using the Metaboanalyst database to identify metabolic pathways involving metabolites that account for differences between groups. For functional analysis, we selected molecular ions that most contributed to the differences between groups, according to the OPLS-DA. The identified metabolic pathways are shown in Table 4. P values were obtained using Fisher's exact test. In embryo culture media, more metabolites and, accordingly, metabolic pathways differing between groups were identified. Thus, the results included metabolic pathways of amino acids (methionine, cysteine, and tryptophan), sugars, and fatty acids. Metabolic pathways of drugs and xenobiotics have also been identified.
Discussion
Omics technologies are relatively new areas of research offering the opportunity to assess the functioning of cellular structures at various levels - from DNA and genes to metabolites. Among numerous omics technologies, metabolomics is often used to profile small endogenous molecules or metabolites present in biological samples [20, 21]. Molecular profiling identifies compounds participating in the pathways of biologically significant organic molecule transformation.
In recent years, this approach has been used to identify specific molecular markers that could clarify the pathogenesis of endometriosis and better understand the causes and mechanisms of infertility. However, changes in endogenous FF metabolites and embryo culture media composition in endometriosis remain poorly understood [22].
One of the most versatile profiling methods widely used in clinical laboratories over the past 10–15 years is HPLC-MS. It allows a highly accurate determination of their molecular weights by separating molecular components [23]. The specificity of the assay using HPLC-MS outperforms immunological methods and classical high-performance liquid chromatography, allowing the separation of complex multicomponent mixtures and detecting a wider range of different low molecular weight compounds. This method often requires minimal sample preparation, leading to high analytical efficiency.
Our study used FFs obtained during transvaginal follicular puncture and spent euploid embryo culture media of day 5 embryo of excellent and good categories in patients included in the study and undergoing the IVF/ICSI with PGT. We used samples only from euploid embryos since earlier Zorina I.M. et al. reported no significant differences between the metabolic profiles of euploid and aneuploid embryos, which allowed us to exclude embryo ploidy as a factor in embryonic metabolic disorders [24].
We found no significant differences in the profiles of FF and embryo culture medium metabolites between the study groups. Using human metabolites (HMDB) databases, we performed the primary identification of compounds that contribute to the differences between groups [25]. We also analyzed metabolic pathways in which potential metabolites may be involved.
It is known that EGE develops in the presence of relative and absolute hyperestrogenism and progesterone resistance. In women with endometriosis, type A progesterone receptors predominate, the shortened form of which acts as an inhibitor of type B progesterone receptors, which are responsible for developing the anti-inflammatory effect of progesterone [26].
Patients with EGE were found to have impaired steroidogenesis. An increased level of cholesterol in FF, which is a precursor of progesterone synthesis, and a decrease in the end metabolites of progesterone metabolism in culture media indicate a hormone deficiency.
One of the side effects of progesterone deficiency is dysregulation of the endocannabinoid system (ESC). ECS consists of endocannabinoids, including anandamide (AEA) and 2-arachidonoylglycerol, their receptors, and regulatory enzymes [27]. Endocannabinoids are endogenous lipids. A decrease in progesterone levels leads to a loss of type 1 cannabinoid receptors (CB1-R) expression and a reduction in endocannabinoid levels [28]. In support of this, we revealed decreased levels of anandamide in embryo culture media of patients with EGE. AEA plays an essential role in folliculogenesis, preovulatory follicle maturation, oocyte maturation, and ovulation [29]. El-Talatini M.R. et al., in their study during IVF/ICSI programs, found that successful implantation and progression of pregnancy were associated with increased AEA plasma levels during ovulation and decreased levels during implantation [30].
Changes in lipid and fatty acid metabolism in FF and culture media of patients with EGE were also revealed. Lipids and fatty acids play an essential role in the body's metabolism since they are structural components, substrates for energy metabolism, and precursors of various mediators. Several studies have shown that disorders in the metabolism of omega-3 and omega-6 polyunsaturated fatty acids (PUFAs) and their oxidized derivatives (oxylipins) may be one of the pathogenetic factors in the development of endometriosis [31]. Hopeman M.M. et al. reported that the plasma levels of eicosapentaenoic acid (omega-3), a substrate for oxylipin synthesis with low inflammatory activity, were reduced in the blood plasma of patients with EGE who underwent IVF/ICSI treatment. Decreased levels of eicosapentaenoic acid and other omega-3 PUFAs can increase the production of oxylipins from omega-6 PUFAs, which are highly inflammatory. Thus, it was shown that endometriosis is associated with increased production of the oxidized derivative of arachidonic acid (omega-6) prostaglandin E2, which is one of the critical factors in the development of inflammatory reactions [32, 33]. Culture media from patients with EGE had an increased level of arachidonic acid and decreased level of docosapentaenoic acid (omega-3) and oxidized derivatives of decosahexaenoic acid (omega-3), which may indicate an imbalance in lipid mediators production. In addition to participating in inflammatory reactions, various oxylipins provide autocrine and paracrine regulation of vital cell processes. The imbalance of oxylipin production by embryos can lead to disruption of intercellular communication processes and a decrease in implantation potential.
