Clinical manifestations of mild and moderate novel coronavirus disease in pregnant women in epidemic dynamics
Objective: To investigate the clinical manifestations of novel coronavirus infection (COVID-19) in pregnant women in epidemic dynamics. Materials and methods: This is a single-center prospective comparative study of three independent groups. The study enrolled 823 pregnant and postpartum women with mild to moderate COVID-19. Patients in Group 1 (n=186), Group 2 (n=412), and Group 3 (n=225) were hospitalized during the first, second, and third waves of the epidemic, respectively. The groups were comparable in age and gestational age. Results: During the epidemic, women of higher parity were exposed to infection. During the first, second, and third waves of the epidemic, 56.45%, 70.29%, and 78.22% of the multiparous women were infected, which could be explained by the possibility of the spread of infection among family members from children. Distinctive features of COVID-19 clinical manifestations included the predominance of pneumonia with scanty clinical symptoms in the second wave increase of exudative symptoms of the respiratory and gastrointestinal tract. The incidence of mild to severe disease progression was increasing: 1/186 (0.5%), 9/412 (2.2%), and 18/225 (8%) pregnant women during the first, second, and third waves, respectively. There was an increased risk of hospitalization for pregnant women with the moderate COVID-19 during the second and third waves (OR=3.9 (95% CI 1.7;8.8); p<0.05). There was an acceleration of the progression of the disease in female patients with each new phase of the epidemic: on the average, by day 9, 6, and 4 days during the first, second, and third waves, respectively, which may indicate an increase in the virus pathogenicity in the epidemic dynamics. Conclusion: In pregnant women, COVID-19 tends to increase the rate of progression and severity of the disease in the dynamics of the epidemic process.Malgina G.B., Dyakova M.M., Bychkova S.V., Pepelyaeva N.A., Olkov S.S., Melkozerova O.A., Bashmakova N.V., Davydenko N.B.
Keywords
The COVID-19 pandemic is still claiming lives every day. With each new stage of the epidemic process, the aggressiveness of SARS-CoV-2 is gaining momentum, the virulence and transmissibility of the virus are increasing, the incubation period is shortening, resulting in superproliferation of the pathogen [1–4]. Despite the actions taken by governments and health authorities in many countries, the spread of COVID-19 continues to grow, affecting all populations [5].
Pregnant women are one of the most vulnerable groups due to their unique immunological status, physiological changes in the body, high susceptibility to pathogens causing acute respiratory viral infections, and pneumonia; there is a risk of adverse effects on the fetus. Therefore, currently an extremely challenging problem is to carry out a pregnancy in the context of COVID-19 pandemic as it is a serious test for the woman's body.
To date, research on the course of COVID-19 in pregnant women, the pathogenesis of detected complications and possible treatments continue to be summarized. Summarizing the studies by international and domestic authors, the following can be concluded: infection was asymptomatic in 10–27% of women, with 53–75% having a mild course; 3–16% of patients had moderate COVID-19, and 3–6% of pregnant women had a severe or extremely severe form [6-11]. There are sporadic publications of comparative analysis of the course of COVID-19 in pregnant women during the epidemic process [12].
Currently, there are several studies, mostly devoted to the analysis of severe COVID-19 in pregnant women [13–16]. However, it is necessary to pay attention not only to the severe course of COVID-19, but also to the mild and moderate ones, as these forms of the disease are associated with an increased risk of pregnancy complications and adverse perinatal outcomes. However, this information is fragmentary in the available literature [17], which explains the relevance of this work.
The present study aimed to investigate the clinical manifestations of mild and moderate COVID-19 in pregnant women during the first, second, and third waves of the epidemic process.
Materials and methods
This is a single-center prospective comparative study of three independent groups conducted according to the STROBE observational study reporting guidelines [18] at the Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia. The study enrolled 823 pregnant and postpartum women with mild to moderate COVID-19. Patients in Group 1 (n=186, first trimester 43, second trimester 60, third trimester 83), Group 2 (n=412, first trimester 66, second trimester 143, third trimester 203), and Group 3 (n=225, first trimester 22, second trimester 74, third trimester 128) were hospitalized during the first, second, and third waves of the epidemic, respectively. Waves 1, 2 and 3 were June-August, 2020, November 2020 to February 2021, and August-September 2021. Third-trimester patients predominated.
The gestational age of the patients ranged from 2 to 41 weeks. The mean gestational age of the patients was 31 (5.1) weeks (3–41 weeks), 25 (8.5) weeks (3–41 weeks), and 27 (8.2) weeks (2–41 weeks) in groups 1, 2, and 3, respectively. The groups were comparable in gestational age.
Inclusion criteria were pregnant and puerperal women with confirmed diagnosis of mild to moderate COVID-19 and signed informed consent to participate in the study.
COVID-19 infection was confirmed by polymerase chain reaction (PCR) with detection of SARS-CoV-2 antigen in an upper respiratory tract swab sampling (nasopharyngeal/ oropharyngeal exudate).
Disease severity was determined at admission, according to the criteria outlined in versions 2, 3, and 4 of the Methodological Recommendations on Organization of Medical Care for Pregnant Women, Women in Labor, Mothers, and Newborns with COVID-19 [19–21].
Clinical and demographic characteristics included age, gestational age, epidemiological history, possible source of infection, symptoms on admission, comorbidities, and characteristic features of pregnancy, delivery, and perinatal outcomes.
The study was reviewed and approved by the Research Ethics Committee of the Ural Research Institute of Maternity and Child Care (Ref. No: №12 of 21.09.2021). The main limitation of this study is the possibility of systematic selection error due to the single-center nature of the study.
Statistical analysis
Statistical analysis was performed using Microsoft Excel (2010), SPSS Statistics version 22.0 (IBM, Microsoft, USA), and Multifactor Dimensionality Reduction 2.0 beta 8 statistical software. Quantitative variables showing normal distribution were expressed as means (M) and standard deviation (SD) and presented as M (SD). Categorical variables were presented as counts and percentages and compared with the χ2 test and odds ratio (OR). Bonferroni correction was applied for multiple comparisons with adjusted significance thresholds of p<0.017.
Results and discussion
The mean age of the patients was 29.7 (4.7), 30.0 (4.9), and 30.0 (4.8) years in the first, second, and third wave, respectively. There were no statistically significant differences in patient age (p1-2=0.495, p1-3=0.428, p2-3= 0.752).
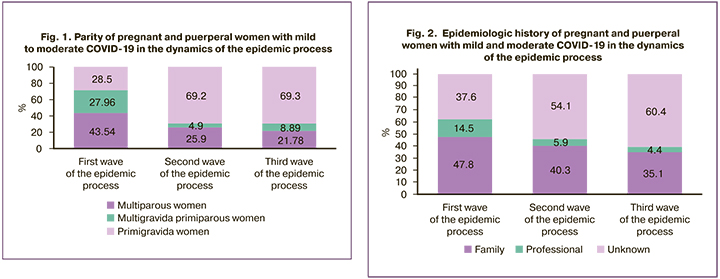
Figure 1 shows the parity of the observed patients. In the first wave, there were 81/186 (43.54%) primigravida women, 52/186 (27.96%) multigravida women, and 53/186 (28.5%) multiparous women. In the second wave there were 107/412 (25.9%) primigravida women, 20/412 (4.9%) multigravida women, and 285/412 (69.2%) multiparous women. In the third wave, there were 49/225 (21.78%) primigravida women, 20/225 (8.89%) multigravida women and 156/225 (69.3%) multiparous women.
During the first wave, patients were hospitalized at an average on day 4.3 (1.2) of illness due to longer PCR testing for COVID-19 in the outpatient phase, in contrast to the second and third waves in which they were admitted on mean day 2.5 (1.4) of illness. Longer testing in the first wave was associated with the need to confirm test results in reference centers, so hospitalization of patients was delayed.