An important factor affecting the quality of gametes in couples with infertility is oxidative stress, which develops in the settings of insufficient activity of antioxidant defense systems [34]. Excessive production of reactive oxygen species can also exert a toxic effect on the embryo, leading to developmental disorders and decreasing the capacity to implant [35–37]. According to current concepts, oxidative stress is one of the critical factors in the pathogenesis of endometriosis. An imbalance in reactive oxygen species production stimulates the proliferation of endometriotic heterotopic cells and the formation of a pro-inflammatory environment around them, which may be one of the factors in reducing fertility in patients with endometriosis at the initial stages of the disease [38].
In their study, Halpern G. et al. discuss the importance of antioxidant deficiency on the progression of endometriosis [39]. The essential components of the antioxidant system include vitamin E, indole derivatives, and vitamin D, which are capable of exerting anti-inflammatory and immunomodulatory effects [40]. Vitamin D reduces the synthesis of pro-inflammatory oxylipins by suppressing the expression of cyclooxygenase-2 [41]. We observed changes in the metabolites of vitamins D and E in VF and embryo culture media in the EGE group.
Conclusion
Findings of our study demonstrated changes in the FF metabolome and embryo culture media of patients with EGE, suggesting that the change in the FF composition can impair gametogenesis and fertilization due to the formation of a pro-inflammatory microenvironment and weakening antioxidant defense. Differences in embryo culture media composition may be associated with the pro-inflammatory microenvironment of oocytes leading to epigenetic reprogramming of metabolic pathways, which also persists in the early stages of embryogenesis. Together, these factors can lead to decreased fertility in patients with endometriosis. It seems promising to continue research in this direction since understanding the causes of changes in metabolic processes may contribute to improving the embryological stage of ART in patients with endometriosis.
References
- Vander Borght M., Wyns C. Fertility and infertility: definition and epidemiology. Clin. Biochem. 2018; 62: 2-10. https://dx.doi.org/10.1016/j.clinbiochem.2018.03.012.
- Рябова М.Г. Индивидуально-психологические особенности женщин с различными типами нарушения репродуктивной функции. Вестник Тамбовского университета. Серия: Гуманитарные науки. 2013; 9: 190-8. [Ryabova M.G. Individual psychological characteristics of women with different types of reproductive disorders. Tambov University Reports. 2013; 9(125):190-8. (in Russian)].
- Всемирная организация здравоохранения. Информационные бюллетени. Бесплодие. 2020. Доступно по: https://www.who.int/ru/news-room/fact-sheets/detail/infertility [World Health Organization. Newsletters. Infertility. 2020. https://www.who.int/ru/news-room/fact-sheets/detail/infertility]
- Gleicher N., Kushnir V.A, Barad D.H. Worldwide decline of IVF birth rates and its probable causes. Hum. Reprod. Open. 2019; 2019(3): hoz017. https://dx.doi.org/10.1093/hropen/hoz017.
- Женское бесплодие (современные подходы к диагностике и лечению). Клинические рекомендации (Протокол лечения). № 15-4/И/2-1913. 2019. 118c. [Female infertility (modern approaches to diagnosis and treatment). Clinical guidelines (Treatment protocol). 2019; 11-12. (in Russian)].
- Polat M., Yaralı İ., Boynukalın K., Yaralı H. In vitro fertilization for endometriosis-associated infertility. Womens Health (Lond.). 2015; 11(5):633-41. https://dx.doi.org/10.2217/whe.15.50.
- Пономаренко И.В., Полоников А.В., Чурносов М.И. Молекулярные механизмы и факторы риска развития эндометриоза. Акушерство и гинекология. 2019; 3: 26-31. [Ponomarenko I.V., Polonikov A.V., Churnosov M.I. Molecular mechanisms and risk factors for the development of endometriosis. Obstetrics and Gynecology. 2019; 3: 26-31. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.3.26-31.
- Vercellini P., Viganò P., Somigliana E., Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014; 10(5): 261-75. https://dx.doi.org/10.1038/nrendo.2013.255.