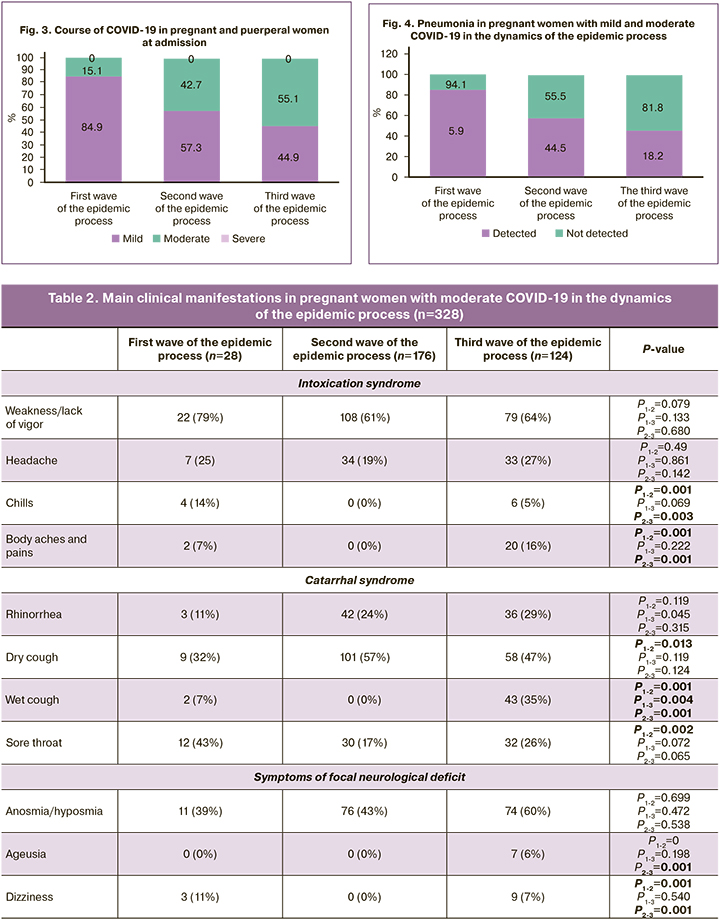
According to our results, at admission to the hospital in the first wave of the epidemic process, 158/186 (84.9%) and 28/186 (15.1%) patients had a mild form and moderate form, respectively. During the second wave, a mild course on admission was recorded in 236/412 (57.3%) patients, a moderate course in 176/412 (42.7%) pregnant women. In the third wave, 124/225 (55.1%) pregnant women had moderate COVID-19 and 101/225 (44.9%) patients had mild COVID-19. In the second and third waves, more pregnant patients were recorded as having moderate COVID-19: 47.2% in the second wave and 55.1% in the third wave (p2-3<0.0001), in contrast to 15.1% of patients in the first phase of the epidemic, where the proportion of mild forms was greater (p1-2<0.0001, p1-3<0.0001). The course of COVID-19 in pregnant women is shown in Figure 2.
There was a significant increase in the proportion of hospitalized patients with moderate COVID-19 in the second and third waves of the epidemic process, compared with the first wave: 1/186 (0.5%) in the first wave, 9/412 (2.18%) in the second wave and 18/225 (8.0%) in the third wave of the epidemic process (χ2=12.86 between the first and third waves; p<0.001 and χ2=12.126 between the second and third waves; p<0.001). In the second and third waves, a proportion of patients with mild COVID-19 received outpatient treatment due to changes in clinical guidelines [21], in contrast to the first wave, when all pregnant patients with confirmed COVID-19 were hospitalized.
The main clinical manifestations of COVID-19 in pregnant women with mild to moderate COVID-19 are presented in Tables 1 and 2. The most common symptoms included nasal congestion and rhinorrhea (49.5% in the first wave, 66% in the second wave, 72.9% in the third wave), weakness (56.5% in the first wave, 51.2% in the second wave, 64.4% in the third wave) anosmia and hyposmia (39.8% in the first wave, 46.6% in the second wave, 58.7% in the third wave), dry cough (32.3% in the first wave, 41.9% in the second wave, 40.9% in the third wave).
In the first wave of the epidemic process, no fever was observed in 119/186 (63.98%) patients. In 45/186 (24.19%) pregnant and puerperal women the temperature increased to subfebrile figures, 20/186 (10.75%) patients had body temperature from 38 to 39°С, and 2/186 (1.07%) had pyretic fever. In the second wave of the epidemic process, 193/412 (46.8%) patients had normal body temperature (p1-2=0.001), 161/412 (39.07%) had subfebrile temperature (p1-2=0.001), 48/412 (11.7%) pregnant and puerperal women had fever; 10/412 (2.4%) patients had pyretic fever. In the third wave of the epidemic process, normothermia (p1-3=0.003) was recorded in 111/225 (49.3%) pregnant and puerperal women, subfebrile fever in 68/225 (30.2%) patients, febrile fever in 42/225 (18.7%) women (p2-3=0.015), and pyretic fever in 4/225 (1.7%) patients.
Comparing the clinical manifestations of mild and moderate COVID-19 in the dynamics of the epidemic process, an increase in clinical symptoms in the third wave was noted, especially in comparison with the second wave. Rhinorrhea was the most frequent clinical symptom in patients of the third wave, followed by weakness, anosmia/hyposmia, dry and wet cough. There was a tendency to increase with symptoms of focal neurological deficit (anosmia/hyposmia, ageusia) in the dynamics of the epidemic process.
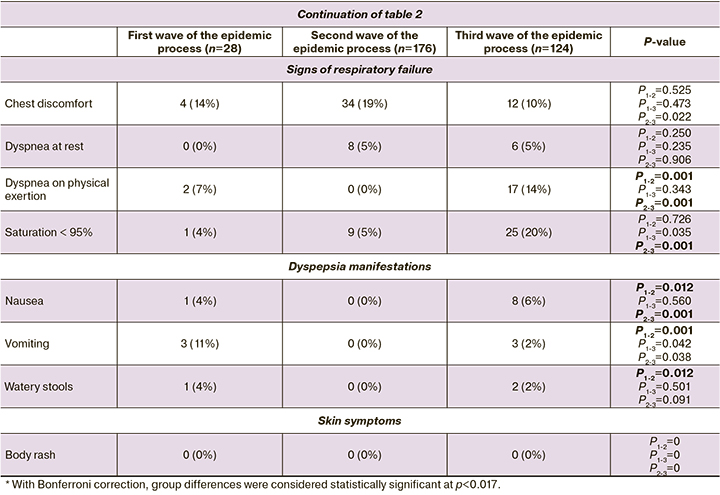
In the second wave of the epidemic process, 185/412 (44.5%) patients had pneumonia, compared to patients from the first and third waves of the epidemic process, where the proportion of pneumonia detected was 11/186 (5.9%) and 41/225 (18.2%), respectively.
The incidence of pneumonia in pregnant and puerperal women of the first wave was 11/186 (5.9%), in the second wave 183/412 (44.4%), and 41/225 (18.2%) in the third wave of the epidemic process. Thus, the highest relative risk (RR) for pneumonia was observed in pregnant and puerperal women with COVID-19 during the second wave, as compared to those in the first wave (RR1-2=7.593, 95% CI [4.24; 13.61]), and the third wave (RR2-3=1.473, 95% CI [1.33; 1.64]). No statistical significance was found when comparing the data of patients in the first and third waves (Fig. 3).
According to our results, the transition of mild COVID-19 to severe was observed in 1/186 (0.5%) first-term pregnant patients in the first wave of pregnancy, in 9/412 (2.2%) women in the second wave, and in 18/225 (8.0%) pregnant women in the third wave. In the second wave, these were predominantly second-trimester patients, 6/9 (66.6%) and only 3/9 (33.3%) in the third trimester. In contrast, in the third wave, there were predominantly multigravida patients, 17/18 (94.4%), and 7/18 (61.1%) in the third trimester. In the first wave, the disease worsening of the course was registered on day 9 after the onset of the disease and on day 5 after admission to the hospital.
In the second wave, deterioration of the disease course was observed 6.5 (0.3) days after the onset of the disease and 3.1 (0.2) days from admission; in the third wave, 4.4 (0.3) days from the disease and on 2.2 (0.4) days from the time of treatment in the infection department.
Patients in the third wave compared to patients in the first wave (OR=16.09, 95% CI [2.13; 121.69]), patients in the third wave compared to patients in the second wave (OR=3.89, 95% CI [1.72; 8.82]) had a higher risk of deterioration. No statistical significance was found when comparing data from the second and first waves (95% CI [0.47; 29.8]).
Conclusion
The study findings suggest that the characteristics of hospitalized pregnant women with COVID-19 varied in the dynamics of the epidemic process during 2020-2021 (first, second, and third waves of the epidemic process).