- Адамян Л.В., Кулаков В.И., Андреева Е.Н. Эндометриозы. Руководство для врачей. 2-е изд. М.: Медицина; 2006. 410c. [Adamyan L.V., Kulakov V.I., Andreeva E.N. Endometriosis: A Guide for Physicians. M.: Medicine. 2006; 411p. (in Russian)].
- Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil. Steril. 2012; 98(3): 591-8. https://dx.doi.org/10.1016/j.fertnstert.2012.05.031.
- Адамян Л.В., Сонова М.М., Арсланян К.Н., Логинова О.Н., Харченко Э.И. Окислительный стресс и эндометриоз: обзор литературы. Лечащий врач. 2019; 12: 20-5. [Adamyan L.V., Sonova M.M., Arslanyan K.N., Loginova O.N., Kharchenko E.I. Oxidative stress and endometriosis: review of the literature. The Lechaschi Vrach Journal. 2019; 12: 20-5. (in Russian)].
- Li Y., Li R., Ouyang N., Dai K., Yuan P., Zheng L. et al. Investigating the impact of local inflammation on granulosa cells and follicular development in women with ovarian endometriosis. Fertil. Steril. 2019; 112(5): 882-91. https://dx.doi.org/10.1016/j.fertnstert.2019.07.007.
- Gardner D.K., Schoolcraft W.B. In Vitro Culture of Human Blastocyst. In: Jansen R. and Mortimer D., eds. Towards Reproductive Certainty: Infertility and Genetics Beyond. Parthenon Press, Carnforth. 1999: 377-88.
- Gardner D.K., Schoolcraft W.B. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999; 11(3): 307-11. https://dx.doi.org/10.1097/00001703-199906000-00013.
- Smith C.A., Want E.J., O’Maille G., Abagyan R., Siuzdak G. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006; 78(3): 779-87. https://dx.doi.org/10.1021/ac051437y.
- Tautenhahn R., Böttcher C., Neumann S. Highly sensitive feature detection for high resolution LC/MS. BMC Bioinformatics. 2008; 9: 504. https://dx.doi.org/10.1186/1471-2105-9-504.
- Bylesjo M., Rantalainen M., Cloarec O., Nicholson J.K., Holmes E., Trygg J. OPLS discriminant analysis: combining the strengths of PLS-DA and SIMCA classification. J. Chemometr. 2006; 20(8-10): 341-51. https://dx.doi.org/10.1002/CEM.1006.
- Li S., Park Y., Duraisingham S., Strobel F.H., Khan N., Soltow Q.A. et al. Predicting network activity from high throughput metabolomics. PLoS Comput. Biol. 2013; 9(7): e1003123. https://dx.doi.org/10.1371/journal.pcbi.1003123.
- Pang Z., Chong J., Zhou G., de Lima Morais D.A., Chang L., Barrette M. et al. MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021; 49(W1): W388-W396. https://dx.doi.org/10.1093/nar/gkab382.
- Podgorsky V.V. Метаболомика: анализ биохимического ответа живых систем вчера, сегодня, завтра. Глава 1. 2019. 17с. https://dx.doi.org/10.13140/RG.2.2.22959.07840. [Metabolomics: Analyzing the Biochemical Response of Living Systems Yesterday, Today, Tomorrow. 2019. (in Russian)]. https://dx.doi.org/10.13140/RG.2.2.22959.07840.
- Jordan K.W., Nordenstam J., Lauwers G.Y., Rothenberger D.A., Alavi K., Garwood M. et al. Metabolomic characterization of human rectal adenocarcinoma with intact tissue magnetic resonance spectroscopy. Dis. Colon Rectum. 2009; 52(3): 520-5. https://dx.doi.org/10.1007/DCR.0b013e31819c9a2c.
- Борисова А.В., Козаченко А.В., Стародубцева Н.Л., Бугрова А.Е., Франкевич В.Е., Адамян Л.В. Диагностика наружного генитального эндометриоза с помощью методов масс-спектрометрии (обзор литературы). Проблемы репродукции. 2015; 21(6): 67-76. [Borisova A.V., Kozachenko A.V., Starodubtseva N.L., Bugrova A.E., Frankevich V.E., Adamyan L.V. The diagnosis of endometriosis with the help of mass spectrometry (a review). Problems of Reproduction. 2015; 21(6): 67-76. (in Russian)]. https://doi.org/10.17116/repro201521659-68.
- Grebe S.K., Singh R.J. LC-MS/MS in the clinical laboratory – where to from here? Clin. Biochem. Rev. 2011; 32(1): 5-31.
- Зорина И.М., Эльдаров Ч.М., Ярыгина С.А., Макарова Н.П., Трофимов Д.Ю., Смольникова В.Ю., Калинина Е.А., Бобров М.Ю. Профилирование метаболитов в питательных средах пятидневных эмбрионов человека. Биомедицинская химия. 2017; 63(5): 385-91. [Zorina I.M., Eldarov C.M., Yarigina S.A., Makarova N.P., Trofimov D.Yu., Smolnikova V.Yu., Kalinina E.A., Bobrov M.Yu. Metabolomic profiling in culture media of day-5 human embryos. Biomedical chemistry. 2017; 63(5): 385-91 (in Russian)]. https://dx.doi.org/10.18097/PBMC20176305385.
- Wishart D.S., Feunang Y.D., Marcu A., Guo A.C., Liang K., Vázquez-Fresno R. et al. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 2018; 46(D1): D608-D617. https://dx.doi.org/10.1093/nar/gkx1089.
- Herington J.L., Bruner-Tran K.L., Lucas J.A., Osteen K.G. Immune interactions in endometriosis. Expert Rev. Clin. Immunol. 2011; 7(5): 611-26. https://dx.doi.org/10.1586/eci.11.53.
- Sugiura T., Waku K. Cannabinoid receptors and their endogenous ligands. J. Biochem. 2002; 132(1): 7-12. https://dx.doi.org/10.1093/oxfordjournals.jbchem.a003200.
- Resuehr D., Glore D.R., Taylor H.S., Bruner-Tran K.L., Osteen K.G. Progesterone-dependent regulation of endometrial cannabinoid receptor type 1 (CB1-R) expression is disrupted in women with endometriosis and in isolated stromal cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Fertil. Steril. 2012; 98(4): 948-56.e1. https://dx.doi.org/10.1016/j.fertnstert.2012.06.009.
- Bouaziz J., Bar On A., Seidman D.S., Soriano D. The clinical significance of endocannabinoids in endometriosis pain management. Cannabis Cannabinoid Res. 2017; 2(1): 72-80. https://dx.doi.org/10.1089/can.2016.0035.
- El-Talatini M.R., Taylor A.H., Konje J.C. Fluctuation in anandamide levels from ovulation to early pregnancy in in-vitro fertilization-embryo transfer women, and its hormonal regulation. Hum. Reprod. 2009; 24(8): 1989-98. https://dx.doi.org/10.1093/humrep/dep065.
- Khanaki K., Nouri M., Ardekani A.M., Ghassemzadeh A., Shahnazi V., Sadeghi M.R. et al. Evaluation of the relationship between endometriosis and omega-3 and omega-6 polyunsaturated fatty acids. Iran. Biomed. J. 2012; 16(1): 38-43. https://dx.doi.org/10.6091/ibj.1025.2012.
- Hopeman M.M., Riley J.K., Frolova A.I., Jiang H., Jungheim E.S. Serum polyunsaturated fatty acids and endometriosis. Reprod. Sci. 2015; 22(9): 1083-7. https://dx.doi.org/10.1177/1933719114565030.
- Pereira F.E.X.G., das Chagas Medeiros F., Rocha H.A.L., da Silva K.S. Effects of omega-6/3 and omega-9/6 nutraceuticals on pain and fertility in peritoneal endometriosis in rats. Acta Cir. Bras. 2019; 34(4): e201900405. https://dx.doi.org/10.1590/s0102-865020190040000005.
- Смольникова В.Ю., Агаджанян Д.С., Красный А.М. Активные формы кислорода и компоненты системы антиоксидантной защиты как маркеры прогнозирования качества эмбрионов у супружеских пар с различными типами бесплодия. Акушерство и гинекология. 2020; 11: 55-60. [Smolnikova V.Yu., Agadzhanyan D.S., Krasnyi A.M. Reactive oxygen species and components of antioxidant defense system as embryo quality prediction markers in couples with various types of infertility. Obstetrics and Gynecology. 2020; 11: 55-60. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.11.55-60.
- Banwell K., Lane M., Russel D., Kind K., Thompson J.G. Oxygen concentration during mouse oocyte in vitro maturation affects embryo and fetal development. Hum. Reprod. 2007; 22(10): 2768-75. https://dx.doi.org/10.1093/humrep/dem203.
- Lin X., Dai Y., Tong X., Xu W., Huang Q., Jin X. et al. Excessive oxidative stress in cumulus granulosa cells induced cell senescence contributes to endometriosis-associated infertility. Redox Biol. 2020; 30: 101431. https://dx.doi.org/10.1016/j.redox.2020.101431.