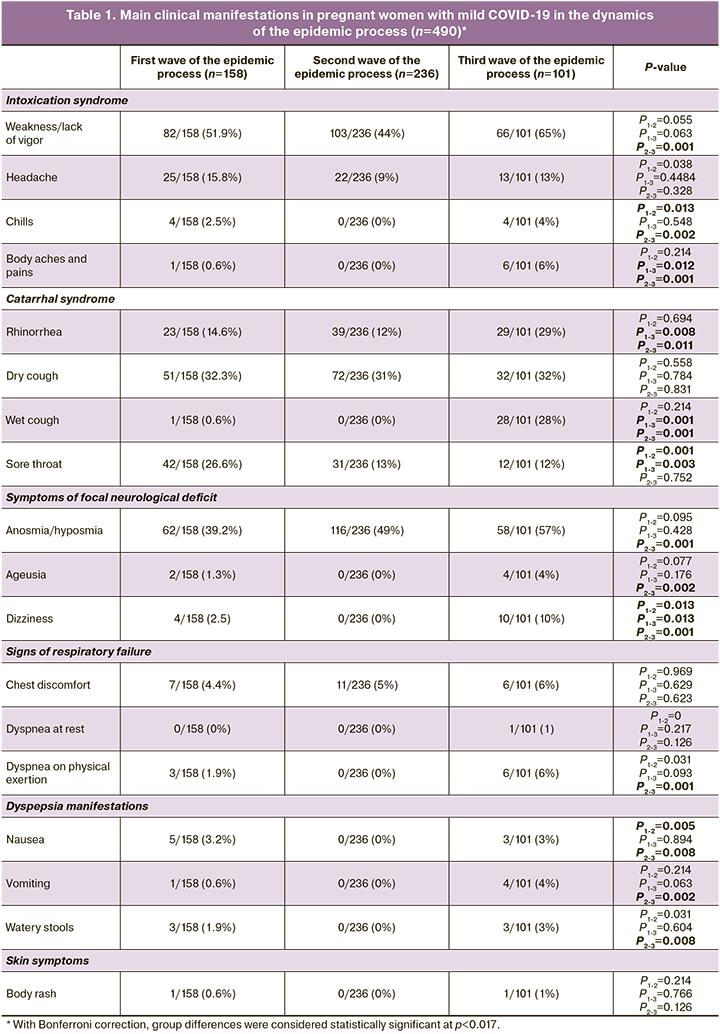
With each new stage of the epidemic process, there was a decrease in the frequency of accurate identification of the source of infection. Statistically significant in the first wave included mainly symptoms of general intoxication (fever, weakness, headache, and myalgia). In the second wave of the epidemic process, catarrhal manifestations of the disease (rhinorrhea, cough, sore throat) often occurred in pregnant and puerperal women. In the third wave, gastrointestinal problems appeared (nausea, vomiting, watery stools), although the classical symptoms remained (loss or change in smell, loss or change of taste, increased body temperature, cough, chills, myalgia). Pregnant and puerperal women of the second wave had higher rates of pneumonia, compared to patients of the first and third waves of the epidemic process. The number of detected pneumonias in the first and second waves was approximately the same. There was a tendency to increase the proportion of hospitalized patients with moderate COVID-19 in the second and third waves of the epidemic process, compared to the first wave.
References
1. Jamieson D.J., Honein M.A., Rasmussen S.A., Williams J.L., Swerdlow D.L., Biggerstaff M.S. et al. Novel Influenza A (H1N1) Pregnancy Working Group. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009; 374(9688): 451-8. https://dx.doi.org/10.1016/S0140-6736(09)61304-0.
2. Yao L., Wang J., Zhao J., Cui J., Zhihang Hu Z. Asymptomatic COVID-19 infection inpregnant woman in the third trimester: a case report. Chin. J. Perinat. Med. 2020; 23. https://dx.doi.org/10.3760/cma.j.cn113903-2020221-00143.
3. Zhou R., Chen Y., Lin C., Li H., Cai, X-Y., Cai Z-W., Lin G. Asymptomatic COVID-19 in pregnant woman with typical chest CT manifestation: a case report. Chin. J. Perinat. Med. 2020; 23. https://dx.doi.org/10.3760/ cma.j.cn113903-20200220-00134.
4. Schwartz D.A., Graham A.L. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020; 12(2): 194. https://dx.doi.org/10.3390/v12020194.
5. WHO. Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19). 2020.
6. Li N., Han L., Peng M., Lv Y., Ouyang Y., Liu K. et al. Maternal and neonatal outcomes of pregnant women with coronavirus desease 2019 (COVID-19) pneumonia: A case-control study. Clin. Infect. Dis. 2020; 71(16): 2035-41. https://dx.doi.org/10.1093/cid/ciaa352.
7. Lowe B., Bopp B. COVID-19 vaginal delivery — A case report. Aust. N. Z. J. Obstet. Gynaecol. 2020; 60(3): 465-6. https://dx.doi.org/10.1111/ajo.13173.
8. Xiong X., Wei H., Zhang Z., Chang J., Ma X., Gao X. et al. Vaginal delivery report of a healthy neonate born to a convalescent mother with COVID-19. J. Med. Virol. 2020; 92(9): 1657-9. https://dx.doi.org/10.1002/jmv.25857.
9. Lee D.H., Lee J., Kim E., Woo K., Park H.Y., An J. Emergency cesarean section performed in a patient with confirmed severe acute respiratory syndrome Coronavirus-2 -a case report. Korean J. Anesthesiol. 2020; 73(4): 347-51. https://dx.doi.org/10.4097/kja.20116.
10. Iqbal S.N., Overcash R., Mokhtari N., Saeed H., Gold S., Auguste T. et al. An Uncomplicated delivery in a patient with Covid-19 in the United States. N. Engl. J. Med. 2020; 382(16): e34. https://dx.doi.org/10.1056/NEJMc2007605.
11. Кравченко Е.Н., Куклина Л.В., Овчинникова Е.М., Чебакова В.Ю., Выжлова Е.Н., Баранов И.И. COVID-19 во время беременности: особенности течения и рациональная терапия с использованием препаратов рекомбинантного интерферона альфа-2Ь. Российский вестник акушера-гинеколога. 2021; 21(5): 96-101. [Kravchenko E.N., Kuklina L.V., Ovchinnikova E.M., Chebakova V.Yu., Vyzhlova E.N., Baranov I.I. COVID- 19 in pregnancy: special characteristics of the course and rational therapy with recombinant interferon alfa-2b formulations. Russian Bulletin of Obstetrician-Gynecologist. 2021; 21(5): 96-101. (in Russian)]. https://dx.doi.org/10.17116/ rosakush20212105196.
12. Белокриницкая Т.Е., Фролова Н.И., Колмакова К.А., Шаметова Е.А. Факторы риска и особенности течения COVID-19 у беременных: сравнительный анализ эпидемических вспышек 2020 и 2021 г. Гинекология. 2021; 23(5): 421-7. [Belokrinitskaya T.E., Frolova N.I., Kolmakova K.A., Shametova E.A. Risk factors and features of COVID-19 course in pregnant women: a comparative analysis of epidemic outbreaks in 2020 and 2021. Gynecology. 2021; 23(5): 421-7. (in Russian)]. https://dx.doi.org/10.26442/ 20795696.2021.5.201107.
13. Alfaraj S.H., Al-Tawfiq J.A., Memish Z.A. Middle East respiratory syndrome coronavirus (MERS-CoV) infection during pregnancy: report of two cases & review of the literature. Microbiol. Immunol. Infect. 2019; 52(3): 501-3. https://dx.doi.org/10.1016/j.jmii.2018.04.005.
14. Chen S., Huang B., Luo D.J., Li X., Yang F., Zhao Y. et al. Pregnancy with new coronavirus infection: a clinical characteristics and placental pathological analysis of three cases. Zhonghua Bing Li Xue Za Zhi. 2020; 49(5): 418-23. https://dx.doi.org/10.3760/cma.j.cn112151-20200225-00138.
15. Fan C., Lei D., Fang C., Li C., Wang M., Yuling Liu Y. et al. Perinatal transmission of COVID-19 associated SARS-CoV-2: should we worry? Clin. Infect. Dis. 2020; 72(5). https://dx.doi.org/10.1093/cid/ciaa226.
16. Liu Y., Chen H., Tang K., Guo Y. Withdrawn: Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J. Infect. 2020; Mar 5: S0163-4453(20)30109-2. https://dx.doi.org/10.1016/j.jinf.2020.02.028.
17. Белокриницкая Т.Е., Артымук Н.В., Филиппов О.С., Фролова Н.И. Клиническое течение, материнские и перинатальные исходы новой коронавирусной инфекции COVID-19 у беременных Сибири и Дальнего Востока. Акушерство и гинекология. 2021; 2: 48-54. [Belokrinitskaya T.E., Artymuk N.V., Filippov O.S., Frolova N.I. Clinical course, maternal and perinatal outcomes of the COVID-19 in pregnant women of Siberia and the Far East. Obstetrics and gynecology. 2021; 2: 48-54. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.2.48-54.
18. von Elm E., AltmanD.G., EggerM., PocockS.J., G0tzschePC., Vandenbroucke J.P.; STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 2008; 61(4): 344-9. https://dx.doi.org/10.1016/ j.jclinepi.2007.11.008.