- Prieto L., Quesada J.F., Cambero O., Pacheco A., Pellicer A., Codoceo R. et al. Analysis of follicular fluid and serum markers of oxidative stress in women with infertility related to endometriosis. Fertil. Steril. 2012; 98(1): 126-30. https://dx.doi.org/10.1016/j.fertnstert.2012.03.052.
- Anastasiu C.V., Moga M.A., Neculau A.E., Bălan A., Scârneciu I., Dragomir R.M. et al. Biomarkers for the noninvasive diagnosis of endometriosis: state of the art and future perspectives. Int. J. Mol. Sci. 2020; 21(5): 1750. https://dx.doi.org/10.3390/ijms21051750.
- Halpern G., Schor E., Kopelman A. Nutritional aspects related to endometriosis. Rev. Assoc. Med. Bras. (1992). 2015; 61(6): 519-23. https://dx.doi.org/10.1590/1806-9282.61.06.519.
- Ярмолинская М.И., Денисова А.С., Толибова Г.Х., Беспалова О.Н., Траль Т.Г., Закураева К.А., Пьянкова В.О. Анализ экспрессии рецепторов витамина D у больных наружным генитальным эндометриозом. Акушерство и гинекология. 2021; 3: 117-23. [Yarmolinskaya M.I., Denisova A.S., Tolibova G.Kh., Bespalova O.N., Tral T.G., Zakuraeva K.A., Pyankova V.O. Analysis of the expression of vitamin D receptors in patients with external genital endometriosis. Obstetrics and Gynecology. 2021; 3: 117-23. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.3.117-123.
- Бахарева И.В. Витамин D и эндометриоз: в поиске новых возможностей. Российский вестник акушера-гинеколога. 2018; 18(4): 35-43. [Bakhareva I.V. Vitamin D and endometriosis: looking for new opportunities. Russian Bulletin of Obstetrician-Gynecologist. 2018; 18(4): 35-43. (in Russian)]. https://doi.org/10.17116/rosakush201818435.
Received 17.08.2021
Accepted 13.10.2021
About the Authors
Luiza K. Ibragimova, Post-Graduate Student at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P,Ministry of Health of Russia, +7(916)524-55-39, ibragimova_luisa0693@mail.ru, https://orcid.org/0000-0002-3090-5922, 117997, Russia, Moscow, Academica Oparina str., 4.
Veronika Yu. Smol’nikova, Dr. Med. Sci., Leading Researcher at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov
NMRC for OG&P, Ministry of Health of Russia, veronika.smolnikova@mail.ru, 117997, Russia, Moscow, Academica Oparina str., 4.
Chupalav M. El’darov, Ph.D. (Bio), Senior Researcher at the Laboratory of Molecular Pathophysiology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
117997, Russia, Moscow, Academica Oparina str., 4.
Mikhail Yu. Bobrov, Ph.D. (Chemistry), Head of the Laboratory of Molecular Pathophysiology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
117997, Russia, Moscow, Academica Oparina str., 4.; Institute of Bioorganic Chemistry, RAS, 117997, Russia, Moscow, Miklukho-Maclay str., 16/10, mbobr@mail.ru
Diana S. Agadzhanyan, Post-graduate Student at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, agadzhanyan@inbox.ru, 117997, Russia, Moscow, Academica Oparina str., 4.
Evgeny A. Romanov, Embryologist at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia, e_romanov@oparina4.ru, 117997, Russia, Moscow, Academica Oparina str., 4.
Elena A. Kalinina, Dr. Med. Sci., Professor, Head of the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-13-41, e_kalinina@oparina4.ru, 117997, Russia, Moscow, Academica Oparina str., 4.
Corresponding author: Luiza K. Ibragimova, ibragimova_luisa0693@mail.ru
Authors' contributions: Ibragimova L.K. – material collection and data analysis, sample preparation, manuscript drafting; Smol’nikova V.Yu., Agadzhanyan D.S., Kalinina E.A. – conception and design of the study, patient selection, manuscript editing; Romanova E.A. – embryo cultivation, material collection; El’darov Ch.M. – HPLC-MS analysis, bioinformatics data processing; Bobrov M.Yu. – conception of the study, data processing, manuscript drafting and editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Ibragimova L.K., Smol'nikova V.Yu., El'darov Ch.M., Bobrov M.Yu., Agadzhanyan D.S., Romanov E.A., Kalinina E.A. Metabolomic profile of follicular fluid and embryo culture media in patients with extragenital endometriosis.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 11: 114-124 (in Russian)
https://dx.doi.org/10.18565/aig.2021.11.114-124