19. Министерство здравоохранения Российской Федерации. Методические рекомендации. Организация оказания медицинской помощи беременным, роженицам, родильницам и новорожденным при новой коронавирусной инфекции COVID-19. Версия 2 от 28.05.2020. [Ministry of Health of the Russian Federation. Guidelines. Organization of medical care for pregnant women, women in labor, new mothers and newborns with new coronavirus infection COVID-19. Version 2 of 28.05.2020. (in Russian)].
20. Министерство здравоохранения Российской Федерации. Методические рекомендации. Организация оказания медицинской помощи беременным, роженицам, родильницам и новорожденным при новой коронавирусной инфекции COVID-19. Версия 3 от 25.01.2021. [Ministry of Health of the Russian Federation. Guidelines. Organization of medical care for pregnant women, women in labor, new mothers and newborns with new coronavirus infection COVID-19. Version 3 of 25.01.2021. (in Russian)].
21. Министерство здравоохранения Российской Федерации. Методические рекомендации. Организация оказания медицинской помощи беременным, роженицам, родильницам и новорожденным при новой коронавирусной инфекции COVID-19. Версия 4 от 05.07.2021. [Ministry of Health of the Russian Federation. Guidelines. Organization of medical care for pregnant women, women in labor, new mothers and newborns with new coronavirus infection COVID-19. Version 4 of 05.07.2021. (in Russian)].
Received 17.12.2021
Accepted 21.02.2022
About the Authors
Galina B. Malgina, Dr. Med. Sci., Director of the Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia, galinamalgina@mail.ru, https://orcid.org/0000-0002-5500-6296, 620028, Russia, Ekaterinburg, Repin str., 1.Maria M. Dyakova, Physician, Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia, mariadakova40@mail.ru, https://orcid.org/0000-0001-7911-6783, 620028, Russia, Ekaterinburg, Repin str., 1.
Svetlana V. Bychkova, PhD, Leading Researcher, Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia, simomm@mail.ru, https://orcid.org/0000-0002-8892-7785, 620028, Russia, Ekaterinburg, Repin str., 1.
Natalia A. Pepelyaeva, PhD, Head of the Department, Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia, pepelyaevana@niiomm.ru, https://orcid.org/0000-0003-3278-2249, 620028, Russia, Ekaterinburg, Repin str., 1.
Sergey S. Olkov, PhD, Deputy Head of the Pediatrics Clinic, Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia, olkovss@niiomm.ru, https://orcid.org/0000-0002-6142-370, 620028, Russia, Ekaterinburg, Repin str., 1.
Oksana A. Melkozerova, Dr. Med. Sci., Deputy Director for Science, Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia, abolmed@mail.ru, ORCID https://orcid.org/0000-0002-4090-0578, 620028, Russia, Ekaterinburg, Repin str., 1.
Nadezhda V. Bashmakova, Dr. Med. Sci., Professor, Chief Researcher, Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia, bashmakovanv@niiomm.ru, https://orcid.org/0000-0001-5746-316X, 620028, Russia, Ekaterinburg, Repin str., 1.
Natalia B. Davydenko, PhD, Head of the Department, Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia, orgomm@mail.ru, https://orcid.org/0000-0002-1617-5521, 620028, Russia, Ekaterinburg, Repin str., 1.
Corresponding authors: Maria M. Dyakova, mariadakova40@gmail.com; Svetlana V. Bychkova, simomm@mail.ru
Authors' contributions: Malgina G.B. - conception and design of the study, drafting and editing of the manuscript;
Dyakova M.M. - material collection and processing, drafting of the manuscript; Bychkova S.V. - review of the relevant literature, drafting and editing of the manuscript; Pepelyaeva N.A., Olkov S.S. - material collection; Melkozerova O.A., Bashmakova N.V. - manuscript editing; Davydenko N.B. - review of the relevant literature.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Ural Research Institute of Maternity and Child Care (Ref. No: №12 of 21.09.2021).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Malgina G.B., Dyakova M.M., Bychkova S.V., Pepelyaeva N.A., Olkov S.S., Melkozerova O.A., Bashmakova N.V., Davydenko N.B. Clinical manifestations of mild and moderate novel coronavirus disease in pregnant women in epidemic dynamics.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 3: 23-31 (in Russian)
https://dx.doi.org/10.18565/aig.2022.3.23-31



